Removal of Ochratoxin A from Grape Juice by Clarification: A Response Surface Methodology Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grape Juice Preparation
2.3. Addition of Clarifier to Grape Juice
2.4. OTA Clean-Up and Measurement
2.5. Apparatus of OTA Analysis
2.6. Validation of the OTA Analysis
2.7. Measurement of Total Phenolic Acid Content
2.8. Measurement of Total Flavonoid Content
2.9. Antioxidant Capacity Measurement
2.10. Experimental Design and Statistical Analysis of Data
2.11. Optimization
3. Results and Discussion
3.1. Method Validation
3.2. OTA Content, Antioxidant Content, and Capacity of Unclarified Grape Juice
3.3. The Effect of Grape Juice Clarification on the Reduction of OTA
3.4. The Effect of Grape Juice Clarification on the Reduction of TPC
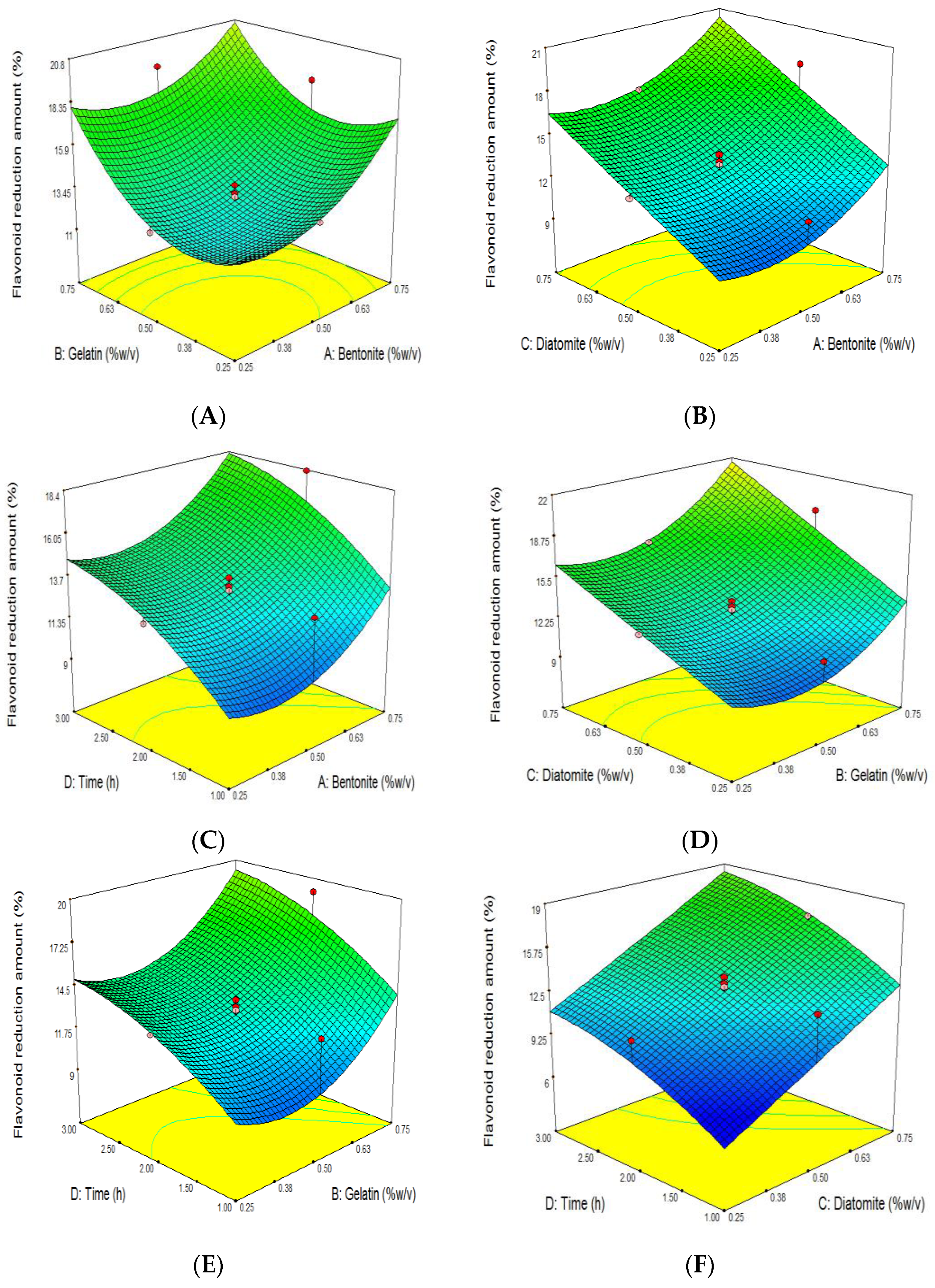
3.5. The Effect of Grape Juice Clarification on the Reduction of TFC
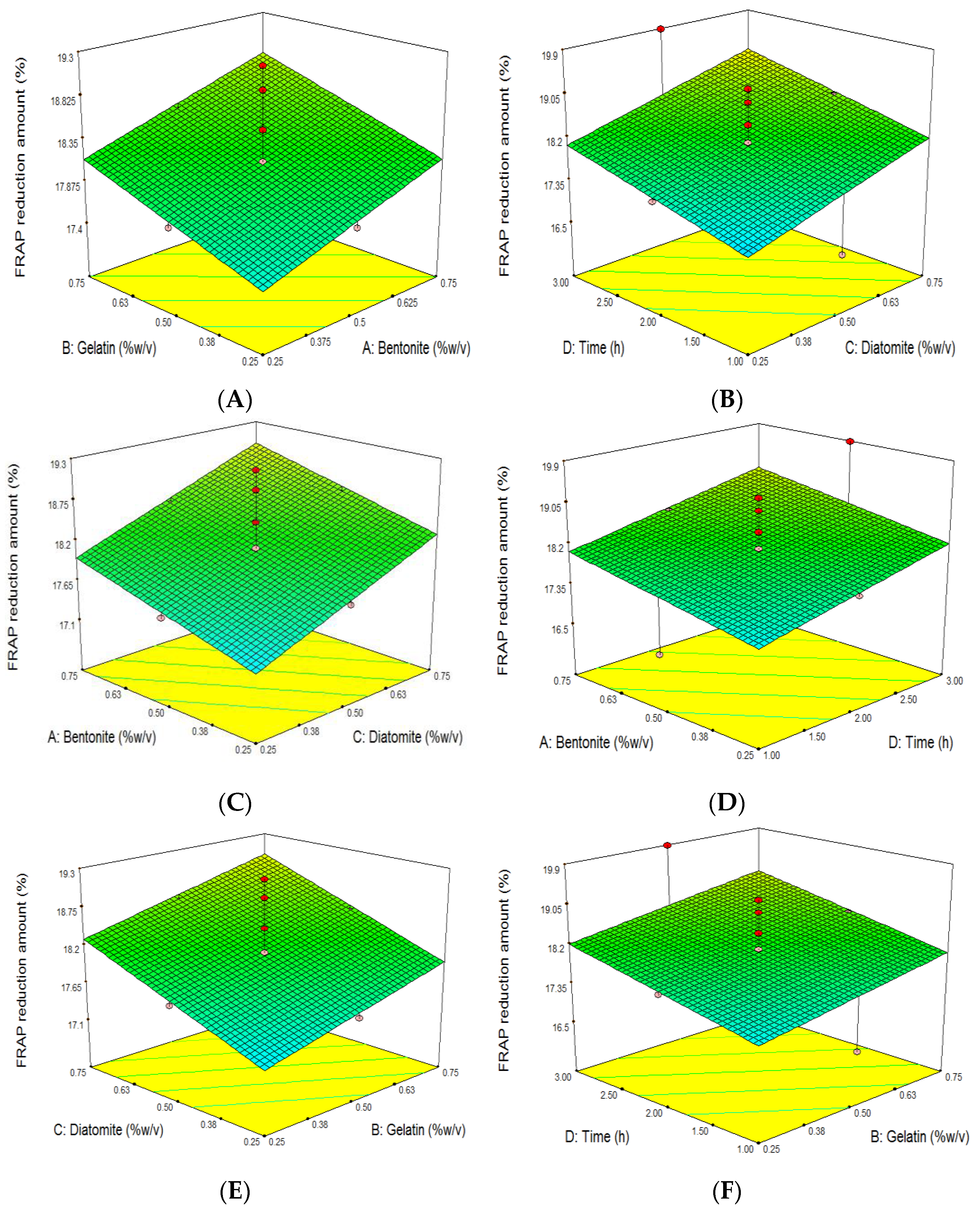
3.6. The Effect of Grape Juice Clarification on the Reduction of Antioxidant Capacity
3.7. Process Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balali-Mood, M.; Riahi-Zanjani, B.; Mahdizadeh, A.; Moradi, V.; Fazeli-Bakhtiyari, R. Arsenic and lead contaminations in commercial fruit juices of markets in Mashhad, Iran. Iran. J. Toxicol. 2018, 12, 15–20. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Robledo, P.; Manríquez, D.; Molina, R.; Defilippi, B.G. Characterization of sugars and organic acids in commercial varieties of table grapes. Chil. J. Agric. Res. 2011, 71, 452. [Google Scholar] [CrossRef] [Green Version]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic compounds and antioxidant activity in grape juices: A chemical and sensory view. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Dutra, M.d.C.P.; Rodrigues, L.L.; de Oliveira, D.; Pereira, G.E.; dos Santos Lima, M. Integrated analyses of phenolic compounds and minerals of Brazilian organic and conventional grape juices and wines: Validation of a method for determination of Cu, Fe and Mn. Food Chem. 2018, 269, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Heshmati, A.; Nili-Ahmadabadi, A.; Rahimi, A.; Vahidinia, A.; Taheri, M. Dissipation behavior and risk assessment of fungicide and insecticide residues in grape under open-field, storage and washing conditions. J. Clean. Prod. 2020, 270, 122287. [Google Scholar] [CrossRef]
- Dutra, M.d.C.P.; Viana, A.C.; Pereira, G.E.; Nassur, R.d.C.M.R.; dos Santos Lima, M. Whole, concentrated and reconstituted grape juice: Impact of processes on phenolic composition,“foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chem. 2021, 343, 128399. [Google Scholar] [CrossRef]
