Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Material, Experimental Design, and Extraction Process
2.3. Moisture
2.4. Total Phenol Content Assay
2.5. VOCs Analysis
2.5.1. Headspace-Gas Chromatography Mass Spectrometry (HS-GC/MS)
2.5.2. Gas Chromatography Mass Spectrometry (GC/MS) of Hexanoic Extract
2.6. Cell Viability Assay
2.6.1. Eukaryotic Cell Culture
2.6.2. Cytotoxicity Assay
2.7. Antibacterial Assay
2.7.1. Prokaryotic Cell Culture
2.7.2. Agar Diffusion Method
2.8. Antioxidant Activities
2.8.1. DPPH• Scavenging Activity
2.8.2. ABTS•+ Scavenging Activity
2.8.3. FRAP Assay
2.9. Statistical Analysis
3. Results
3.1. General Aspects
3.2. HS-GC/MS Identification of Volatile Compounds
3.3. GC/MS Identification of Hexanoic Extract Compounds
3.4. Biological Activities of Raspberry Leaf Distillate
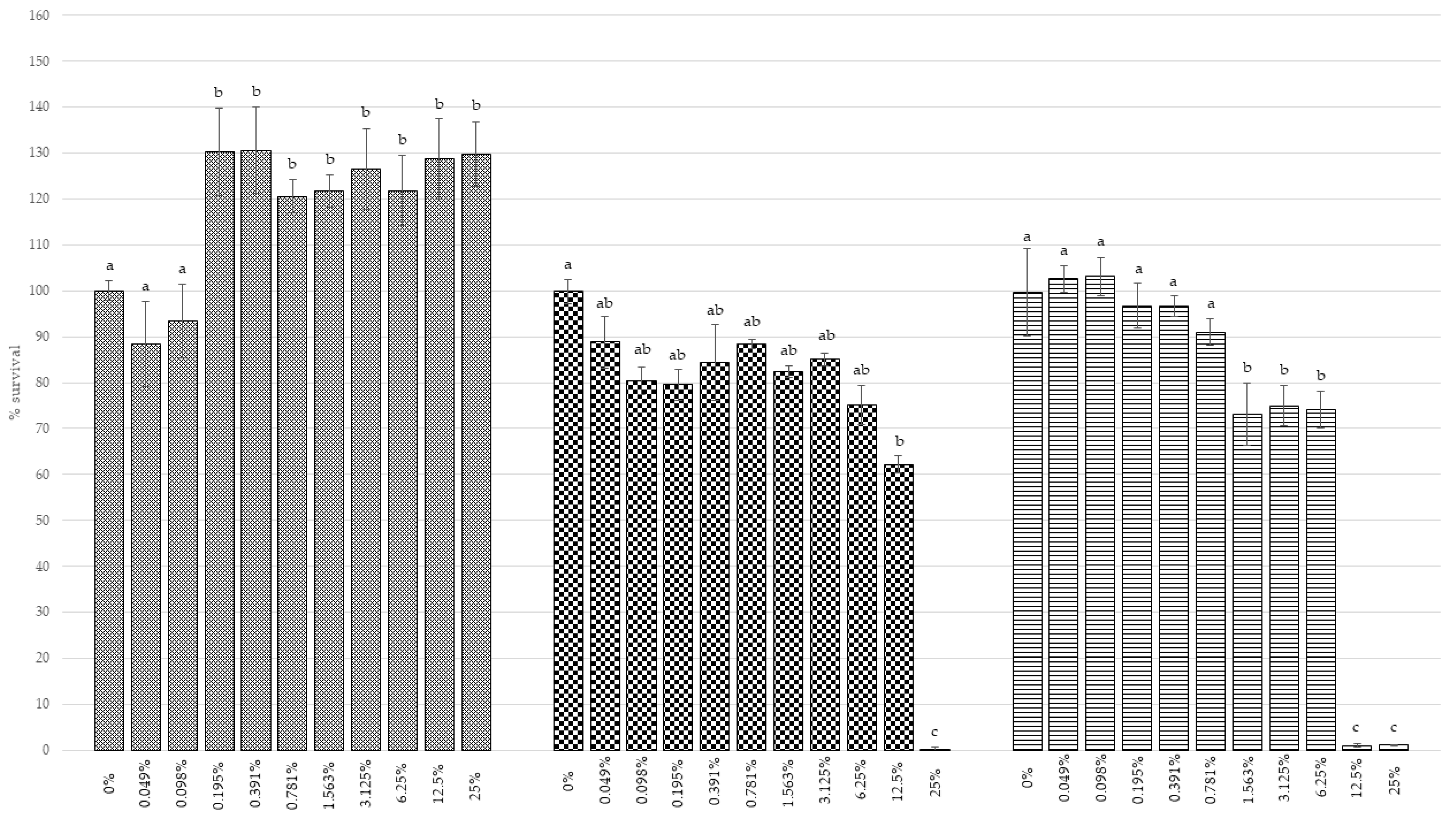
3.4.1. Cell Viability
3.4.2. Antibacterial Activity
3.4.3. TPC and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Crops and Livestock Products—Raspberries Production. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 14 April 2022).
