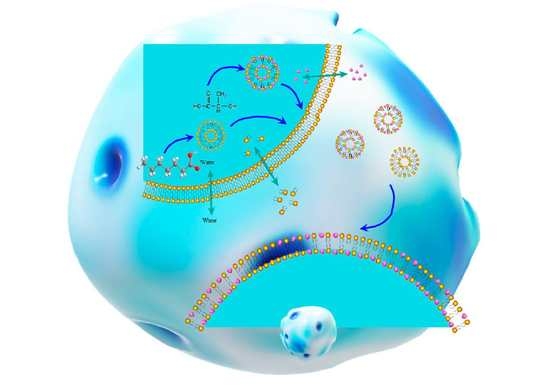
Effection of Lactic Acid Dissociation on Swelling-Based Short-Chain Fatty Acid Vesicles Nano-Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Vesicle Preparation
2.2. Dynamic Light Scattering (DLS) and Zeta-Potential Measurements
2.3. Morphology Study of Assembled Structures
2.4. Calculation Dissociation Constant
2.5. Cell Culture and Membrane Dipole Potential
2.6. Infrared Spectroscopy and Computational Methods
3. Results and Discussion
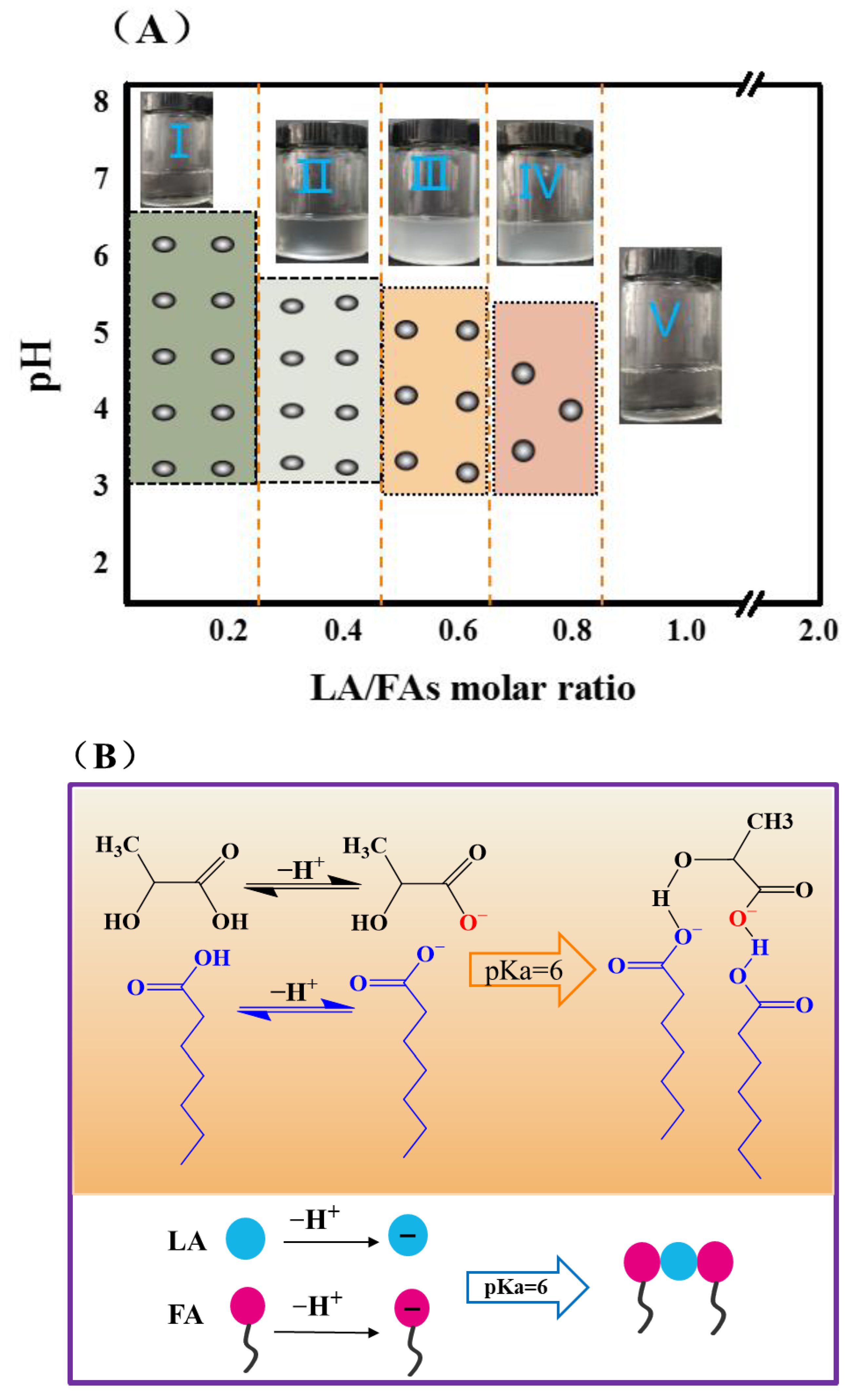
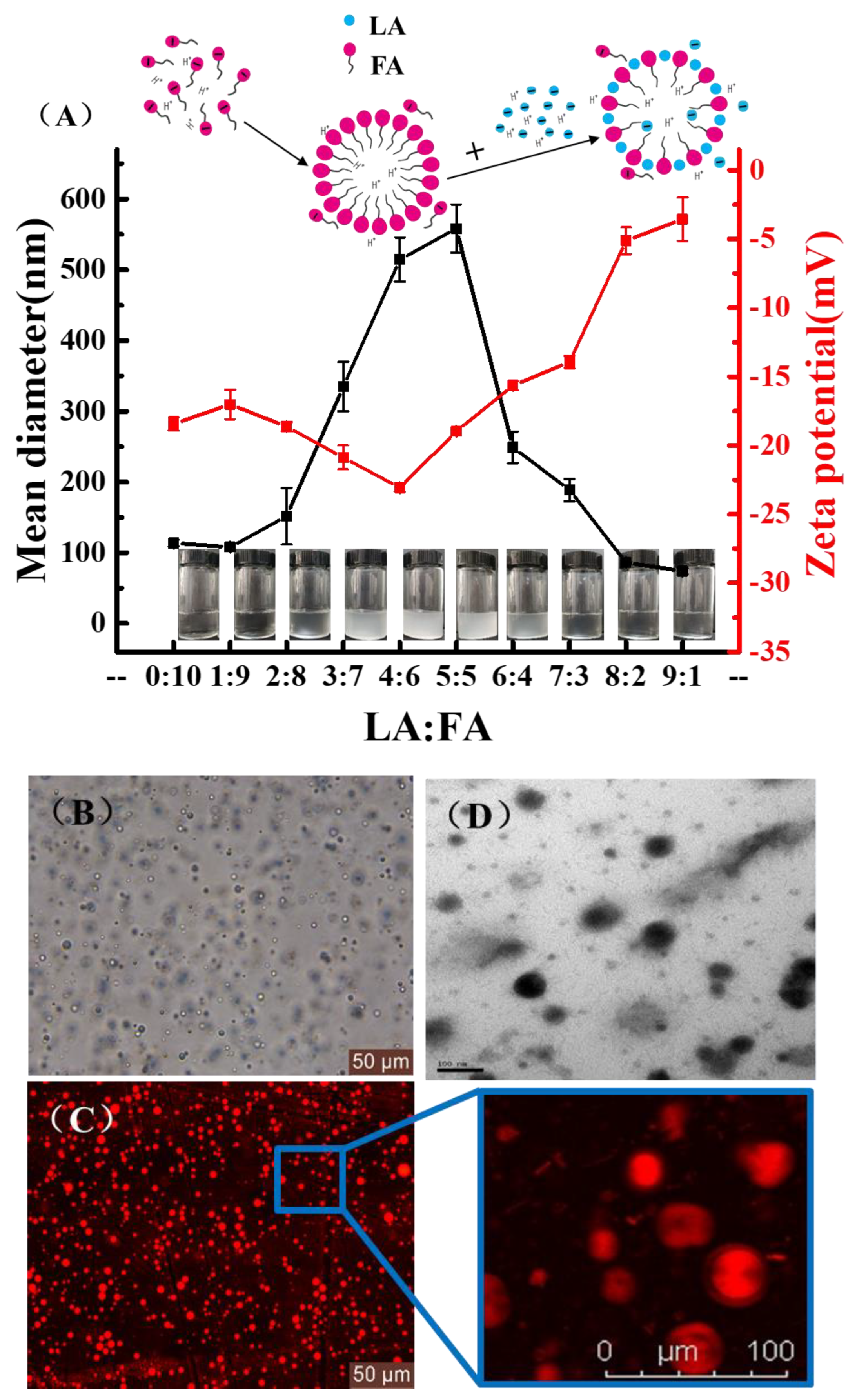
3.1. Fatty Acid Vesicular Properties
3.2. The pH Causes Ion Concentration Changes
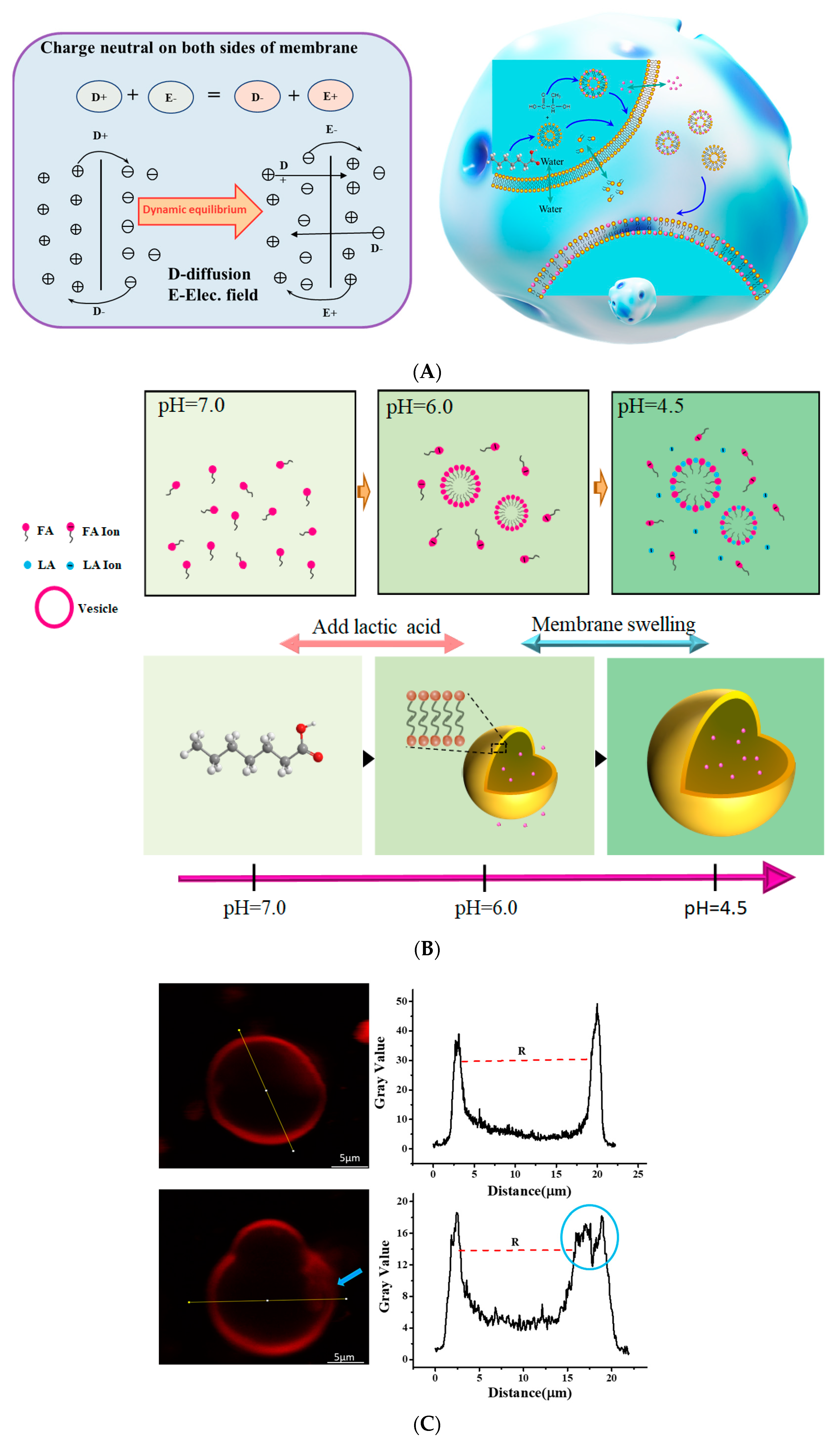
3.3. Changes in Cell Membrane Properties Caused by LA
3.4. Intermolecular Interaction between FA and LA
3.5. Weak Acid Changes the Distribution of Ions in LA/FA Vesicles System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kelestemur, S.; Cobandede, Z.; Culha, M. Biofilm formation of clinically important microorganisms on 2D and 3D poly (methyl methacrylate) substrates: A surface-enhanced Raman scattering study. Colloids Surf. B Biointerfaces 2020, 188, 110761–110765. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.G.; Jiang, Y.W.; Chen, Z.; Yu, Z.W. Folding behaviors of protein (lysozyme) confined in polyelectrolyte complex micelle. Langmuir 2016, 32, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alfonso, T.; Ryan, T.A. The efficiency of the synaptic vesicle cycle at central nervous system synapses. Trends Cell Biol. 2006, 16, 413–420. [Google Scholar] [CrossRef]
- Smith, S.M.; Renden, R.; von Gersdorff, H. Synaptic vesicle endocytosis: Fast and slow modes of membrane retrieval. Trends Neurosci. 2008, 31, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Hinckelmann, M.V.; Virlogeux, A.; Niehage, C.; Poujol, C.; Choquet, D.; Hoflack, B.; Zala, D.; Saudou, F. Self-propelling vesicles define glycolysis as the minimal energy machinery for neuronal transport. Nat. Commun. 2016, 7, 13233–13240. [Google Scholar] [CrossRef]
