Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Antibody Development
2.4. Indirect Non-Competitive Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Isolation of Chicken Serum Albumin
2.6. Gel Electrophoresis
2.7. Western Blot
2.8. Statistical Analysis
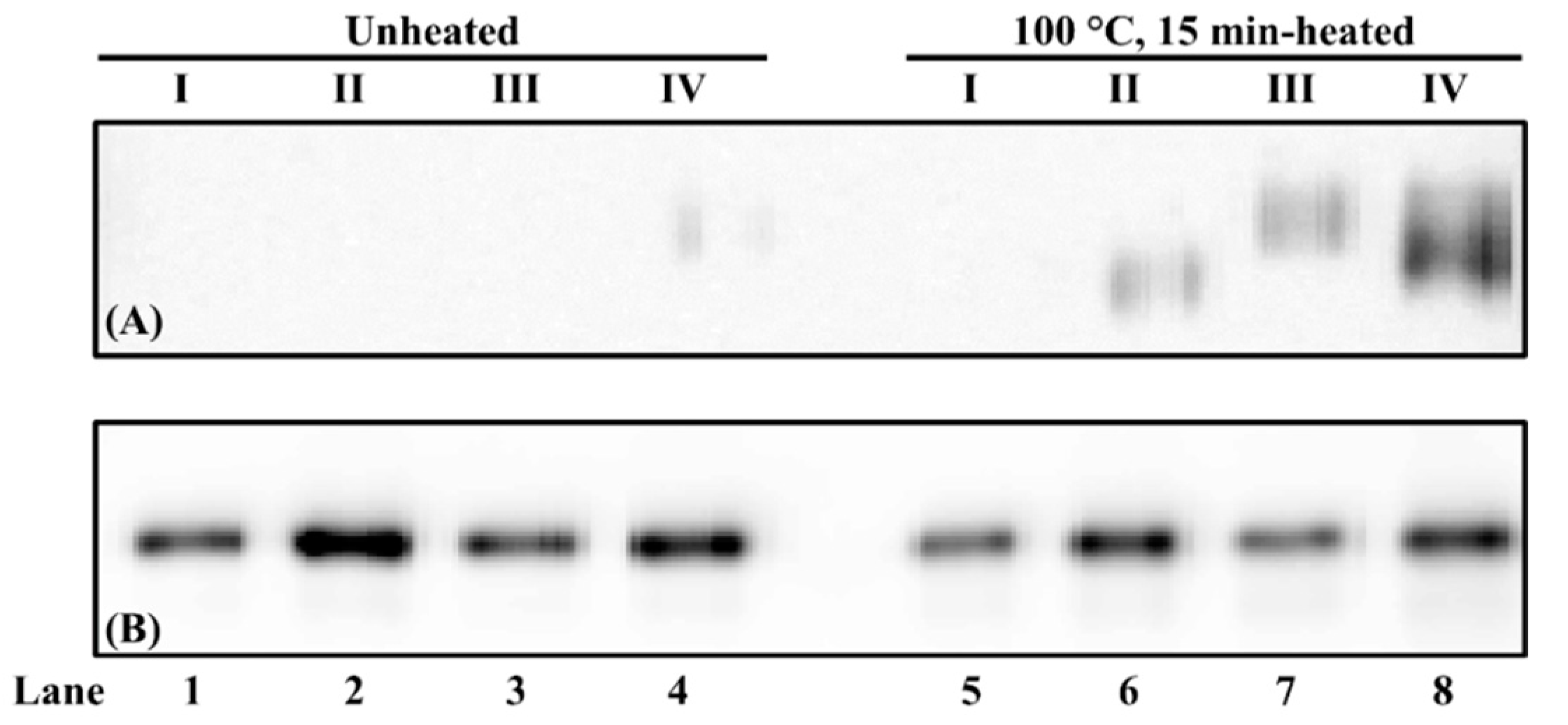
3. Results and Discussion
3.1. Antibody Characterization
3.1.1. Antibody Target Analyte
3.1.2. Antibody Species Selectivity
3.2. Chicken Serum Albumin Isolation
3.3. Effect of Extraction Buffer Composition on Chicken Serum Albumin Extractability and Immunoreactivity
3.4. Effect of Extraction Buffer pH on Chicken Serum Albumin Solubility and Immunoreactivity
3.5. Effect of Buffer Composition on Chicken Serum Albumin Thermostability from Different Food Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FDA. Food Allergen Labeling and Consumer Protection Act of 2004. Available online: https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM179394.pdf (accessed on 14 April 2022).