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, A.; Viškelis, J. Valorisation of raspberries by-products for food and pharmaceutical industries. Adv. Agric. Harti. Ento. AAHE-102 2019. [Google Scholar]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Ilić, N.; Teslić, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT 2020, 130, 109627. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Ćetković, G.S.; Tumbas Šaponjac, V.T.; Vulić, J.J.; Čanadanović-Brunet, J.M.; Djilas, S.M. Bioactivity of Meeker and Willamette raspberry (Rubus idaeus L.) pomace extracts. Food Chem. 2015, 166, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Chwil, M.; Kostryco, M. Bioactive compounds and antioxidant activity of Rubus idaeus L. Leaves. Acta Sci. Pol. Hortorum Cultus 2018, 17, 135–147. [Google Scholar] [CrossRef]
- Veljković, B.; Dordević, N.; Dolićanin, Z.; Ličina, B.; Topuzović, M.; Stanković, M.; Zlatić, N.; Dajić-Stevanović, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. 2019, 47, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Komes, D.; Benković, M.; Karlović, S.; Hečimović, I.; Ježek, D.; Bauman, I. Innovative formulations of chocolates enriched with plant polyphenols from Rubus idaeus L. leaves and characterization of their physical, bioactive and sensory properties. Food Res. Int. 2012, 48, 820–830. [Google Scholar] [CrossRef]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Predan, G.M.I. Botanical characterization, phytochemical analysis and antioxidant activity of indigenous red raspberry (Rubus Idaeus L.) leaves. Stud. Univ. Vasile Goldis Arad Ser. Stiint. Vietii 2016, 26, 463–472. [Google Scholar]
- Durgo, K.; Belščak-Cvitanović, A.; Stančić, A.; Franekić, J.; Komes, D. The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon adenocarcinoma. J. Med. Food 2012, 15, 258–268. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus l.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, A.R.; Parekh, S.; Rathbone, J.; Del Mar, C.B.; Hoffmann, T.C. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2016, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, H.; Shojaee-Aliabadi, S.; Torbati, M.; Alizadeh, A.M.; Alizadeh, M. Drug resistance and the prevention strategies in food borne bacteria: An update review. Adv. Pharm. Bull. 2019, 9, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Acar, J.F.; Moulin, G. Antimicrobial resistance at farm level Resistant bacterial clones on the farm. Rev. Sci. Tech. Off. Int. Epiz. 2006, 25, 775–792. [Google Scholar] [CrossRef]
- Mensah, S.E.P.; Koudandé, O.D.; Sanders, P.; Laurentie, M.; Mensah, G.A.; Abiola, F.A. Antimicrobial residues in foods of animal origin in Africa: Public health risks. Rev. Sci. Tech. 2014, 33, 987–996. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS analysis and investigation of antibacterial, antioxidant and cytotoxic activity of essential oils and hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef]
- Rodolfi, M.; Chiancone, B.; Liberatore, C.M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in chemical profile of Cascade hop cones according to the growing area. J. Sci. Food Agric. 2019, 99, 6011–6019. [Google Scholar] [CrossRef]
- Robertson, G.W.; Griffiths, D.W.; Woodford, J.A.T.; Birch, A.N.E. Changes in the chemical composition of volatiles released by the flowers and fruits of the red raspberry (Rubus idaeus) cultivar glen prosen. Phytochemistry 1995, 38, 1175–1179. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456, pp. 544–545. [Google Scholar]