- Hanczyc, M.M. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Božič, B.; Zemljič Jokhadar, Š.; Kristanc, L.; Gomišček, G. Cell volume changes and membrane ruptures induced by hypotonic electrolyte and sugar solutions. Front. Physiol. 2020, 11, 582781. [Google Scholar] [CrossRef]
- Niu, D.; Wu, Y.; Lei, Z.; Zhang, M.; Xie, Z.; Tang, S. Lactic acid, a driver of tumor-stroma interactions. Int. Immunopharmacol. 2022, 106, 108597. [Google Scholar] [CrossRef]
- Syed, M.; Kammala, A.K.; Callahan, B.; Oskeritzian, C.A.; Subramanian, H. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell. Immunol. 2021, 368, 104422. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Liu, B.; Liu, Z. Tumor microenvironment-responsive dynamic inorganic nanoassemblies for cancer imaging and treatment. Adv. Drug Deliv. Rev. 2021, 179, 114004. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Ealey, K.N.; Tetsu, H.; Kiniwa, T.; Motomura, Y.; Moro, K.; Koyasu, S. Tumor-derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep. 2020, 30, 2743–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-H.; Peng, W.-B.; Zhang, P.; Yang, X.-P.; Zhou, Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine 2021, 73, 103627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Peralta, R.M.; Avina-Ochoa, N.; Delgoffe, G.M.; Kaech, S.M. Metabolic regulation of T cells in the tumor microenvironment by nutrient availability and diet. Semin. Immunol. 2021, 52, 101485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Li, Q.; Li, L.; Jia, Y.; Geng, F.; Zhou, J.; Yin, T. Tumor microenvironment remodeling-based penetration strategies to amplify nanodrug accessibility to tumor parenchyma. Adv. Drug Deliv. Rev. 2021, 172, 80–103. [Google Scholar] [CrossRef]
