Comparative Evaluation on the Bioaccessibility of Citrus Fruit Carotenoids In Vitro Based on Different Intake Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Determination of Vitamin C (VC) Content
2.4. Determination of Total Phenolics
2.5. In Vitro Digestion
2.5.1. Mouth Phase Digestion
2.5.2. Stomach Phase Digestion
2.5.3. Small Intestine Phase Digestion
2.6. Extraction of Carotenoids
2.7. HPLC Analysis
2.8. Particle size Distribution Analysis
2.9. Determination of Viscosity
2.10. Calculation of Carotenoid Retention Ratio (CRR)
2.11. Calculation of Carotenoid Bioaccessibility (CBA)
2.12. Statistical Analysis
3. Results and Discussion
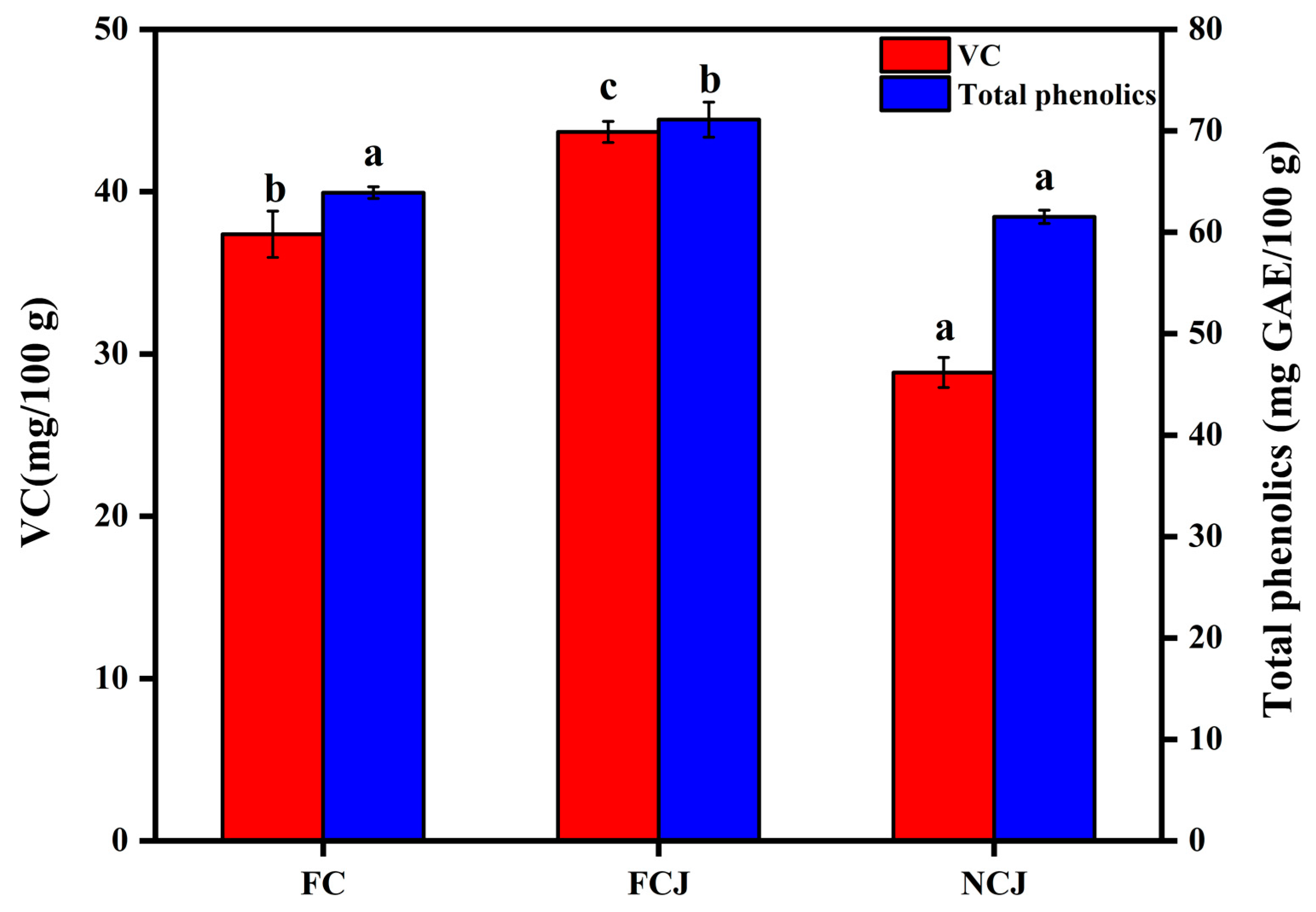
3.1. Contents of Vitamin C (VC) and Total Phenolics
3.2. Carotenoid Analysis
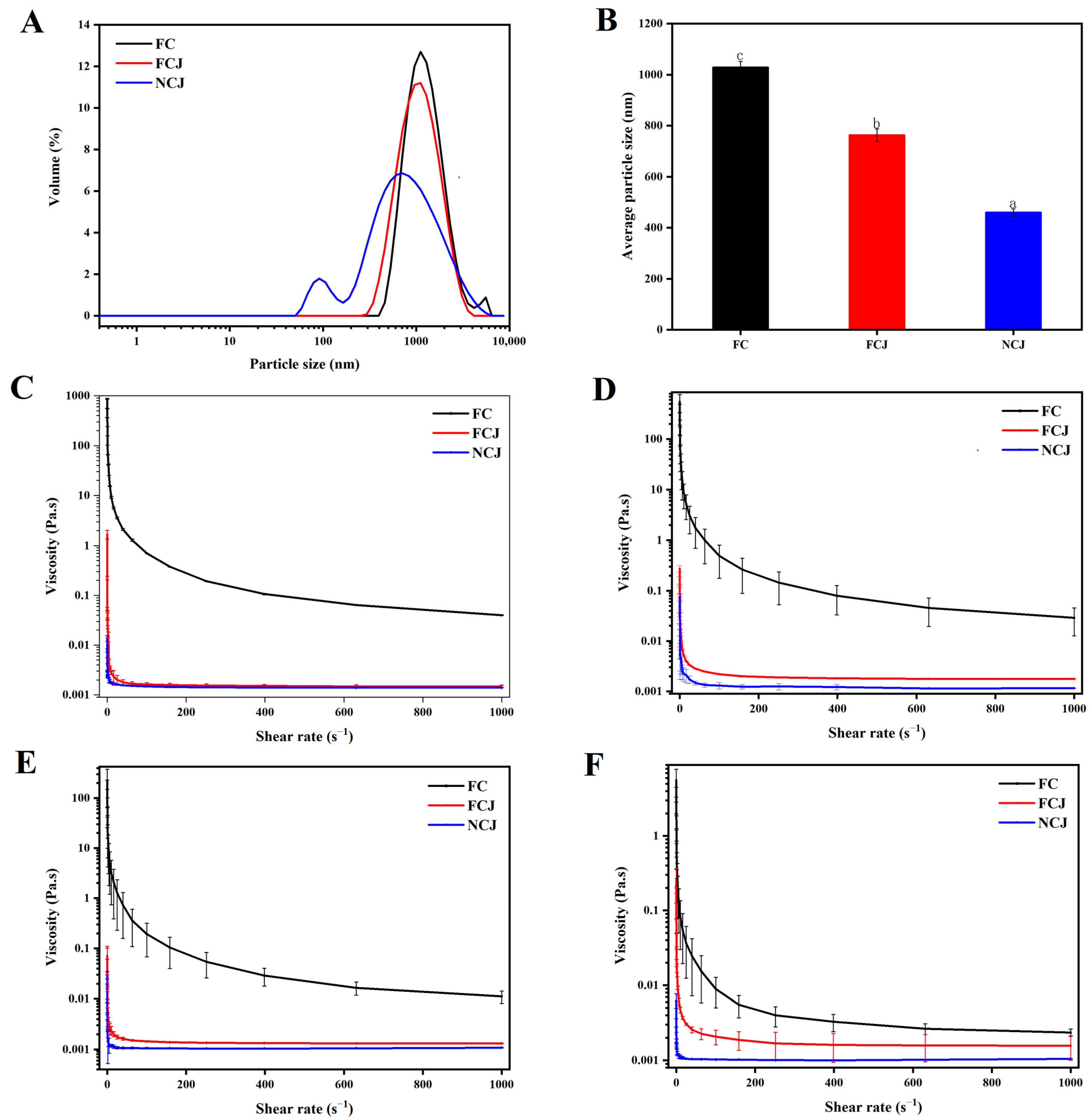
3.3. Particle Size Distribution and Viscosity Analysis
3.4. Carotenoid Retention Ratio (CRR) in the Small Intestine
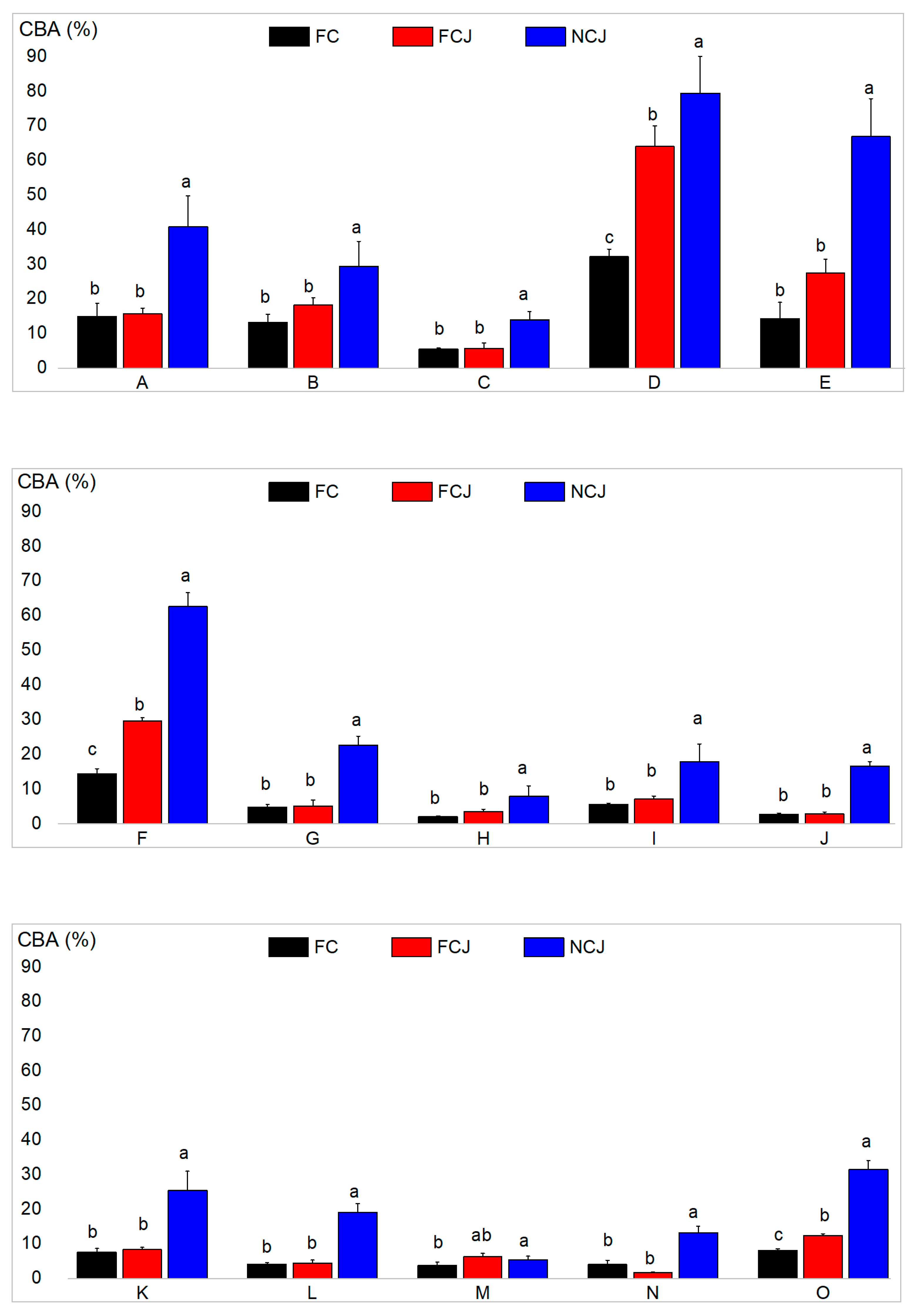
3.5. Carotenoid Bioaccessibility (CBA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rošul, M.; Đerić, N.; Mišan, A.; Pojić, M.; Šimurina, O.; Halimi, C.; Nowicki, M.; Cvetković, B.; Mandić, A.; Reboul, E. Bioaccessibility and uptake by Caco-2 cells of carotenoids from cereal-based products enriched with butternut squash (Cucurbita moschata L.). Food Chem. 2022, 385, 132595. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Ying, P.; Chunhua, Z.; Siyi, P. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara Cara. Food Chem. 2018, 265, 39–48. [Google Scholar]
- Von Lintig, J. Metabolism of Carotenoids and Retinoids Related to Vision. J. Biol. Chem. 2012, 287, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retinal Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Zhang, L.C.; Kudaka, R.; Inaba, H.; Murakami, K.; Yamamoto, M.; Kojima, N.; Yahata, M.; Matsumoto, H.; Kato, M. Auxin induced carotenoid accumulation in GA and PDJ-treated citrus fruit after harvest. Postharvest Biol. Technol. 2021, 181, 9. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, X.J.; Lv, S.; Pan, S. Carotenoid profiling of red navel orange “Cara Cara” harvested from five regions in China. Food Chem. 2017, 232, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mercadante, A.Z. The bioaccessibility of carotenoids impacts the design of functional foods. Curr. Opin. Food Sci. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Meléndez-Martínez, A. High-pressure homogenization as compared to pasteurization as a sustainable approach to obtain mandarin juices with improved bioaccessibility of carotenoids and flavonoids. J. Clean. Prod. 2020, 262, 121325. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.F.; Liu, X.; Wu, X.Y.; Lyu, J.; Liu, J.N.; Liu, D.Z.; Guo, C.T. Effect of high pressure homogenization on water-soluble pectin characteristics and bioaccessibility of carotenoids in mixed juice. Food Chem. 2022, 371, 130073. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Xie, F.; Gu, X.; Wang, Z. High pressure homogenization versus ultrasound treatment of tomato juice: Effects on stability and in vitro bioaccessibility of carotenoids. LWT—Food Sci. Technol. 2019, 116, 108597. [Google Scholar] [CrossRef]
- Park, S.J.; Nurika, I.; Suhartini, S.; Cho, W.-H.; Moon, K.-D.; Jung, Y.H. Carbonation of not from concentrate apple juice positively impacts shelf-life. LWT—Food Sci. Technol. 2020, 134, 110128. [Google Scholar] [CrossRef]
- Braddock, R.J.; Goodrich, R.M. Processing technologies to enhance fresh flavor of citrus juice. Abstr. Pap. Am. Chem. Soc. 2001, 222, AGFD41. [Google Scholar]
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Characterization of Carotenoids in Juice of Red Navel Orange (Cara Cara). J. Agric. Food Chem. 2001, 49, 2563–2568. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Rong, T. Ultra-performance liquid chromatographic separation of geometric isomers of carotenoids and antioxidant activities of 20 tomato cultivars and breeding lines. Food Chem. 2012, 132, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Chen, L.; Pan, S. Subacute toxicity assessment of carotenoids extracted from citrus peel (Nanfengmiju, Citrus reticulata Blanco) in rats. Regul. Toxicol. Pharmacol. 2012, 62, 16–22. [Google Scholar] [CrossRef]