- Chanson-Rolle, A.; Braesco, V.; Chupin, J.; Bouillot, L. Nutritional composition of orange juice: A comparative study between French commercial and home-made juices. Food Nutr. Sci. 2016, 7, 252. [Google Scholar] [CrossRef]
- Jiménez, M.; Juárez, N.; Jiménez-Fernández, V.; Monribot-Villanueva, J.; Guerrero-Analco, J. Phenolic compounds and antioxidant activity of wild grape (Vitis tiliifolia). Ital. J. Food Sci. 2018, 30, 1. [Google Scholar]
- Khazaei, S.; Talebi Ghane, E.; Bashirian, S.; Mehri, F. The concentration of potentially toxic elements (PTEs) in fruit juices: A global systematic review, meta-analysis and probabilistic health risk assessment. Int. J. Environ. Anal. Chem. 2021, 1–13. [Google Scholar] [CrossRef]
- Genchev, R.; Angelova, G.; Laskova, I.; Gotcheva, V.; Angelov, A. Mycoflora of fresh chokeberry (Aronia melanocarpa) and ochratoxin-producing ability of Penicillium isolates. Qual. Assur. Saf. Crops Foods 2015, 7, 123–131. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Heshmati, A.; Ghadimi, S.; Ranjbar, A.; Khaneghah, A.M. Assessment of processing impacts and type of clarifier on the concentration of ochratoxin A in pekmez as a conventional grape-based product. LWT 2020, 119, 108882. [Google Scholar] [CrossRef]
- Heshmati, A.; Mozaffari Nejad, A.S. Ochratoxin A in dried grapes in Hamadan province, Iran. Food Addit. Contam. Part B 2015, 8, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Villeda, B.; Lobos, O.; Aguilar-Zuniga, K.; Carrasco-Sánchez, V. Ochratoxins in wines: A review of their occurrence in the last decade, toxicity, and exposure risk in humans. Toxins 2021, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y.; Ji, M. Determination of Ochratoxin A contamination in grapes, processed grape products and animal-derived products using ultra-performance liquid chromatography-tandem mass spectroscopy system. Sci. Rep. 2018, 8, 1–8. [Google Scholar]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Coppa, C.F.S.C.; Franco, L.T.; de Oliveira, C.A.F. The concentration and prevalence of ochratoxin A in coffee and coffee-based products: A global systematic review, meta-analysis and meta-regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef]
- Heshmati, A.; Zohrevand, T.; Khaneghah, A.M.; Nejad, A.S.M.; Sant’Ana, A.S. Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: Dietary exposure risk assessment. Food Chem. Toxicol. 2017, 106, 202–208. [Google Scholar] [CrossRef]