- Samady, W.; Warren, C.; Wang, J.; Das, R.; Gupta, R.S. Egg allergy in US children. J. Allergy Clin. Immunol. Pract. 2020, 8, 3066–3073.e6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed]
- Amo, A.; Rodríguez-Pérez, R.; Blanco, J.; Villota, J.; Juste, S.; Moneo, I.; Caballero, M.L. Gal d 6 is the second allergen characterized from egg yolk. J. Agric. Food. Chem. 2010, 58, 7453–7457. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Esparza, Y.; Temelli, F.; Wu, J. Physicochemical and functional properties of livetins fraction from hen egg yolk. Food Biosci. 2017, 18, 38–45. [Google Scholar] [CrossRef]
- Szépfalusi, Z.; Ebner, C.; Pandjaitan, R.; Orlicek, F.; Scheiner, O.; Boltz-Nitulescu, G.; Kraft, D.; Ebner, H. Egg yolk alpha-livetin (chicken serum albumin) is a cross-reactive allergen in the bird-egg syndrome. J. Allergy Clin. Immunol. 1994, 93, 932–942. [Google Scholar] [CrossRef]
- Mandallaz, M.M.; de Weck, A.L.; Dahinden, C.A. Bird-egg syndrome. Int. Arch. Allergy Immunol. 1988, 87, 143–150. [Google Scholar] [CrossRef]
- Urich, K. Plasma proteins, yolk proteins and metal-binding proteins. In Comparative Animal Biochemistry; Springer: Berlin/Heidelberg, Germany, 1994; pp. 184–219. [Google Scholar]
- Hemmer, W.; Klug, C.; Swoboda, I. Update on the bird-egg syndrome and genuine poultry meat allergy. Allergo J. Int. 2016, 25, 68–75. [Google Scholar] [CrossRef]
- Tóthová, C.; Sesztáková, E.; Bielik, B.; Nagy, O. Changes of total protein and protein fractions in broiler chickens during the fattening period. Vet. World 2019, 12, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Quirce, S.; Marañón, F.; Umpiérrez, A.; Heras, M.D.L.; Fernández-Caldas, E.; Sastre, J. Chicken serum albumin (Gal d 5*) is a partially heat-labile inhalant and food allergen implicated in the bird-egg syndrome. Allergy 2001, 56, 754–762. [Google Scholar] [CrossRef]
- Hilger, C.; Swiontek, K.; Hentges, F.; Donnay, C.; de Blay, F.; Pauli, G. Occupational inhalant allergy to pork followed by food allergy to pork and chicken: Sensitization to hemoglobin and serum albumin. Int. Arch. Allergy Immunol. 2010, 151, 173–178. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2017, 49, D480–D489. [Google Scholar] [CrossRef]
- Raoufinia, R.; Mota, A.; Keyhanvar, N.; Safari, F.; Shamekhi, S.; Abdolalizadeh, J. Overview of albumin and its purification methods. Adv. Pharm. Bull. 2016, 6, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Mikolajczak, K.; Mank, N.; Majorek, K.A.; Porebski, P.J.; Minor, W. Serum albumins—Unusual allergens. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5375–5381. [Google Scholar] [CrossRef]
- Anthony-Regnitz, C.M.; Wilson, A.E.; Sweazea, K.L.; Braun, E.J. Fewer exposed lysine residues may explain relative resistance of chicken serum albumin to in vitro protein glycation in comparison to bovine serum albumin. J. Mol. Evol. 2020, 88, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Lehrer, S.B.; Tanaka, L.; Ibanez, M.D.; Pascual, C.; Burks, A.W.; Sussman, G.L.; Goldberg, B.; Lopez, M.; Reese, G. IgE antibody response to vertebrate meat proteins including tropomyosin. Ann. Allergy Asthma Immunol. 1999, 83, 399–405. [Google Scholar] [CrossRef]
- Sousa, R.S.; Coimbra, J.S.R.; Rojas, E.E.G.; Minim, L.A.; Oliveira, F.C.; Minim, V.P.R. Effect of pH and salt concentration on the solubility and density of egg yolk and plasma egg yolk. LWT 2007, 40, 1253–1258. [Google Scholar] [CrossRef]
- deWit, J.N.; Klarenbeek, G. Effects of various heat treatments on structure and solubility of whey proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- MyBioSource. Chicken Serum Albumin ELISA Kit. Available online: https://www.mybiosource.com/alb-chicken-elisa-kits/serum-albumin/2881881 (accessed on 7 September 2020).