- Yu, W.; Lin, R.; He, X.; Yang, X.; Zhang, H.; Hu, C.; Liu, R.; Huang, Y.; Qin, Y.; Gao, H. Self-propelled nanomotor reconstructs tumor microenvironment through synergistic hypoxia alleviation and glycolysis inhibition for promoted anti-metastasis. Acta Pharm. Sin. B 2021, 11, 2924–2936. [Google Scholar] [CrossRef]
- Hu, J.; Guan, Z.; Chen, J. Multifunctional biomaterials that modulate oxygen levels in the tumor microenvironment. Cancer Lett. 2021, 521, 39–49. [Google Scholar] [CrossRef]
- Enoki, T.A.; Wu, J.; Heberle, F.A.; Feigenson, G.W. Investigation of the domain line tension in asymmetric vesicles prepared via hemifusion. Biochim. Biophys. Acta Biomembr. 2021, 1863, 1835–1842. [Google Scholar] [CrossRef]
- Chabanon, M.; Ho, J.C.S.; Liedberg, B.; Parikh, A.N.; Rangamani, P. Pulsatile lipid vesicles under osmotic stress. Biophys. J. 2017, 112, 1682–1691. [Google Scholar] [CrossRef] [Green Version]
- D’Acunto, M. Nanovectors for drug delivery: Long-lived pore dynamics for swelling liposomes. Mech. Res. Commun. 2011, 38, 34–37. [Google Scholar] [CrossRef]
- Cochereau, R.; Renard, D.; Nous, C.; Boire, A. Semi-permeable vesicles produced by microfluidics to tune the phase behaviour of encapsulated macromolecules. J. Colloid Interface Sci. 2020, 580, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Rey-Castro, C.; Herrero, R.; Sastre de Vicente, M.E. Gibbs–Donnan and specific-ion interaction theory descriptions of the effect of ionic strength on proton dissociation of alginic acid. J. Electroanal. Chem. 2004, 564, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Levin, Y.; Idiart, M.A. Pore dynamics of osmotically stressed vesicles. Phys. A Stat. Mech. Appl. 2004, 331, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Idiart, M.A.; Levin, Y. Rupture of a liposomal vesicle. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004, 69, 0619–0622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khmelinskii, I.; Makarov, V.I. On the effects of mechanical stress of biological membranes in modeling of swelling dynamics of biological systems. Sci. Rep. 2020, 10, 8395. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chan Miller, C.; Szostak, J.W. Core-shell modeling of light scattering by vesicles: Effect of size, contents, and lamellarity. Biophys. J. 2019, 116, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Han, Y.; Zhang, L.; Chen, Q.; Ding, M.; Shi, T. Migration and deformation of polyelectrolyte vesicle through a pore in electric field. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 1255–1260. [Google Scholar] [CrossRef]
- Momotake, A.; Mizuguchi, T.; Hishida, M.; Yamamoto, Y.; Yasui, M.; Nuriya, M. Monitoring the morphological evolution of giant vesicles by azo dye-based sum-frequency generation (SFG) microscopy. Colloids Surf. B Biointerfaces 2020, 186, 1107–1116. [Google Scholar] [CrossRef]
- Gabba, M.; Poolman, B. Physiochemical modeling of vesicle dynamics upon osmotic upshift. Biophys. J. 2020, 118, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Che, H.; Cao, S.; van Hest, J.C.M. Feedback-induced temporal control of “breathing” polymersomes to create self-adaptive nanoreactors. J. Am. Chem. Soc. 2018, 140, 5356–5359. [Google Scholar] [CrossRef] [Green Version]
- Rahmanian, M.; Seyfoori, A.; Ghasemi, M.; Shamsi, M.; Kolahchi, A.R.; Modarres, H.P.; Sanati-Nezhad, A.; Majidzadeh-A, K. In-vitro tumor microenvironment models containing physical and biological barriers for modelling multidrug resistance mechanisms and multidrug delivery strategies. J. Control. Release 2021, 334, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Chen, K.X.; Huang, X.Y.; Lou, J.; Li, J.Y.; Deng, S.P. Vesicles from the self-assembly of the ultra-small fatty acids with amino acids under aqueous conditions. Colloids Surf. B Biointerfaces 2018, 173, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Chen, K.X.; Zhang, S.Y.; Deng, S.P. Vesicle formation by ultrashort alkyl-phosphonic acids and serine in aqueous solutions. Colloids Surf. B Biointerfaces 2019, 179, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-S.; Yu, Y.