- Batrinou, A.; Houhoula, D.; Papageorgi, E. Rapid detection of mycotoxins on foods and beverages with enzyme-linked immunosorbent assay. Qual. Assur. Saf. Crops Foods 2020, 12, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- Nourbakhsh, F.; Tajbakhsh, E. Neurotoxicity mechanism of Ochratoxin A. Qual. Assur. Saf. Crops Foods 2021, 13, 34–45. [Google Scholar] [CrossRef]
- Mehri, F.; Esfahani, M.; Heshmati, A.; Jenabi, E.; Khazaei, S. The prevalence of ochratoxin A in dried grapes and grape-derived products: A systematic review and meta-analysis. Toxin Rev. 2020, 41, 1–10. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Khaneghah, A.M.; Campagnollo, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT 2017, 80, 200–207. [Google Scholar] [CrossRef]
- Var, I.; Kabak, B.; Erginkaya, Z. Reduction in ochratoxin A levels in white wine, following treatment with activated carbon and sodium bentonite. Food Control 2008, 19, 592–598. [Google Scholar] [CrossRef]
- Quintela, S.; Villarán, M.C.; de Armentia, I.L.; Elejalde, E. Ochratoxin A removal in wine: A review. Food Control 2013, 30, 439–445. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.H.; Kim, M. Efficient, safe, renewable, and industrially feasible strategy employing Bacillus subtilis with alginate bead composite for the reduction of ochratoxin A from wine. J. Clean. Prod. 2020, 242, 118344. [Google Scholar] [CrossRef]
- Dachery, B.; Hernandes, K.C.; Veras, F.F.; Schmidt, L.; Augusti, P.R.; Manfroi, V.; Zini, C.A.; Welke, J.E. Effect of Aspergillus carbonarius on ochratoxin a levels, volatile profile and antioxidant activity of the grapes and respective wines. Food Res. Int. 2019, 126, 108687. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Inês, A.; Silva, D.; Filipe-Ribeiro, L.; Abrunhosa, L.; Nunes, F.M. Elimination of ochratoxin A from white and red wines: Critical characteristics of activated carbons and impact on wine quality. LWT 2021, 140, 110838. [Google Scholar] [CrossRef]
- Lulamba, T.E.; Stafford, R.A.; Njobeh, P.B. The relative effectiveness of two filter aids in removing ochratoxin A during beer filtration. J. Inst. Brew. 2019, 125, 422–432. [Google Scholar] [CrossRef]
- Sun, X.; Niu, Y.; Ma, T.; Xu, P.; Huang, W.; Zhan, J. Determination, content analysis and removal efficiency of fining agents on ochratoxin A in Chinese wines. Food Control 2017, 73, 382–392. [Google Scholar] [CrossRef]