- Yang, Y.N.; Zheng, F.P.; Yu, A.N.; Sun, B.G. Changes of the free and bound volatile compounds in Rubus corchorifolius L. f. fruit during ripening. Food Chem. 2019, 287, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.H.; Clark, E.R.; Ananthakrishnan, S.; Lenz, K.; Canavan, H.E. How to select the appropriate method (s) of cytotoxicity analysis of mammalian cells at biointerfaces: A tutorial. Biointerphases 2020, 15, 031201. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol author information. Am. Soc. Microbiol. 2009, 15, 1–13. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.Č.D.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.; Huang, M.; Sun, F.; Yang, B.; He, J.; Wang, X.; Xia, P.; Chen, J. Characterization of the antibacterial activity and the chemical components of the volatile oil of the leaves of Rubus parvifolius L. Molecules 2012, 17, 7758–7768. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid identification of flavonoid constituents directly from PTP1B Inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-assisted extraction of bioactive compounds from raspberry (Rubus idaeus L.) pomace. J. Food Sci. 2019, 84, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cui, J.; Chen, J.; Yao, J.; Hao, Y.; Fan, Y.; Liu, Y. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. 2020, 263, 109146. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- De Sousa, J.P.; De Azerêdo, G.A.; De Araújo Torres, R.; Da Silva Vasconcelos, M.A.; Da Conceição, M.L.; De Souza, E.L. Synergies of carvacrol and 1,8-cineole to inhibit bacteria associated with minimally processed vegetables. Int. J. Food Microbiol. 2012, 154, 145–151. [Google Scholar] [CrossRef]
- Moteki, H.; Hibasami, H.; Yamada, Y.; Katsuzaki, H.; Imai, K.; Komiya, T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, butnot a in human stomach cancer cell line. Oncol. Rep. 2002, 9, 757–760. [Google Scholar]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- De Araújo-Filho, H.G.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytoher. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of anti-inflammatory activity of citrus latifolia Tanaka essential oil and limonene in experimental mouse models. Evid.-Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2009, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jiang, M.L.; Shao, F.; Ma, G.Q.; Shi, Q.; Liu, R.H. Chemical composition and antimicrobial activity of the essential oil from Euphorbia helioscopia L. Nat. Prod. Commun. 2020, 15, 549–555. [Google Scholar] [CrossRef]
- Attia, E.Z.; Abd El-Baky, R.M.; Desoukey, S.Y.; El Hakeem Mohamed, M.A.; Bishr, M.M.; Kamel, M.S. Chemical composition and antimicrobial activities of essential oils of Ruta graveolens plants treated with salicylic acid under drought stress conditions. Futur. J. Pharm. Sci. 2018, 4, 254–264. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed Res. Int. 2014, 11, 125704. [Google Scholar]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Mazzanti, G.; Uccella, N.; Castelli, F. Study on the mechanisms of the antibacterial action of some plant α,β-unsaturated aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Kamdem, S.S.; Belletti, N.; Magnani, R.; Lanciotti, R.; Gardini, F. Effects of carvacrol, (E)-2-hexenal, and citral on the thermal death kinetics of listeria monocytogenes. J. Food Prot. 2011, 74, 2070–2078. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y. (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef]
- Ma, W.; Johnson, E.T. Natural flavour (E,E)-2,4-heptadienal as a potential fumigant for control of Aspergillus flavus in stored peanut seeds: Finding new antifungal agents based on preservative sorbic acid. Food Control 2021, 124, 107938. [Google Scholar] [CrossRef]
- Sartori, D.; Gaion, A. Toxicity of polyunsaturated aldehydes of diatoms to Indo-Pacific bioindicator organism Echinometra mathaei. Drug Chem. Toxicol. 2016, 39, 124–128. [Google Scholar] [PubMed]