-X.; Li, Q.-K.; Yu, Z.-W. Interaction of human synovial phospholipase A2 with mixed lipid bilayers: A coarse-grain and all-atom molecular dynamics simulation study. Biochemistry 2013, 52, 1477–1489. [Google Scholar] [CrossRef]
- Dejanovic, B.; Noethig-Laslo, V.; Sentjurc, M.; Walde, P. On the surface properties of oleate micelles and oleic acid/oleate vesicles studied by spin labeling. Chem. Phys. Lipids 2011, 164, 83–88. [Google Scholar] [CrossRef]
- Chen, L.C.; Xie, N.N.; Deng, S.P. Sweetness-induced activation of membrane dipole potential in STC-1 taste cells. Food Chem. 2016, 212, 768–777. [Google Scholar] [CrossRef]
- Chen, L.C.; Wang, H.P.; Deng, Y.H.; Deng, S.P. Vesicle formation by proton transfer driven short-tailed fatty acids of C4-C8 chain length in water. Soft Matter 2017, 13, 1291–1298. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Magazu, S.; Wanderlingh, U.; Barreca, D.; Pasqua, L.; Kiselev, M.A. Soft nanoparticles charge expression within lipid membranes: The case of amino terminated dendrimers in bilayers vesicles. Colloids Surf. B Biointerfaces 2018, 170, 609–616. [Google Scholar] [CrossRef]
- Peterlin, P.; Arrigler, V.; Haleva, E.; Diamant, H. Law of corresponding states for osmotic swelling of vesicles. Soft Matter 2012, 8, 2185. [Google Scholar] [CrossRef] [Green Version]
- Kirouac, M.; Vachon, V.; Fortier, M.; Trudel, M.C.; Berteloot, A.; Schwartz, J.L.; Laprade, R. A mechanical force contributes to the “osmotic swelling” of brush-border membrane vesicles. Biophys. J. 2006, 91, 3301–3312. [Google Scholar] [CrossRef] [Green Version]