- Capanoglu, E.; de Vos, R.C.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol content during production of grape juice concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Dıblan, S.; Özkan, M. Effects of various clarification treatments on anthocyanins, color, phenolics and antioxidant activity of red grape juice. Food Chem. 2021, 352, 129321. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.; Taillandier, P.; Rizk, M.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Analysis of the impact of fining agents types, oenological tannins and mannoproteins and their concentrations on the phenolic composition of red wine. LWT Food Sci. Technol. 2017, 83, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Palencia, L.A.; Hawken, P.; Talcott, S.T. Phytochemical, antioxidant and pigment stability of açai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res. Int. 2007, 40, 620–628. [Google Scholar] [CrossRef]
- Heshmati, A.; Mehri, F.; Nili-Ahmadabadi, A.; Khaneghah, A.M. The fate of ochratoxin A during grape vinegar production. Int. J. Environ. Anal. Chem. 2021, 1–10. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Antioxidant potential of green and black tea determined using the ferric reducing power (FRAP) assay. Int. J. Food Sci. Nutr. 2000, 51, 181–188. [Google Scholar] [CrossRef]
- Stroka, J.; Ambrosio, M.; Doncheva, I.; Lerda, D.; Mischke, C.; Breidbach, A. Validation of an Analytical Method to Determine the Content of Ochratoxin A in Animal Feed. European Commission Joint Research Centre Institute for Reference Materials and Measurements; Office for Official Publications of the European Communities: Luxembourg, 2009; p. 54. [Google Scholar]
- Leong, S.L.L.; Hocking, A.D.; Scott, E.S. The effect of juice clarification, static or rotary fermentation and fining on ochratoxin A in wine. Aust. J. Grape Wine Res. 2006, 12, 245–251. [Google Scholar] [CrossRef]
- Mine Kurtbay, H.; Bekçi, Z.; Merdivan, M.; Yurdakoç, K. Reduction of ochratoxin A levels in red wine by bentonite, modified bentonites, and chitosan. J. Agric. Food Chem. 2008, 56, 2541–2545. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef]
- Nones, J.; Riella, H.G.; Trentin, A.G.; Nones, J. Effects of bentonite on different cell types: A brief review. Appl. Clay Sci. 2015, 105, 225–230. [Google Scholar] [CrossRef]
- Downing, D. Canning of juices, fruit drinks and water. In A Complete Course in Canning, Book III; Woodhead Publishing: Cambridge, UK, 1996. [Google Scholar]
- Al-Ghouti, M.A.; Al-Degs, Y.S.; Khraisheh, M.A.; Ahmad, M.N.; Allen, S.J. Mechanisms and chemistry of dye adsorption on manganese oxides-modified diatomite. J. Environ. Manag. 2009, 90, 3520–3527. [Google Scholar] [CrossRef] [PubMed]