- Adaszyńska, M.; Swarcewicz, M.; Dziȩciol, M.; Dobrowolska, A. Comparison of chemical composition and antibacterial activity of lavender varieties from Poland. Nat. Prod. Res. 2013, 27, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Hǎncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Smith, R.L.; Waddell, W.J.; Cohen, S.M.; Feron, V.J.; Marnett, L.J.; Portoghese, P.S.; Rietjens, I.M.C.M.; Adams, T.B.; Gavin, C.L.; Mcgowen, M.M.; et al. GRAS flavoring substances 24. Food Technol. 2009, 63, 88. [Google Scholar]
- Gadino, A.N.; Walton, V.M.; Lee, J.C. Evaluation of methyl salicylate lures on populations of Typhlodromus pyri (Acari: Phytoseiidae) and other natural enemies in western Oregon vineyards. Biol. Control 2012, 63, 48–55. [Google Scholar] [CrossRef]
- Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Prakash, B. Nanoencapsulated methyl salicylate as a biorational alternative of synthetic antifungal and aflatoxin B1 suppressive agents. Environ. Sci. Pollut. Res. 2019, 26, 18440–18450. [Google Scholar] [CrossRef]
- Lu, X.P.; Liu, J.H.; Weng, H.; Ma, Z.Q.; Zhang, X. Efficacy of binary combinations between methyl salicylate and carvacrol against thrips Anaphothrips obscurus: Laboratory and field trials. Pest Manag. Sci. 2020, 76, 589–596. [Google Scholar] [CrossRef]
- Oloyede, G.K. Toxicity, antimicrobial and antioxidant activities of methyl salicylate dominated essential oils of Laportea aestuans (Gaud). Arab. J. Chem. 2016, 9, S840–S845. [Google Scholar] [CrossRef] [Green Version]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial activity of lauric acid on some selected clinical isolates. Ann. Clin. Lab. Res. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Sado Kamdem, S.; Guerzoni, M.E.; Baranyi, J.; Pin, C. Effect of capric, lauric and α-linolenic acids on the division time distributions of single cells of Staphylococcus aureus. Int. J. Food Microbiol. 2008, 128, 122–128. [Google Scholar] [CrossRef]
- Shen, X.; Chen, W.; Zheng, Y.; Lei, X.; Tang, M.; Wang, H.; Song, F. Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind. Crops Prod. 2017, 96, 110–119. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1,8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; de Felipe, L.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; De Saeger, C.; García de Bravo, M.; Crespo, R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018, 199, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Maczka, W.; Winska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Gu, X.; Yao, X.; Mei, J.; He, H.; Gao, X.; Du, Y.; Zhao, J.; Zhao, L.; Lai, X.; Shi, K. β-caryophyllene, a natural bicyclic sesquiterpene, induces apoptosis by inhibiting inflammation-associated proliferation in MOLT-4 leukemia cells. Pharmacogn. Mag. 2021, 17, 58. [Google Scholar]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.C.; Ho, C.L. Composition, in vitro cytotoxic, and antimicrobial activities of the flower essential oil of Diospyros discolor from Taiwan. Nat. Prod. Commun. 2015, 10, 1311–1314. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhou, R.; Zhou, R.; Li, W.; Weerasinghe, J.; Chen, S.; Rehm, B.H.A.; Zhao, L.; Frentiu, F.D.; Zhang, Z.; et al. Cold atmospheric plasma for preventing infection of viruses that use ACE2 for entry. Theranostics 2022, 12, 2811–2832. [Google Scholar] [CrossRef]