- Jiang, X.Y.; Fuller, D.; Hsieh, Y.-H.P.; Rao, Q.C. Monoclonal antibody-based ELISA for the quantification of porcine hemoglobin in meat products. Food Chem. 2018, 250, 170–179. [Google Scholar] [CrossRef]
- Odunuga, O.O.; Shazhko, A. Ammonium sulfate precipitation combined with liquid chromatography is sufficient for purification of bovine serum albumin that is suitable for most routine laboratory applications. Biochem. Compd. 2013, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Jiang, X.Y.; Wu, M.; Albo, J.; Rao, Q.C. Non-specific binding and cross-reaction of ELISA: A case study of porcine hemoglobin detection. Foods 2021, 10, 1708. [Google Scholar] [CrossRef]
- Rao, Q.C.; Hsieh, Y.-H.P. Competitive enzyme-linked immunosorbent assay for quantitative detection of bovine blood in heat-processed meat and feed. J. Food Prot. 2008, 71, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Chapter 10—Blood. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 167–191. [Google Scholar]
- Peltonen, L.M.; Sankari, S. Ott’s protein osmotic pressure of serum and interstitial fluid in chickens (Gallus gallus): Effect of age and gender. J. Exp. Biol. 2011, 214, 599–606. [Google Scholar] [CrossRef]
- Sunwoo, H.H.; Gujral, N. Chemical composition of eggs and egg products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 331–363. [Google Scholar]
- Mine, Y. Recent advances in egg protein functionality in the food system. World Poult. Sci. J. 2002, 58, 31–39. [Google Scholar] [CrossRef]
- Beretta, B.; Conti, A.; Fiocchi, A.; Gaiaschi, A.; Galli, C.L.; Giuffrida, M.G.; Ballabio, C.; Restani, P. Antigenic determinants of bovine serum albumin. Int. Arch. Allergy Immunol. 2001, 126, 188–195. [Google Scholar] [CrossRef]
- Akita, E.M.; Nakai, S. Production and purification of Fab’ fragments from chicken egg yolk immunoglobulin Y (IgY). J. Immunol. Methods 1993, 162, 155–164. [Google Scholar] [CrossRef]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.O.P.T.; Guedes, M.I.F. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, Q.; Liu, Y.; Jin, Y.; Harlina, P.W.; Ma, M. Highly efficient extraction and purification of low-density lipoprotein from hen egg yolk. Poult. Sci. 2018, 97, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Besse, D.; Siedler, F.; Diercks, T.; Kessler, H.; Moroder, L. The redox potential of selenocystine in unconstrained cyclic peptides. Angew. Chem. Int. Edit. 1997, 36, 883–885. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, I.; Lagrain, B.; Scherf, K.A.; Koehler, P.; Delcour, J.A. Formation and reshuffling of disulfide bonds in bovine serum albumin demonstrated using tandem mass spectrometry with collision-induced and electron-transfer dissociation. Sci. Rep. 2015, 5, 12210. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Ballabio, C.; Cattaneo, A.; Isoardi, P.; Terracciano, L.; Fiocchi, A. Characterization of bovine serum albumin epitopes and their role in allergic reactions. Allergy 2004, 59, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, Y.; Gotoh, Y.; Adachi, Y.; Tsutsumi, M. Improvement of digestibility of bovine serum albumin by chemical treatment and reduction in the antigenicity of the digests. Food Sci. Technol. Res. 2001, 7, 149–153. [Google Scholar] [CrossRef][Green Version]
- Restani, P.; Fiocchi, A.; Beretta, B.; Velona, T.; Giovannini, M.; Galli, C.L. Effects of structure modifications on IgE binding properties of serum albumins. Int. Arch. Allergy Immunol. 1998, 117, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Savin, G.; Pouzot, M.; Schmitt, C.; Mezzenga, R. Structure of heat-induced β-lactoglobulin aggregates and their complexes with sodium-dodecyl sulfate. Biomacromolecules 2008, 9, 2477–2486. [Google Scholar] [CrossRef]
- Ding, Y.; Shu, Y.; Ge, L.; Guo, R. The effect of sodium dodecyl sulfate on the conformation of bovine serum albumin. Colloids Surf. A. Physicochem. Eng. Asp. 2007, 298, 163–169. [Google Scholar] [CrossRef]
- Vicente-Serrano, J.; Caballero, M.L.; Rodríguez-Pérez, R.; Carretero, P.; Pérez, R.; Blanco, J.G.; Juste, S.; Moneo, I. Sensitization to serum albumins in children allergic to cow’s milk and epithelia. Pediatr. Allergy Immunol. 2007, 18, 503–507. [Google Scholar] [CrossRef]
- Fiocchi, A.; Restani, P.; Riva, E.; Mirri, G.P.; Santini, I.; Bernardo, L.; Galli, C.L. Heat treatment modifies the allergenicity of beef and bovine serum albumin. Allergy 1998, 53, 798–802. [Google Scholar] [CrossRef]
- Estey, T.; Kang, J.; Schwendeman, S.P.; Carpenter, J.F. BSA degradation under acidic conditions: A model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 2006, 95, 1626–1639. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.; Lal, J.; An, L.; Huang, Q. Effects of pH on the interactions and conformation of bovine serum albumin: Comparison between chemical force microscopy and small-angle neutron scattering. J. Phys. Chem. B 2008, 112, 3797–3806. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. In Advances in Protein Chemistry; Anfinsen, C.B., Edsall, J.T., Richards, F.M., Eisenberg, D.S., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 45, pp. 153–203. [Google Scholar]
- Mthembu, S.N.; Sharma, A.; Albericio, F.; de la Torre, B.G. Breaking a couple: Disulfide reducing agents. ChemBioChem 2020, 21, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Interchim. DTT (Dithiothreitol). Available online: https://www.interchim.fr/ft/0/054721.pdf (accessed on 14 April 2022).