- Concenco, F.; Brotto, G.F.; Nora, L. Grape wine and juice: Comparison on resveratrol levels. Int. J. Adv. Res. Sci. Eng. Technol. 2019, 6, 368–386. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, F.J.; Jordão, A.M. Influence of different commercial fining agents on proanthocyanidin fraction and antioxidant activity of a red wine from Baga grapes. OENO One 2009, 43, 111–120. [Google Scholar] [CrossRef]
- Balík, J.; Kyseláková, M.; Tříska, J.; Vrchotová, N.; Veverka, J.; Híc, P.; Totušek, J.; Lefnerová, D. The changes of selected phenolic substances in wine technology. Czech J. Food Sci. 2009, 26, S3–S12. [Google Scholar] [CrossRef] [Green Version]
- Dordoni, R.; Galasi, R.; Colangelo, D.; De Faveri, D.M.; Lambri, M. Effects of fining with different bentonite labels and doses on colloidal stability and colour of a Valpolicella red wine. Int. J. Food Sci. Technol. 2015, 50, 2246–2254. [Google Scholar] [CrossRef]
- González-Neves, G.; Favre, G.; Gil, G. Effect of fining on the colour and pigment composition of young red wines. Food Chem. 2014, 157, 385–392. [Google Scholar] [CrossRef]
- Wasila, H.; Li, X.; Liu, L.; Ahmad, I.; Ahmad, S. Peel effects on phenolic composition, antioxidant activity, and making of pomegranate juice and wine. J. Food Sci. 2013, 78, C1166–C1172. [Google Scholar] [CrossRef]
- Bagci, P.O. Effective clarification of pomegranate juice: A comparative study of pretreatment methods and their influence on ultrafiltration flux. J. Food Eng. 2014, 141, 58–64. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Du, W.; Sun, J. Effect of fining and filtration on the haze formation in bayberry (Myrica rubra Sieb. et Zucc.) juice. J. Agric. Food Chem. 2007, 55, 113–119. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Bin Jumah, M.N.; Othman, S.I.; Alruhaimi, R.S.; Al-Khalawi, N.; Salama, Y.F.; Allam, A.A.; Abukhadra, M.R. Synthesis of Chitosan/Diatomite Composite as an Advanced Delivery System for Ibuprofen Drug; Equilibrium Studies and the Release Profile; ACS Omega: Washington, DC, USA, 2021. [Google Scholar]
- Ren, M.; Liu, S.; Li, R.; You, Y.; Huang, W.; Zhan, J. Clarifying effect of different fining agents on mulberry wine. Int. J. Food Sci. Technol. 2020, 55, 1578–1585. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochemistry 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, H.K. Effects of fining agents on antioxidant capacity of red wines. J. Inst. Brew. 2011, 117, 55–60. [Google Scholar] [CrossRef]