- Zaręba, N.; Więcławik, K.; Kizek, R.; Hosnedlova, B.; Kepinska, M. The impact of fullerenes as doxorubicin nano-transporters on metallothionein and superoxide dismutase status in MCF-10A cells. Pharmaceutics 2022, 14, 102. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.M.A.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.S.; Ascrizzi, R.; Bader, A. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1,8-cineole against A2780 ovarian cancer cells. Molecules 2020, 25, 1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.A.; Cazaux, S.; Bouajila, J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017, 55, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Sukapaka, M.; Babu, G.D.K.; Padwad, Y. Chemical composition and in vitro cytotoxicity of essential oils from leaves and flowers of Callistemon citrinus from western himalayas. PLoS ONE 2015, 10, e0133823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, S.; Veeramani, V.; Krishnakumar, G.S.; Sivalingam, U.; Madurai, S.L.; Chellan, R. Evaluation of in vitro anticancer activity of 1,8-Cineole–containing n-hexane extract of Callistemon citrinus (Curtis) Skeels plant and its apoptotic potential. Biomed. Pharmacother. 2017, 93, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-κB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar]
- Brändle, G.; L’Huillier, A.G.; Wagner, N.; Gervaix, A.; Wildhaber, B.E.; Lacroix, L. First report of spontaneous peritonitis in a child. BMC Infect. Dis. 2014, 14, 719. [Google Scholar] [CrossRef] [Green Version]
- Pulcrano, G.; Balzaretti, M.; Grosini, A.; Piacentini, V.; Poddighe, D. First report of Kocuria marina bloodstream infection unrelated to a central venous catheter: A mini-review on an emerging and under-recognized opportunistic pathogen. Infez. Med. 2017, 25, 71–74. [Google Scholar]
- De Paiva Anciens Ramos, G.L.; Vigoder, H.C.; dos Santos Nascimento, J. Kocuria spp. in foods: Biotechnological uses and risks for food safety. Appl. Food Biotechnol. 2021, 8, 79–88. [Google Scholar]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.-R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literatu. Antimicrob. Resist. Infect. Control 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Adewoyin, M.A.; Okoh, A.I. The natural environment as a reservoir of pathogenic and non-pathogenic Acinetobacter species. Rev. Environ. Health 2018, 33, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Ferreira, V.; Silva, J.; Teixeira, P. Enrichment of Acinetobacter spp. from food samples. Food Microbiol. 2016, 55, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Lee, H.H.; Moon, Y.S.; Yun, H.K.; Park, P.J.; Kwak, E.J. Contents of bioactive constituents and antioxidant activities of cultivated and wild raspberries. Korean J. Hortic. Sci. Technol. 2014, 32, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical composition, antioxidant and antimicrobial activity of raspberry, blackberry and raspberry-blackberry hybrid leaf buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Soutinho, S.M.A.; Gonçalves, F.J. Phenolic compounds and antioxidant activity in red fruits produced in organic farming. Croat. J. Food Sci. Technol 2014, 6, 15–26. [Google Scholar]
- Mihailović, N.R.; Mihailović, V.B.; Ćirić, A.R.; Srećković, N.Z.; Cvijović, M.R.; Joksović, L.G. Analysis of wild raspberries (Rubus idaeus L.): Optimization of the ultrasonic-assisted extraction of phenolics and a new insight in phenolics bioaccessibility. Plant Foods Hum. Nutr. 2019, 74, 399–404. [Google Scholar] [CrossRef]