- Knockaert, G.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Changes in β-carotene bioaccessibility and concentration during processing of carrot puree. Food Chem. 2012, 133, 60–67. [Google Scholar] [CrossRef]
- Xuan, L.; Bi, J.; Hang, X.; Mcclements, D.J. Enhancement of Nutraceutical Bioavailability using Excipient Nanoemulsions: Role of Lipid Digestion Products on Bioaccessibility of Carotenoids and Phenolics from Mangoes. J. Food Sci. 2016, 81, N754–N761. [Google Scholar]
- Arena, E.; Fallico, B.; Maccarone, E. Thermal damage in blood orange juice: Kinetics of 5-hydroxymethyl-2-furancarboxaldehyde formation. Int. J. Food Sci. Technol. 2010, 36, 145–151. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.; Hernández-Herrero, M.; Rüfer, C.; Guamis-López, B.; Roig-Sagués, A. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 89–94. [Google Scholar] [CrossRef]
- Saldo, J.; Suárez-Jacobo, á.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X. Use of ultra-high-pressure homogenization to preserve apple juice without heat damage. High. Press. Res. 2009, 29, 52–56. [Google Scholar] [CrossRef]
- Marques, M.C.; Hacke, A.; Neto, C.; Mariu, L.R.B. Impact of phenolic compounds in the digestion and absorption of carotenoids. Curr. Opin. Food Sci. 2021, 39, 190–196. [Google Scholar] [CrossRef]
- Palmero, P.; Panozzo, A.; Colle, I.; Chigwedere, C.; Hendrickx, M.; Loey, A.V. Role of structural barriers for carotenoid bioaccessibility upon high pressure homogenization. Food Chem. 2016, 199, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Etzbach, L.; Pfeiffer, A.; Schieber, A.; Weber, F. Effects of thermal pasteurization and ultrasound treatment on the peroxidase activity, carotenoid composition, and physicochemical properties of goldenberry (Physalis peruviana L.) puree—ScienceDirect. LWT—Food Sci. Technol. 