- Farahmand, M.; Golmakani, M.T.; Mesbahi, G.; Farahnaky, A. Investigating the effects of large-scale processing on phytochemicals and antioxidant activity of pomegranate juice. J. Food Process. Preserv. 2017, 41, e12792. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Wang, W.; Yue, J.; Yue, P.; Gao, X. Anthocyanin profile, color and antioxidant activity of blueberry (Vaccinium ashei) juice as affected by thermal pretreatment. Int. J. Food Prop. 2019, 22, 1035–1046. [Google Scholar] [CrossRef] [Green Version]




| Run | Independent Variables | Dependent Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1: Bentonite (% w/v) | X2: Gelatin (% w/v) | X3: Diatomite (% w/v) | X4: Time (h) | OTA (µg/kg) | Loss of OTA (%) | TPC (µg/mL) | Loss of TPC (%) | TFC (µg/mL) | Loss of TFC (%) | FRAP (µmol/L) | Loss of FRAP (%) | |
| 1 | 0.25 (−1) | 0.25 (−1) | 0.25 (−1) | 1 (−1) | 3.47 | 30.56 | 234.33 | 20.81 | 184.13 | 7.21 | 1138.34 | 16.43 |
| 2 | 0.25 (−1) | 0.25 (−1) | 0.75 (+1) | 1 (−1) | 3.37 | 32.65 | 218.68 | 26.10 | 173.6 | 12.51 | 1122.40 | 17.6 |
| 3 | 0.25 (−1) | 0.75 (+1) | 0.25 (−1) | 1 (−1) | 3.07 | 38.55 | 217.94 | 26.35 | 177.91 | 10.34 | 1126.76 | 17.28 |
| 4 | 0.25 (−1) | 0.75 (+1) | 0.75 (+1) | 1 (−1) | 3.00 | 39.95 | 202.91 | 31.43 | 164.16 | 17.27 | 1113.28 | 18.27 |
| 5 | 0.25 (−1) | 0.5 (0) | 0.5 (0) | 2 (0) | 3.30 | 34.00 | 214.68 | 27.45 | 173.21 | 12.71 | 1120.77 | 17.72 |
| 6 | 0.25 (−1) | 0.25 (−1) | 0.25 (−1) | 3 (+1) | 3.50 | 30.06 | 210.66 | 28.81 | 174.69 | 11.96 | 1128.67 | 17.14 |
| 7 | 0.25 (−1) | 0.25 (−1) | 0.75 (+1) | 3 (+1) | 3.40 | 32.1 | 194.86 | 34.15 | 164.16 | 17.27 | 1115.05 | 18.14 |
| 8 | 0.25 (−1) | 0.75 (+1) | 0.25 (−1) | 3 (+1) | 2.74 | 45.15 | 194.56 | 34.25 | 168.26 | 15.20 | 1119.54 | 17.81 |
| 9 | 0.25 (−1) | 0.75 (+1) | 0.75 (+1) | 3 (+1) | 2.71 | 45.85 | 179.00 | 39.51 | 147.31 | 25.76 | 1105.92 | 18.81 |
| 10 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 1 (−1) | 3.05 | 39.00 | 218.29 | 26.23 | 172.55 | 13.04 | 1137.11 | 16.52 |
| 11 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 3.10 | 38.00 | 221.58 | 25.12 | 176.08 | 11.26 | 1116.41 | 18.04 |
| 12 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 3.09 | 38.24 | 216.81 | 26.73 | 171.41 | 13.62 | 1116.27 | 18.05 |
| 13 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 2.92 | 41.7 | 222.08 | 24.95 | 176.55 | 11.03 | 1115.59 | 18.1 |
| 14 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 2.90 | 42.10 | 216.34 | 26.89 | 172.8 | 12.92 | 1104.83 | 18.89 |
| 15 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 2.93 | 41.50 | 215.96 | 27.02 | 172.37 | 13.13 | 1101.29 | 19.15 |