- Dos Santos, S.S.; Paraíso, C.M.; Rodrigues, L.M.; Madrona, G.S. Agro-industrial waste as a source of bioactive compounds: Ultrasound-assisted extraction from blueberry (Vaccinium myrtillus) and raspberry (Rubus idaeus) pomace. Acta Sci. Technol. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Çekiç, Ç.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compos. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Karlidag, H.; Sengul, M. Characterization of red raspberry (Rubus idaeus L.) genotypes for their physicochemical properties. J. Food Sci. 2009, 74, C575–C579. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, D.; Luzak, B.; Boncler, M.; Rywaniak, J.; Sosnowska, D.; Podsedek, A.; Watala, C. CD39/NTPDase-1 expression and activity in human umbilical vein endothelial cells are differentially regulated by leaf extracts from Rubus caesius and Rubus idaeus. Cell. Mol. Biol. Lett. 2014, 19, 361–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabek-Lejko, D.; Wojtowicz, K. Comparison of antibacterial and antioxidant properties of fruits and leaves of blackberry (Rubus plicatus) and raspberry (Rubus idaeus). J. Microbiol. Biotechnol. Food Sci. 2014, 3, 514–518. [Google Scholar]
- Mîrza, A. Antioxidant actiyity of leaf and fruit extracts from Rubus fruticosus, Rubus idaeus and Rubus loganobaccus growing in the conditions of the Republic of Moldova. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 363–372. [Google Scholar]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Ledwozyw-Smoleń, I.; Augustynowicz, J.; Wyzgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef]
- Dvaranauskaite, A.; Venskutonis, P.R.; Labokas, J. Comparison of quercetin derivatives in ethanolic extracts of red raspberry (Rubus idaeus L.) leaves. Acta Aliment. 2008, 37, 449–461. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Dvaranauskaite, A.; Labokas, J. Radical scavenging activity and composition of raspberry (Rubus idaeus) leaves from different locations in Lithuania. Fitoterapia 2007, 78, 162–165. [Google Scholar] [CrossRef]
- Yu, R.; Chen, L.; Xin, X. Comparative assessment of chemical compositions, antioxidant and antimicrobial activity in ten berries grown in China. Flavour Fragr. J. 2020, 35, 197–208. [Google Scholar] [CrossRef]
- Gülçin, I.; Topal, F.; Çakmakçi, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.R.; Apel, M.A.; Raseira, M.C.B.; Zuanazzi, J.Â.S.; Henriques, A.T. Polyphenol content and evaluation of antichemotactic, antiedematogenic and antioxidant activities of Rubus sp. cultivars. J. Food Biochem. 2011, 35, 1389–1397. [Google Scholar] [CrossRef]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, M.E.; Corona-Angeles, J.A.; Tejeda-Martinez, A.R.; Flores-Guzman, P.A.; Luna-Mujica, I.; Chaparro-Huerta, V.; Viveros-Paredes, J.M. β-Caryophyllene exerts protective antioxidant effects through the activation of NQO1 in the MPTP model of Parkinson’s disease. Neurosci. Lett. 2021, 742, 135534. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Long, N.; Qiu, M.; Liu, Y.; Sun, F.; Dai, M. The inhibitory efficiencies of geraniol as an anti-inflammatory, antioxidant, and antibacterial, natural agent against methicillin-resistant Staphylococcus aureus infection in vivo. Infect. Drug Resist. 2021, 14, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Taheri Mirghaed, A.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Kacaniova, M.; Dincheva, I.; Radoukova, T.; Semerdjieva, I.B.; Astatkie, T.; Schlegel, V. Essential oil composition, antioxidant and antimicrobial activity of the galbuli of six juniper species. Ind. Crops Prod. 2018, 124, 449–458. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice-In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [Green Version]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Dvaranauskaite, A.; Venskutonis, P.R.; Labokas, J. Radical scavenging activity of raspberry (Rubus idaeus L.) fruit extracts. Acta Aliment. 2006, 35, 73–83. [Google Scholar] [CrossRef]