2019, 100, 69–74. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.; Vicario, I.M.; Heredia, F.J. Review: Analysis of carotenoids in orange juice. J. Food Compos. Anal. 2007, 20, 638–649. [Google Scholar] [CrossRef]
- Santiago, J.S.J.; Salvia-Trujillo, L.; Zucca, R.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. In vitro digestibility kinetics of oil-in-water emulsions structured by water-soluble pectin-protein mixtures from vegetable purées. Food Hydrocoll. 2018, 80, 231–244. [Google Scholar] [CrossRef]
- Zacherl, C.; Eisner, P.; Engel, K.H. In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibres. Food Chem. 2011, 126, 423–428. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; Liu, X.; Liu, D.; Wu, X.; Lyu, J.; Ding, Y. Effects of pectins and sugars on β-carotene bioaccessibility in an in vitro simulated digestion model. J. Food Compos. Anal. 2020, 91, 103537. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Połaska, M.; Marszałek, K. High pressure homogenization with a cooling circulating system: The effect on physiochemical and rheological properties, enzymes, and carotenoid profile of carrot juice. Food Chem. 2022, 370, 131023. [Google Scholar] [CrossRef]
- Gouma, M.; Alvarez, I.; Condon, S.; Gayan, E. Pasteurization of carrot juice by combining UV-C and mild heat: Impact on shelf-life and quality compared to conventional thermal treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 12. [Google Scholar] [CrossRef]
- Courraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Wellala, C.K.D.; Bi, J.F.; Liu, X.; Liu, J.N.; Lyu, J.; Zhou, M.; Marszalek, K.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 13. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of in vitro digestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Verrijssen, T.A.J.; Verkempinck, S.H.E.