Moringa oleifera Seeds Characterization and Potential Uses as Food
Abstract
:1. Introduction
2. Materials and Methods
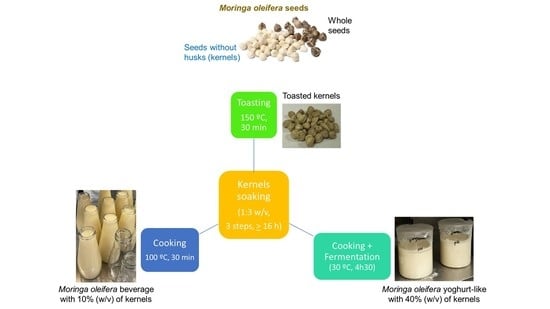
2.1. M. oleifera Toasted Seed Preparation
2.2. M. oleifera-Based Beverage Preparation
2.3. M. oleifera Yoghurt-like Preparation
2.4. Physico-Chemical Analysis
2.5. Sensory Evaluation of Samples
2.6. Rheological Measurements
2.7. Statistical Analysis
3. Results and Discussion
3.1. Progression on the Processing Techniques Used for the Development of Moringa oleifera Seed-Based Foods
3.2. Physico-Chemical Parameters of the Optimized M. oleifera Seed-Based Foods
3.3. Evaluation of the M. oleifera-Based Beverage and Yoghurt-like Rheology Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Rockwood, J.; Anderson, B.; Casamatta, D. Potential uses of Moringa oleifera and an examination of antibiotic efficacy conferred by M. oleifera seed and leaf extracts using crude extraction techniques available to underserved indigenous populations. Int. J. Phytother. Res. 2013, 3, 61–71. [Google Scholar]
- Oyeyinka, A.T.; Oyeyinka, S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018, 17, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Quintanilha, G.E.O.; Baptista, A.T.A.; Gomes, R.G.; Vieira, A.M.C. Yogurt production added ultrafiltered seed extract of Moringa oleifera Lam. Biocatal. Agric. Biotechnol. 2021, 37, 102159. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo-Branco, D.S.C.M.; Cordeiro, R.A.; Sampaio, C.M.S.; Paiva, M.A.N.; Santos, J.B.F.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asia Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, R.; Kachhwaha, S.; Kothari, S.L. Nutritional and medicinal applications of Moringa oleifera Lam.—Review of current status and future possibilities. J. Herb. Med. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Bancessi, A.; Bancessi, Q.; Baldé, A.; Catarino, L. Present and potential uses of Moringa oleifera as a multipurpose plant in Guinea-Bissau. S. Afr. J. Bot. 2020, 129, 206–208. [Google Scholar] [CrossRef]
- Agoyi, E.E.; Okou, F.A.Y.; Assogbadjo, E.A.; Sinsin, B. Medicinal uses of Moringa oleifera in southern Benin (West Africa). Acta Hortic. 2017, 1158, 303–308. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Özcan, M.M. Moringa spp.: Composition and bioactive properties. S. Afr. J. Bot. 2018, 129, 25–31. [Google Scholar] [CrossRef]
- Lalas, S.; Tsaknis, J. Characterization of Moringa oleifera seed oil variety “Periyakulam 1”. J. Food Compos. Anal. 2002, 15, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.N.; Mellon, F.A.; Foid, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Barthet, V.J.; Daun, J.K. Chapter 5—Seed Morphology, Composition, and Quality. In Canola, 1st ed.; Daun, J.K., Eskin, N.A.M., Hickling, D., Eds.; AOCS Press of the American Oil Chemists’ Society: Champaign, IL, USA, 2011; pp. 119–162. [Google Scholar]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.A.; Carvalho, A.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.P.; Singh, P.; Singh, S. Processing of Moringa oleifera leaves for human consumption. Bull. Environ. Pharmacol. Life Sci. 2012, 2, 28–31. [Google Scholar]
- Ijarotimi, O.S.; Adeoti, O.A.; Ariyo, O. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci. Nutr. 2013, 1, 452–463. [Google Scholar] [CrossRef]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume beverages from chickpea and lupin, as new milk alternatives. Foods 2020, 9, 1458. [Google Scholar] [CrossRef]
- Pan, Z.; Tangratanavalee, W. Characteristics of soybeans as affected by soaking conditions. LWT Food Sci. Technol. 2003, 36, 143–151. [Google Scholar] [CrossRef]
- Khandelwal, S.; Udipi, S.A.; Ghugre, P. Polyphenols and tannins in Indian pulses: Effect of soaking, germination and pressure cooking. Food Res. Int. 2010, 43, 526–530. [Google Scholar] [CrossRef]
- Nelson, A.I.; Steinberg, M.P.; Wei, L.S. Illinois process for preparation of soymilk. J. Food Sci. 1976, 41, 57–61. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 2446. Milk—Determination of Fat Content, 2nd ed.; ISO: Geneva, Switzerland, 2008; pp. 1–12. [Google Scholar]
- International Organization of Vine and Wine. Type I methods: Total Acidity (OIV-MA-AS313-01: R2015). In Compendium of International Methods of Wine and Must Analysis; IOVW: Paris, France, 2019; Volume I. [Google Scholar]
- AOAC (Association of Analytical Chemists). Ash of flour (direct method) 923.03. In Official Methods of Analysis, 18th ed.; Horowitz, W., Ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- European Commission. Regulation (EC) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1169&from=pt (accessed on 25 September 2019).