- Buřičová, L.; Andjelkovic, M.; Čermáková, A.; Réblová, Z.; Jurček, O.; Kolehmainen, E.; Verhé, R.; Kvasnička, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Frías-Moreno, M.N.; Parra-Quezada, R.Á.; Ruíz-Carrizales, J.; González-Aguilar, G.A.; Sepulveda, D.; Molina-Corral, F.J.; Jacobo-Cuellar, J.L.; Olivas, G.I. Quality, bioactive compounds and antioxidant capacity of raspberries cultivated in northern Mexico. Int. J. Food Prop. 2021, 24, 603–614. [Google Scholar] [CrossRef]
- Costea, T.; Lupu, A.R.; Vlase, L.; Nencu, I.; Gîrd, C.E. Phenolic content and antioxidant activity of a raspberry leaf dry extract. Rom. Biotechnol. Lett. 2016, 21, 11346–11356. [Google Scholar]
- Gramza-Michałowska, A.; Bueschke, M.; Kulczyński, B.; Gliszczyńska-Świgło, A.; Kmiecik, D.; Bilska, A.; Purłan, M.; Wałęsa, L.; Ostrowski, M.; Filipczuk, M.; et al. Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 2019, 13, 1739–1747. [Google Scholar] [CrossRef] [Green Version]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, bioactive compounds, antioxidant capacity, and enzymes of raspberries at different maturity stages, effects of organic vs. Conventional fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.H. Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-κβ and stimulation of TGF-β/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 185, 241–253. [Google Scholar] [CrossRef]
- Kafkas, E.; Ozgen, M.; Ozogul, Y.; Turemis, N. Phytochemical and Fatty Acid Profile of Selected Red Raspberry cultivars: A comparative study. J. Food Qual. 2008, 31, 67–78. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Ozarda, O.; Barla Demirkoz, A.; Özdemir, M. Sensory characteristics and antioxidant capacity of red raspberry extract as a preservative in fruity flavoured beverages. J. Food Sci. Technol. 2015, 52, 6687–6694. [Google Scholar] [CrossRef] [Green Version]
- Radočaj, O.; Vujasinović, V.; Dimić, E.; Basić, Z. Blackberry (Rubus fruticosus L.) and raspberry (Rubus idaeus L.) seed oils extracted from dried press pomace after longterm frozen storage of berries can be used as functional food ingredients. Eur. J. Lipid Sci. Technol. 2014, 116, 1015–1024. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Peter Yurawecz, M.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Miletic, N.; Leposavic, A.; Popovic, B.; Mitrovic, O.; Kandic, M. Chemical and antioxidant properties of fully matured raspberry fruits (Rubus idaeus L.) picked in different moments of harvesting season. Acta Hortic. 2015, 1099, 211–218. [Google Scholar] [CrossRef]
- Marhuenda, J.; Alemán, M.D.; Gironés-vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and In Vitro Availability of Four Different Berries. J. Chem. 2016, 7. [Google Scholar]
- Farias-Cervantes, V.S.; Chávez-Rodríguez, A.; García-Salcedo, P.A.; García-López, P.M.; Casas-Solís, J.; Andrade-González, I. Antimicrobial effect and in vitro release of anthocyanins from berries and Roselle obtained via microencapsulation by spray drying. J. Food Process. Preserv. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.Z.; Cong, Q.; Wang, W.; Qi, X.L.; Peng, Y.; Song, S.J. Bioactive lignans and flavones with in vitro antioxidant and neuroprotective properties from Rubus idaeus rhizome. J. Funct. Foods 2017, 32, 160–169. [Google Scholar] [CrossRef]
- Konić-Ristić, A.; Šavikin, K.; Zdunić, G.; Janković, T.; Juranic, Z.; Menković, N.; Stanković, I. Biological activity and chemical composition of different berry juices. Food Chem. 2011, 125, 1412–1417. [Google Scholar] [CrossRef]
- Krivokapić, S.; Vlaović, M.; Vratnica, B.D.; Perović, A.; Perovic, S. Biowaste as a potential source of bioactive compound-a case study of raspberry fruit pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef]