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. The effect of pectin on in vitro β-carotene bioaccessibility and lipid digestion in low fat emulsions. Food Hydrocoll. 2015, 49, 73–81. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.d.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, T.; Sawada, Y.; Wang, J.; Nagaoka, S.; Yanase, E. Molecular Mechanism by Which Tea Catechins Decrease the Micellar Solubility of Cholesterol. J. Agric. Food Chem. 2019, 67, 7128–7135. [Google Scholar] [CrossRef]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
- Chamnansilpa, N.; Aksornchu, P.; Adisakwattana, S.; Thilavech, T.; Mäkynen, K.; Dahlan, W.; Ngamukote, S. Anthocyanin-rich fraction from Thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon 2020, 6, e05408. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Granado-Lorencio, F.; de Ancos, B.; Sánchez-Moreno, C.; Martín-Belloso, O.; Blanco, I.; Herrero-Barbudo, C.; Elez-Martínez, P.; Plaza, L.; Cano, M.P. Greater bioavailability of xanthophylls compared to carotenes from orange juice (high-pressure processed, pulsed electric field treated, low-temperature pasteurised, and freshly squeezed) in a crossover study in healthy individuals. Food Chem. 2022, 371, 130821. [Google Scholar] [CrossRef] [PubMed]

| Carotenoids | Sample | ||
|---|---|---|---|
| FC | FCJ | NCJ | |
| 9-cis-violaxanthin | 0.65 ± 0.04 b | 0.77 ± 0.09 a | 0.42 ± 0.01 c |
| 9-cis-antheraxanthin | 0.83 ± 0.03 a | 0.65 ± 0.05 b | 0.40 ± 0.05 c |
| 9-cis-zeaxanthin | 0.40 ± 0.01 a | 0.36 ± 0.03 a | 0.23 ± 0.03 b |
| 13- or 15-cis-β-cryptoxanthin | 0.37 ± 0.02 b | 0.39 ± 0.05 b | 1.23 ± 0.14 a |
| α-cryptoxanthin | 0.63 ± 0.04 a | 0.62 ± 0.11 a | 0.74 ± 0.04 a |
| β-cryptoxanthin | 12.79 ± 1.24 a | 11.78 ± 0.57 a | 12.94 ± 1.74 a |
| β-carotene | 2.99 ± 0.35 b | 3.56 ± 0.10 a | 3.03 ± 0.15 b |
| 9-cis-violaxanthin-C12:0-C12:0 | 1.17 ± 0.12 a | 1.00 ± 0.04 b | 0.77 ± 0.05 c |
| 9-cis-violaxanthin-C12:0-C14:0 | 1.03 ± 0.11 a | 1.15 ± 0.05 a | 1.01 ± 0.17 a |