| 16 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 2 (0) | 2.91 | 41.75 | 216.58 | 26.81 | 174.04 | 12.29 | 1110.83 | 18.45 |
| 17 | 0.5 (0) | 0.5 (0) | 0.5 (0) | 3 (+1) | 2.94 | 41.15 | 212.05 | 28.34 | 173.47 | 12.58 | 1091.21 | 19.89 |
| 18 | 0.75 (+1) | 0.25 (−1) | 0.75 (+1) | 1 (−1) | 3.62 | 27.65 | 202.34 | 31.62 | 164.16 | 17.27 | 1113.14 | 18.28 |
| 19 | 0.5 (0) | 0.25 (−1) | 0.5 (0) | 2 (0) | 3.52 | 29.60 | 215.6 | 27.14 | 172.08 | 13.28 | 1120.77 | 17.72 |
| 20 | 0.5 (0) | 0.5 (0) | 0.25 (−1) | 2 (0) | 2.81 | 43.80 | 212.91 | 28.05 | 176.34 | 11.13 | 1123.22 | 17.54 |
| 21 | 0.5 (0) | 0.5 (0) | 0.75 (+1) | 2 (0) | 2.88 | 42.45 | 199.98 | 32.42 | 165.82 | 16.43 | 1109.60 | 18.54 |
| 22 | 0.5 (0) | 0.75 (+1) | 0.5 (0) | 2 (0) | 2.60 | 47.97 | 200.15 | 32.36 | 160.48 | 19.13 | 1111.64 | 18.39 |
| 23 | 0.75 (+1) | 0.25 (−1) | 0.25 (−1) | 1 (−1) | 2.83 | 43.35 | 216.43 | 26.86 | 180.04 | 9.27 | 1126.76 | 17.28 |
| 24 | 0.75 (+1) | 0.25 (−1) | 0.25 (−1) | 3 (+1) | 2.79 | 44.23 | 194.83 | 34.16 | 166.19 | 16.25 | 1119.54 | 17.81 |
| 25 | 0.75 (+1) | 0.75 (+1) | 0.75 (+1) | 1 (−1) | 2.54 | 49.23 | 187.22 | 36.73 | 156.86 | 20.95 | 1104.15 | 18.94 |
| 26 | 0.75 (+1) | 0.75 (+1) | 0.25 (−1) | 1 (−1) | 2.56 | 48.75 | 203.35 | 31.28 | 173.61 | 12.51 | 1117.50 | 17.96 |
| 27 | 0.75 (+1) | 0.75 (+1) | 0.25 (−1) | 3 (+1) | 2.53 | 49.32 | 180.95 | 38.85 | 161.72 | 18.50 | 1110.42 | 18.48 |
| 28 | 0.75 (+1) | 0.75 (+1) | 0.75 (+1) | 3 (+1) | 2.47 | 50.56 | 163.58 | 44.72 | 147.21 | 25.81 | 1096.80 | 19.48 |
| 29 | 0.75 (+1) | 0.25 (−1) | 0.75 (+1) | 3 (+1) | 3.12 | 37.54 | 178.49 | 39.68 | 152.55 | 23.12 | 1105.65 | 18.83 |
| 30 | 0.75 (+1) | 0.5 (0) | 0.5 (0) | 2 (0) | 3.27 | 34.67 | 198.82 | 32.81 | 162.03 | 18.34 | 1138.34 | 18.39 |
| Spiked Concentration (µg/kg) | Recovery | Linearity (µg mL−1) | LOD (µg kg−1) | LOQ (µg kg−1) | |||
|---|---|---|---|---|---|---|---|
| Intra-Day | RSD | Inter-Day | RDS | ||||
| 2.5 | 98.25 | 12.56 | 99.78 | 14.87 | |||
| 5 | 101.23 | 11.87 | 100.85 | 15.87 | 0.05–25 | 0.07 | 0.23 |
| 10 | 97.54 | 9.87 | 96.09 | 10.76 | |||
| Response | Source | Sum of Square | DF | Mean Square | F Value | Probe > F | Model |
|---|---|---|---|---|---|---|---|
| OTA reduction amount (%) | Model | 872.19 | 4 | 218.05 | 16.96 | <0.0001 | Linear |
| Lack of fit | 303.42 | 20 | 15.17 | 4.24 | 0.0580 | ||
| Pure error | 17.91 | 5 | 3.58 | ||||
| Total | 49,307.99 | 30 | 1643.60 | ||||
| R2 | 0.7308 | ||||||
| TPC reduction amount (%) | Model | 207.72 | 4 | 51.93 | 26.57 | <0.0001 | Quadratic |