- Malone, N. Strawberries-Cultivation, Antioxidant Properties and Health Benefits; Nova Publishers: New York, NY, USA, 2014; ISBN 9781631172557. [Google Scholar]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. 2010, 119, 592–599. [Google Scholar] [CrossRef]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic content estimated from a red raspberry factorial experiment. J. Am. Soc. Hortic. Sci. 2005, 130, 40. [Google Scholar] [CrossRef] [Green Version]

| Peak | LRI | LRIlit | Component | Class | Amount (%) |
|---|---|---|---|---|---|
| 1 | 915 | 914 | butanal, 3-methyl | Aldehyde | 0.2 |
| 2 | 970 | 968 | pentanal | Aldehyde | 2.9 |
| 3 | 995 | 993 | 2,3-butanedione | Ketone | 0.4 |
| 4 | 1025 | 1022 | 2-butanol | Alcohol | 0.4 |
| 5 | 1093 | 1095 | 1-propanol, 2-methyl- | Alcohol | 0.4 |
| 6 | 1148 | 1146 | 3-carene | Monoterpene | 16.3 |
| 7 | 1160 | 1157 | β-myrcene | Monoterpene | 0.1 |
| 8 | 1200 | 1198 | limonene | Monoterpene | 1.0 |
| 9 | 1210 | 1207 | 1-butanol, 2-methyl- | Alcohol | 1.6 |
| 10 | 1214 | 1209 | 1,8-cineole | Oxygenated monoterpene | 50.8 |
| 11 | 1298 | 1296 | 2-heptanol | Alcohol | 10.3 |
| 12 | 1395 | 1389 | 3-hexen-1-ol | Alcohol | 1.5 |
| 13 | 1398 | 1394 | 2-hexen-1-ol | Alcohol | 0.2 |
| 14 | 1472 | 1469 | 5-hepten-2-ol, 6-methyl- | Alcohol | 0.9 |
| 15 | 1531 | 1528 | camphor | Oxygenated monoterpene | 2.9 |
| 16 | 1585 | 1586 | hotrienol | Alcohol | 0.2 |
| 17 | 1618 | 1619 | terpinyl acetate | Oxygenated monoterpene | 3.7 |
| 18 | 1676 | 1675 | α-terpineol | Oxygenated monoterpene | 5.2 |
| 19 | 2014 | 2011 | methyl eugenol | Phenylpropanoid | 0.5 |
| Total | 99.0 | ||||
| Peak | LRI 1 | Compound | Class | Amount (%) |
|---|---|---|---|---|
| 1 | 829 | 2-hexenal | Aldehyde | 8.79 |
| 2 | 866 | 2-heptanone | Ketone | 1.49 |
| 3 | 876 | 2-hexanol-3-methyl | Alcohol | 1.59 |
| 4 | 914 | 4-heptanol-3-ethyl | Alcohol | 1.56 |
| 5 | 924 | 3-hexanol-5-methyl | Alcohol | 1.26 |
| 6 | 969 | 2,4 heptadienal | Aldehyde | 3.13 |
| 7 | 984 | 1,8-cineole | Oxygenated monoterpene | 2.47 |
| 8 | 1029 | 2-nonanone | Ketone | 6.88 |
| 9 | 1035 | β-linalool | Oxygenated monoterpene | 6.15 |
| 10 | 1135 | geraniol | Oxygenated monoterpene | 4.45 |
| 11 | 1145 | citral | Terpenoid | 1.00 |
| 12 | 1162 | unknown | - | 1.68 |
| 13 | 1195 | methyl salicylate | Ester | 1.94 |
| 14 | 1198 | n-decanoic acid | Acid | 1.15 |
| 15 | 1210 | unknown | - | 6.71 |
| 16 | 1256 | unknown | - | 2.80 |
| 17 | 1269 | unknown | - | 2.11 |
| 18 | 1281 | α-terpinen-7-al | Monoterpenoid | 1.19 |
| 19 | 1289 | unknown | - | 1.15 |
| 20 | 1308 | unknown | - | 6.65 |
| 21 | 1352 | dodecanoic acid | Acid | 1.37 |
| 22 | 1410 | τ-muurolol | Sesquiterpene | 1.09 |
| 23 | 1437 | unknown | - | 3.30 |
| 24 | 1466 | caryophyllene | Sesquiterpene | 2.61 |
| Total identified | 72.52 | |||
| Agar Diffusion Method | RH (10 µL) Inhibition Halo (mm) ± sd (mm) | Gentamicin (10 µL) Inhibition Halo (mm) ± sd (mm) |
|---|---|---|
| E. coli | NE | 24.8 ± 0.4 |
| K. marina | NE | 26 ± 6 |
| P. fluorescens | NE | 22 ± 1 |
| B. cereus | 7.67 ± 1 | 22 ± 3 |
| A. bohemicus | 12 ± 3 | 34 ± 2 |
| TPC | DPPH | ABTS | FRAP | |
|---|---|---|---|---|
| RH | 6.25 ± 0.54 | 6746 ± 555 | 13.57 ± 0.56 | 307.48 ± 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, D.; Carbone, K.; Garzoli, S.; Laghezza Masci, V.; Turchetti, G. Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract. Foods 2022, 11, 1455. https://doi.org/10.3390/foods11101455
De Santis D, Carbone K, Garzoli S, Laghezza Masci V, Turchetti G. Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract. Foods. 2022; 11(10):1455. https://doi.org/10.3390/foods11101455
Chicago/Turabian StyleDe Santis, Diana, Katya Carbone, Stefania Garzoli, Valentina Laghezza Masci, and Giovanni Turchetti. 2022. "Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract" Foods 11, no. 10: 1455. https://doi.org/10.3390/foods11101455