- Castellar, M.; Obón, J.; Fernández-López, J. The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia stricta fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- International Standard Organization. ISO 11035—Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach, 1st ed.; ISO: Geneva, Switzerland, 1994; pp. 1–26. [Google Scholar]
- Favaro-Trindade, C.; Terzi, S.; Trugo, L.; Modesta, R.; Couri, S. Development and sensory evaluation of soymilk based yogurt. Arch. Latinoam. Nutr. 2001, 51, 100–104. [Google Scholar]
- Barnes, H.A. Chapter 9: Shear-thinning liquids. In Handbook of Elementary Rheology, 1st ed.; Institute of Non-Newtonian Fluid Mechanics, Department of Mathematics, University of Wales: Aberystwyth, UK, 2000; pp. 55–61. [Google Scholar]
- PortFIR. Composição de Alimentos. Available online: http://portfir.insa.pt/foodcomp/search (accessed on 28 April 2022).
- Fontana, A.J. Water activity’s role in food safety and quality. Food Safety Magazine. 1 February 2001. Available online: https://www.food-safety.com/articles/4420-water-activitye28099s-role-in-food-safety-and-quality (accessed on 22 April 2022).
- Duarte, C.M.; Mota, J.; Assunção, R.; Martins, C.; Ribeiro, A.C.; Lima, A.I.; Raymundo, A.; Nunes, M.C.; Ferreira, R.B.; Sousa, I. New alternatives to milk from pulses: Chickpea and lupin beverages with improved digestibility and potential bioactivities for human health. Front. Nutr. 2022; in press. [Google Scholar]
- Ogunsina, B.S.; Radha, C.; Indrani, D. Quality characteristics of bread and cookies enriched with debittered Moringa oleifera seed flour. Int. J. Food Sci. Nutr. 2011, 62, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Béal, C.; Helinck, S. Fabrication des yaourts et des laits fermentés. Tech. Ing. Bioprocédés 2019, F6315 v2, 1–23. [Google Scholar] [CrossRef]
- Asante, W.J.; Nasare, I.L.; Tom-Dery, D.; Ochire-Boadu, K.; Kentil, K.B. Nutrient composition of Moringa oleifera leaves from two agro ecological zones in Ghana. Afr. J. Plant Sci. 2014, 8, 65–71. [Google Scholar]
- The National Academies Press. Dietary Reference Intakes (DRIs): Elements. Retrieved from Food and Nutrition Board, Institute of Medicine, National Academies. 2019. Available online: https://www.nap.edu (accessed on 29 April 2022).
- Makinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef]
- Raymundo, A.; Batista, A.; Sousa, I. Chapter 4: Rheology applied to food product design. In Advances in Rheology Research, 1st ed.; Pérez, M.D.T., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 63–118. [Google Scholar]
- Shaker, R.R.; Jumah, R.Y.; Abu-Jdayil, B. Rheological properties of plain yogurt during coagulation process: Impact of fat content and preheat treatment of milk. J. Food Eng. 2000, 44, 175–180. [Google Scholar] [CrossRef]


| Task Description | Results/Sensory Evaluation | Further Steps | Decision Support |
|---|---|---|---|
| SEED TOASTING | |||
| 65 °C for 30 min | Still sour, but less than raw seed; sweet after flavor. | Increase toasting temperature to diminish or eliminate the sourness. | Control of temperature will help the removal of bitterness. |
| 200 °C for 1 h 40 min + 260 °C for 16 min | Too much toasted, very bad taste (burnt). | Reduce toasting time. | |
| 200 °C for 30 min | Seed without color change; still bitter and sweet. | Reduce toasting temperature. | |
| 150 °C for 30 min | Too sweet, too bitter, not burnt. | Soak the kernels first. | |
| After soaking: 100 °C, for 30 min | Taste the same as raw, still too sweet and too bitter. | Keep soaking and increase the temperature to 150 °C. | |
| After soaking: 150 °C, for 30 min | Very crunchy, good taste (similar to toasted groundnuts). | Not necessary. | OK. Best performing procedure (BPP). |
| SEED SOAKING | |||
| Soaking raw seeds (1:3 w/v). 3 stages: first two warm water (30–35 °C), last with cold water (15–20 °C) overnight. | Taste the same as the raw kernels, still too sour and too sweet. | Cook the seeds. | Soaking and cooking help to release the anti-nutrients, such as the bitter compounds, to the water. |
| Before cooking: Soaked seeds cooked in hot water (30 min, 100 °C) | The sweetness was lost, still a little bit of bitterness. | Addition of flavors to improve beverage taste. | OK. Sensory testing with new flavors. |
| SOAKED SEED MILLING | |||
| Before cooking Food processor (4 min, 20,500 rpm) followed by Ultraturrax (1 min, 20,500 rpm) + addition of tap water to help milling efficiency and beverage homogenization. | Very strong bitter/raw taste. Sandy mouthfeel. | Cook the soaked seeds. To reduce sandy mouthfeel use the colloidal mill. | |
| After cooking Food processor (4 min, 20,500 rpm) followed by colloidal milling (70 rpm, 15 min) + addition of tap water to help milling efficiency and beverage homogenization. | Smooth and pleasant taste, with a slight bitterness on final beverage. | Not necessary. | OK. BPP. |
| FERMENTATION | |||
| MO “cream”—MO beverage with 40% (w/v) of seeds, with 18% (w/v) of soy yoghurt incubated at 30 °C for 4 h 30 min | Pleasant taste and smell. Liquid cream appearance as beverage. | Not necessary. | OK. BPP. |
| Incubation time of 21 h | Not so nice smell as the previous one: “green” smell; no sweetness. Phase separation, layers with air in between. | ||
| aw | Moisture (%) | Total Acidity (mEq of acid/L) | Fat (% w/w) | Protein (% w/w) | |
|---|---|---|---|---|---|
| Raw MO seeds | 0.56 ± 0.00 | 4.74 ± 0.05 a | 3.70 ± 0.29 | 37.24 ± 0.83 | 36.82 ± 0.20 a |