- Campelo, F.; Arnarez, C.; Marrink, S.J.; Kozlov, M.M. Helfrich model of membrane bending: From Gibbs theory of liquid interfaces to membranes as thick anisotropic elastic layers. Adv. Colloid Interface Sci. 2014, 208, 25–33. [Google Scholar] [CrossRef]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094–2097. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, L.; Wang, M. Applicability of donnan equilibrium theory at nanochannel-reservoir interfaces. J. Colloid Interface Sci. 2015, 452, 78–88. [Google Scholar] [CrossRef]
- Maeda, H. The Gibbs-duhem type relation for ionic/nonionic mixed micelles—An alternative approach to Hall’s method. J. Colloid Interface Sci. 2011, 364, 413–416. [Google Scholar] [CrossRef]
- Chng, C.P.; Sadovsky, Y.; Hsia, K.J.; Huang, C. Curvature-regulated lipid membrane softening of nano-vesicles. Extreme Mech. Lett. 2021, 43, 101174. [Google Scholar] [CrossRef]
- Martin-Molina, A.; Lue, L.; Quesada-Perez, M.; Bohinc, K. Interaction between charged lipid vesicles and point- or rod-like trivalent ions. Colloids Surf. B Biointerfaces 2019, 178, 525–529. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhao, H.; Xue, S.; Chen, K.; Zhang, Y. Effection of Lactic Acid Dissociation on Swelling-Based Short-Chain Fatty Acid Vesicles Nano-Delivery. Foods 2022, 11, 1630. https://doi.org/10.3390/foods11111630
Chen L, Zhao H, Xue S, Chen K, Zhang Y. Effection of Lactic Acid Dissociation on Swelling-Based Short-Chain Fatty Acid Vesicles Nano-Delivery. Foods. 2022; 11(11):1630. https://doi.org/10.3390/foods11111630
Chicago/Turabian StyleChen, Lichun, Huimin Zhao, Songwen Xue, Kexian Chen, and Yue Zhang. 2022. "Effection of Lactic Acid Dissociation on Swelling-Based Short-Chain Fatty Acid Vesicles Nano-Delivery" Foods 11, no. 11: 1630. https://doi.org/10.3390/foods11111630
APA StyleChen, L., Zhao, H., Xue, S., Chen, K., & Zhang, Y. (2022). Effection of Lactic Acid Dissociation on Swelling-Based Short-Chain Fatty Acid Vesicles Nano-Delivery. Foods, 11(11), 1630. https://doi.org/10.3390/foods11111630