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of thermal denaturation and aggregation of bovine serum albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef] [PubMed]
- Maciążek-Jurczyk, M.; Janas, K.; Pożycka, J.; Szkudlarek, A.; Rogóż, W.; Owczarzy, A.; Kulig, K. Human serum albumin aggregation/fibrillation and its abilities to drugs binding. Molecules 2020, 25, 618. [Google Scholar] [CrossRef] [PubMed]
- Babajimopoulos, M.; Mikolajcik, E.M. Influence of heat treatment on antigenicity of bovine serum albumin in milk and model systems. J. Dairy Sci. 1978, 61, 1380–1383. [Google Scholar] [CrossRef]
- Steinhoff, M.; Fischer, M.; Paschke-Kratzin, A. Comparison of extraction conditions for milk and hen’s egg allergens. Food Addit. Contam. Part A 2011, 28, 373–383. [Google Scholar] [CrossRef]





| Reaction Unheated | Heated (100 °C, 15 min) | |
|---|---|---|
| Animal whole blood | ||
| Bovine | − | − |
| Goat | − | − |
| Horse | − | − |
| Porcine | + a | − |
| Rabbit | − | − |
| Chicken | + | ++ |
| Turkey | − | ++ |
| Animal meat | ||
| Bovine | − | − |
| Pork | − | − |
| Salmon | − | − |
| Chicken | − | − |
| Turkey | − | − |
| Hen egg | ||
| Egg white | − | − |
| Egg yolk | + | +++ |
| Extraction Buffer | I: PBS (Mean ± SEM *, %) | IV: PBS-SDS-DTT (Mean ± SEM *, %) | ||||
|---|---|---|---|---|---|---|
| Sample Model | Model 1: Chicken Serum Albumin | Model 2: Chicken Blood Plasma | Model 3: Hen Egg Yolk | Model 1: Chicken Serum Albumin | Model 2: Chicken Blood Plasma | Model 3: Hen Egg Yolk |
| Relative protein solubility | ||||||
| Unheated | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| 60 °C/15 min | 98.3 ± 0.5 a | 94.2 ± 0.9 b | 91.8 ± 1.6 b | 93.6 ± 0.5 a | 99.3 ± 1.9 a | 90.5 ± 3.9 a |
| 60 °C/30 min | 97.4 ± 1.9 a | 90.9 ± 0.9 b | 92.2 ± 1.0 b | 88.6 ± 4.4 a | 99.0 ± 1.0 a | 97.5 ± 0.8 a |
| 100 °C/15 min | 84.8 ± 0.3 b | 77.7 ± 2.3 c | 78.1 ± 0.7 c | 85.1 ± 5.6 a | 95.4 ± 7.2 a | 89.3 ± 4.6 a |
| 100 °C/15 min | 79.8 ± 0.7 c | 73.9 ± 1.1 c | 74.1 ± 0.9 c | 92.7 ± 8.9 a | 93.5 ± 2.9 a | 95.6 ± 10.1 a |
| Relative immunoreactivity | ||||||
| Unheated | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| 60 °C/15 min | 94.1 ± 2.7 a | 115.7 ± 2.2 b | 91.0 ± 3.4 ab | 111.7 ± 3.5 ab | 103.3 ± 4.0 a | 86.5 ± 1.8 ac |
| 60 °C/30 min | 101.8 ± 2.4 ac | 111.7 ± 1.9 b | 88.0 ± 3.0 b | 111.9 ± 6.7 ab | 103.1 ± 1.8 a | 84.2 ± 1.5 b |
| 100 °C/15 min | 110.7 ± 2.7 bc | 117.1 ± 1.5 b | 76.4 ± 2.9 c | 120.0 ± 4.5 b | 92.3 ± 4.7 a | 70.7 ± 0.9 b |
| 100 °C/15 min | 98.2 ± 1.1 a | 113.1 ± 2.2 b | 89.5 ± 3.8 b | 107.4 ± 3.5 ab | 91.8 ± 5.3 a | 55.2 ± 1.7 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Mu, H.; Hsieh, Y.-H.P.; Rao, Q. Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5). Foods 2022, 11, 1637. https://doi.org/10.3390/foods11111637
Jiang X, Mu H, Hsieh Y-HP, Rao Q. Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5). Foods. 2022; 11(11):1637. https://doi.org/10.3390/foods11111637
Chicago/Turabian StyleJiang, Xingyi, Han Mu, Yun-Hwa Peggy Hsieh, and Qinchun Rao. 2022. "Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5)" Foods 11, no. 11: 1637. https://doi.org/10.3390/foods11111637
APA StyleJiang, X., Mu, H., Hsieh, Y.-H. P., & Rao, Q. (2022). Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5). Foods, 11(11), 1637. https://doi.org/10.3390/foods11111637