| Toasted MO seeds | 0.48 ± 0.03 | 0.33 ± 0.05 b | 3.50 ± 0.00 | 34.95 ± 1.34 | 40.21 ± 0.18 b |
| pH | Total Acidity (mEq acid/L) | Fat (%) | Moisture (%) | Ashes (%) | Protein * (%) | Carbohydrate Estim. (%) | Energy (kcal) (kJ) | |
|---|---|---|---|---|---|---|---|---|
| MO beverage 10% (w/v) dry seed | 6.03 ± 0.00 | 10.00 ± 1.32 | 2.97 ± 0.12 | 92.67 ± 0.05 | 0.24 ± 0.05 | 3.68 ± 0.02 | 0.44 | 43.19 ± 0.83 (179.87 ± 3.38) |
| MO yoghurt-like (4 h 30 min) | 5.45 ± 0.02 | 46.70 ± 2.00 | 7.25 ± 0.47 | 81.10 ± 0.43 | 0.75 ± 0.05 | 14.73 ± 0.08 | 0.00 | 124.13 ± 4.46 (518.51 + 18.38) |
| Raw MO Seed (mg/100 g) | % DRI | Toasted MO Seed (mg/100 g) | % DRI | MO Beverage (mg/100 mL) | % DRI | MO Yoghurt (mg/100 g) | % DRI | |
|---|---|---|---|---|---|---|---|---|
| Na | 6.82 ± 0.19 a | 0.45 | 9.01 ± 0.78 b | 0.60 | 3.14 ± 0.04 | 0.21 | 5.01 ± 0.04 | 0.33 |
| K | 763.49 ± 6.83 a | 38.17 | 747.56 ± 6.38 b | 37.38 | 38.08 ± 0.40 | 1.90 | 28.05 ± 0.14 | 1.40 |
| Ca | 139.64 ± 0.76 a | 17.45 | 163.64 ± 1.46 b | 20.45 | 17.86 ± 0.08 | 2.23 | 13.29 ± 0.12 | 1.66 |
| Mg | 302.51 ± 2.99 a | 80.67 | 310.47 ± 3.34 b | 82.79 | 29.31 ± 0.04 | 7.82 | 15.98 ± 0.15 | 4.26 |
| P | 771.19 ± 3.92 a | 110.17 | 790.68 ± 4.89 b | 112.95 | 72.24 ± 1.01 | 10.32 | 40.53 ± 0.19 | 5.79 |
| S | 1994.06 ± 51.42 | ___ | 1977.58 ± 23.44 | ___ | 156.54 ± 3.90 | ___ | 85.88 ± 1.20 | ___ |
| Fe | 9.97 ± 0.07 a | 71.18 | 17.01 ± 0.05 b | 121.52 | 0.50 ± 0.00 | 3.57 | 0.26 ± 0.00 | 1.84 |
| Cu | 0.86 ± 0.01 a | 85.82 | 0.91 ± 0.02 b | 90.52 | 0.08 ± 0.00 | 8.13 | 0.04 ± 0.00 | 3.85 |
| Zn | 5.20 ± 0.03 a | 52.00 | 6.38 ± 0.02 b | 63.81 | 0.56 ± 0.00 | 5.61 | 0.31 ± 0.00 | 3.09 |
| Mn | 1.25 ± 0.01 a | 62.35 | 1.44 ± 0.00 b | 72.17 | 0.15 ± 0.00 | 7.32 | 0.08 ± 0.00 | 3.77 |
| B | 0.58 ± 0.01 a | 2.88 | 0.50 ± 0.01 b | 2.49 | 0.00 ± 0.00 | ___ | 0.02 ± 0.00 | 0.11 |
| η0 (Pa·s) | η∞ (Pa·s) | c (s−1) | |
|---|---|---|---|
| MO yoghurt_4 h 30 min_8 °C | 21,465.7 ± 2723.5 b,c | 3.3 × 10−2 ± 0.3 × 10−2 a | 6.7 × 10−4 ± 0.4 × 10−4 a |
| Low fat dairy yoghurt_8 °C | 15,127.2 + 2013.4 a,b | 1.4 × 10−2 + 0.4 × 10−2 a,b | 26.6 × 10−4 ± 3.0 × 10−4 a,b |
| Soy yoghurt_8 °C | 3381.2 ± 277.1 a,c | 2.7 × 10−2 ± 0.2 × 10−2 b | 11.0 × 10−4 ± 1.9 × 10−4 b |
| Lupin beverage | 658.1 ± 34.6 d,e | 2.9 × 10−2 ± 0.1 × 10−2 c,d | 2.0 × 10−4 ± 0.5 × 10−4 c |
| Chickpea beverage | 176.4 ± 22.4 d,f | 1.3 × 10−2 ± 0.1 × 10−2 c,e | 5.6 × 10−4 ± 1.1 × 10−4 |
| MO beverage | 17.4 ± 3.1 e,f | 0.6 × 10−2 ± 0.0 × 10−2 d,e | 8.1 × 10−4 ± 1.9 × 10−4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautier, A.; Duarte, C.M.; Sousa, I. Moringa oleifera Seeds Characterization and Potential Uses as Food. Foods 2022, 11, 1629. https://doi.org/10.3390/foods11111629
Gautier A, Duarte CM, Sousa I. Moringa oleifera Seeds Characterization and Potential Uses as Food. Foods. 2022; 11(11):1629. https://doi.org/10.3390/foods11111629
Chicago/Turabian StyleGautier, Adèle, Carla Margarida Duarte, and Isabel Sousa. 2022. "Moringa oleifera Seeds Characterization and Potential Uses as Food" Foods 11, no. 11: 1629. https://doi.org/10.3390/foods11111629
APA StyleGautier, A., Duarte, C. M., & Sousa, I. (2022). Moringa oleifera Seeds Characterization and Potential Uses as Food. Foods, 11(11), 1629. https://doi.org/10.3390/foods11111629