| 9-cis-violaxanthin-C12:0-C18:1 | 6.90 ± 0.32 a | 7.09 ± 0.20 a | 7.24 ± 0.40 a |
| 9-cis-violaxanthin-C14:0-C14:0 | 1.00 ± 0.07 b | 1.28 ± 0.10 a | 1.05 ± 0.11 b |
| Mixture of 9-cis-violaxanthin-C12:0-C16:0 and 9-cis-violaxanthin-C14:0-C18:1 | 5.60 ± 0.26 b | 6.55 ± 0.30 a | 5.60 ± 0.40 b |
| Mixture of Violaxanthin-C14:0-C16:0 and Violaxanthin-C16:0-C18:1 | 3.58 ± 0.50 b | 3.53 ± 0.07 b | 6.87 ± 1.08 a |
| β-cryptoxanthin-C18:0 | 2.81 ± 0.27 c | 4.38 ± 0.26 a | 3.61 ± 0.30 b |
| Total carotenoid | 40.75 ± 1.45 b | 43.12 ± 0.87 ab | 45.16 ± 2.17 a |
| Carotenoids | Sample | ||
|---|---|---|---|
| FC | FCJ | NCJ | |
| 9-cis-violaxanthin | 0.52 ± 0.07 a | 0.57 ± 0.05 a | 0.30 ± 0.02 b |
| 9-cis-antheraxanthin | 0.62 ± 0.06 a | 0.55 ± 0.03 a | 0.36 ± 0.05 b |
| 9-cis-zeaxanthin | 0.23 ± 0.02 a | 0.27 ± 0.02 a | 0.14 ± 0.03 b |
| 13- or 15-cis-β-cryptoxanthin | 0.24 ± 0.02 c | 0.36 ± 0.02 b | 0.78 ± 0.09 a |
| α-cryptoxanthin | 0.51 ± 0.03 a | 0.53 ± 0.07 a | 0.57 ± 0.08 a |
| β-cryptoxanthin | 10.1 ± 0.43 ab | 10.18 ± 0.62 a | 9.17 ± 0.32 b |
| β-carotene | 2.04 ± 0.21 b | 2.98 ± 0.50 a | 1.80 ± 0.09 b |
| 9-cis-violaxanthin-C12:0-C12:0 | 0.90 ± 0.06 a | 0.83 ± 0.05 a | 0.66 ± 0 b |
| 9-cis-violaxanthin-C12:0-C14:0 | 0.71 ± 0.07 c | 1.08 ± 0.05 a | 0.79 ± 0.07 b |
| 9-cis-violaxanthin-C12:0-C18:1 | 6.04 ± 0.81 a | 6.64 ± 0.24 a | 5.92 ± 0.28 a |
| 9-cis-violaxanthin-C14:0-C14:0 | 0.86 ± 0.12 ab | 1.01 ± 0.05 a | 0.72 ± 0.05 b |
| Mixture of 9-cis-violaxanthin-C12:0-C16:0 and 9-cis-violaxanthin-C14:0-C18:1 | 3.70 ± 0.04 c | 5.55 ± 0.07 a | 4.96 ± 0.13 b |
| Mixture of Violaxanthin-C14:0-C16:0 and Violaxanthin-C16:0-C18:1 | 2.59 ± 0.61 b | 2.38 ± 0.03 b | 3.59 ± 0.21 a |
| β-cryptoxanthin-C18:0 | 1.59 ± 0.18 c | 4.18 ± 0.21 a | 2.93 ± 0.11 b |
| Total carotenoid | 30.67 ± 0.59 c | 37.12 ± 0.46 a | 32.69 ± 1.11 b |
| Carotenoids | Sample | ||
|---|---|---|---|
| FC | FCJ | NCJ | |
| 9-cis-violaxanthin | 0.08 ± 0.01 b | 0.09 ± 0.01 b | 0.12 ± 0.02 a |