| Lack of fit | 24.80 | 10 | 2.48 | 2.75 | 0.1380 | ||
| Pure error | 4.51 | 5 | 0.90 | ||||
| Total | 28,908.91 | 30 | 963.63 | ||||
| R2 | 0.9651 | ||||||
| TFC reduction amount (%) | Model | 78.65 | 4 | 19.66 | 6.73 | 0.0026 | Quadratic |
| Lack of fit | 38.24 | 10 | 3.83 | 3.51 | 0.0892 | ||
| Pure error | 5.47 | 5 | 1.09 | ||||
| Total | 7423.38 | 30 | 247.45 | ||||
| R2 | 0.9282 | ||||||
| FRAP reduction amount (%) | Model | 12.38 | 4 | 3.09 | 14.14 | <0.001 | Linear |
| Lack of fit | 4.34 | 20 | 0.22 | 0.96 | 0.5809 | ||
| Pure error | 1.13 | 5 | 0.23 | ||||
| Total | 9880.13 | 30 | 329.34 | ||||
| R2 | 0.6935 |
| Coefficients | Reduction of OTA (%) | p-Value | Reduction of TPC (%) | p-Value | Reduction of TFC (%) | p-Value | Reduction of FRAP (%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Intercept (X0) | +40.05 | - | +27.07 | - | +13.06 | - | +18.13 | - |
| X1 | +3.14 | 0.0010 | +2.66 | <0.0001 | +1.77 | 0.0005 | +0.35 | 0.0042 |
| X2 | +5.98 | <0.0001 | +2.56 | <0.0001 | +2.07 | 0.0001 | +0.34 | 0.0045 |
| X3 | −0.88 | 0.3092 | +2.61 | <0.0001 | +3.56 | <0.0001 | +0.51 | 0.0001 |
| X4 | +1.46 | 0.0965 | +3.61 | <0.0001 | +2.56 | <0.0001 | +0.43 | 0.0006 |
| X1X2 | - | - | −0.15 | 0.6726 | −0.49 | 0.2735 | - | - |
| X1X3 | - | - | +0.039 | 0.9118 | +0.16 | 0.7159 | - | - |
| X1X4 | - | - | −0.069 | 0.8453 | +0.051 | 0.9073 | - | - |
| X2X3 | - | - | +0.047 | 0.8951 | +0.48 | 0.2742 | - | - |
| X2X4 | - | - | +8.125 × 10−3 | 0.9818 | +0.12 | 0.7888 | - | - |
| X3X4 | - | - | +0.088 | 0.8044 | +0.086 | 0.8431 | - | - |
| X12 | - | - | +2.25 | 0.0207 | +1.78 | 0.1135 | - | - |
| X22 | - | - | +1.87 | 0.0484 | +2.46 | 0.0350 | - | - |
| X32 | - | - | +2.35 | 0.0162 | +0.040 | 0.9703 | - | - |
| X42 | - | - | −0.60 | 0.5012 | −0.93 | 0.3938 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behfar, M.; Heshmati, A.; Mehri, F.; Khaneghah, A.M. Removal of Ochratoxin A from Grape Juice by Clarification: A Response Surface Methodology Study. Foods 2022, 11, 1432. https://doi.org/10.3390/foods11101432
Behfar M, Heshmati A, Mehri F, Khaneghah AM. Removal of Ochratoxin A from Grape Juice by Clarification: A Response Surface Methodology Study. Foods. 2022; 11(10):1432. https://doi.org/10.3390/foods11101432
Chicago/Turabian StyleBehfar, Majid, Ali Heshmati, Freshteh Mehri, and Amin Mousavi Khaneghah. 2022. "Removal of Ochratoxin A from Grape Juice by Clarification: A Response Surface Methodology Study" Foods 11, no. 10: 1432. https://doi.org/10.3390/foods11101432