| 9-cis-antheraxanthin | 0.08 ± 0.01 a | 0.10 ± 0.01 a | 0.1 ± 0.03 a |
| 9-cis-zeaxanthin | 0.01 ± 0 b | 0.02 ± 0 ab | 0.02 ± 0 a |
| 13- or 15-cis-β-cryptoxanthin | 0.08 ± 0 c | 0.23 ± 0.03 b | 0.62 ± 0.01 a |
| α-cryptoxanthin | 0.07 ± 0.02 c | 0.15 ± 0.03 b | 0.37 ± 0.01 a |
| β-cryptoxanthin | 1.46 ± 0.21 c | 3.02 ± 0.14 b | 5.73 ± 0.3 a |
| β-carotene | 0.10 ± 0.01 c | 0.15 ± 0.03 b | 0.41 ± 0.03 a |
| 9-cis-violaxanthin-C12:0-C12:0 | 0.02 ± 0 c | 0.03 ± 0 b | 0.05 ± 0.02 a |
| 9-cis-violaxanthin-C12:0-C14:0 | 0.04 ± 0c | 0.08 ± 0.01 b | 0.14 ± 0.05 a |
| 9-cis-violaxanthin-C12:0-C18:1 | 0.17 ± 0.01 b | 0.2 ± 0.03 b | 0.99 ± 0.08 a |
| 9-cis-violaxanthin-C14:0-C14:0 | 0.06 ± 0 b | 0.08 ± 0 b | 0.18 ± 0.04 a |
| Mixture of 9-cis-violaxanthin-C12:0-C16:0 and 9-cis-violaxanthin-C14:0-C18:1 | 0.15 ± 0.02 b | 0.25 ± 0.04 b | 0.94 ± 0.11 a |
| Mixture of Violaxanthin-C14:0-C16:0 and Violaxanthin-C16:0-C18:1 | 0.09 ± 0.01 c | 0.15 ± 0.02 b | 0.19 ± 0.02 a |
| β-cryptoxanthin-C18:0 | 0.06 ± 0.01 b | 0.07 ± 0 b | 0.39 ± 0.07 a |
| Total carotenoid | 2.49 ± 0.07 c | 4.61 ± 0.13 b | 10.27 ± 0.74 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Hu, T.; Hu, H.; Xiong, S.; Shi, K.; Zhang, N.; Mu, Q.; Xu, G.; Zhang, P.; Pan, S. Comparative Evaluation on the Bioaccessibility of Citrus Fruit Carotenoids In Vitro Based on Different Intake Patterns. Foods 2022, 11, 1457. https://doi.org/10.3390/foods11101457
Xu Y, Hu T, Hu H, Xiong S, Shi K, Zhang N, Mu Q, Xu G, Zhang P, Pan S. Comparative Evaluation on the Bioaccessibility of Citrus Fruit Carotenoids In Vitro Based on Different Intake Patterns. Foods. 2022; 11(10):1457. https://doi.org/10.3390/foods11101457
Chicago/Turabian StyleXu, Yang, Tan Hu, Haijuan Hu, Sihui Xiong, Kaixin Shi, Nawei Zhang, Qier Mu, Gang Xu, Peipei Zhang, and Siyi Pan. 2022. "Comparative Evaluation on the Bioaccessibility of Citrus Fruit Carotenoids In Vitro Based on Different Intake Patterns" Foods 11, no. 10: 1457. https://doi.org/10.3390/foods11101457







