Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Equipment
2.3. Preparation and Purification of DOP
2.4. Determination of Molecular Weight of DOP
2.5. Monosaccharide Composition of DOP
2.6. Total Polysaccharide Content of DOP
2.7. In Vitro Fermentation
2.8. 16S rDNA Sequencing
2.9. Determination of Short Chain Fatty Acid Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Purification of DOP
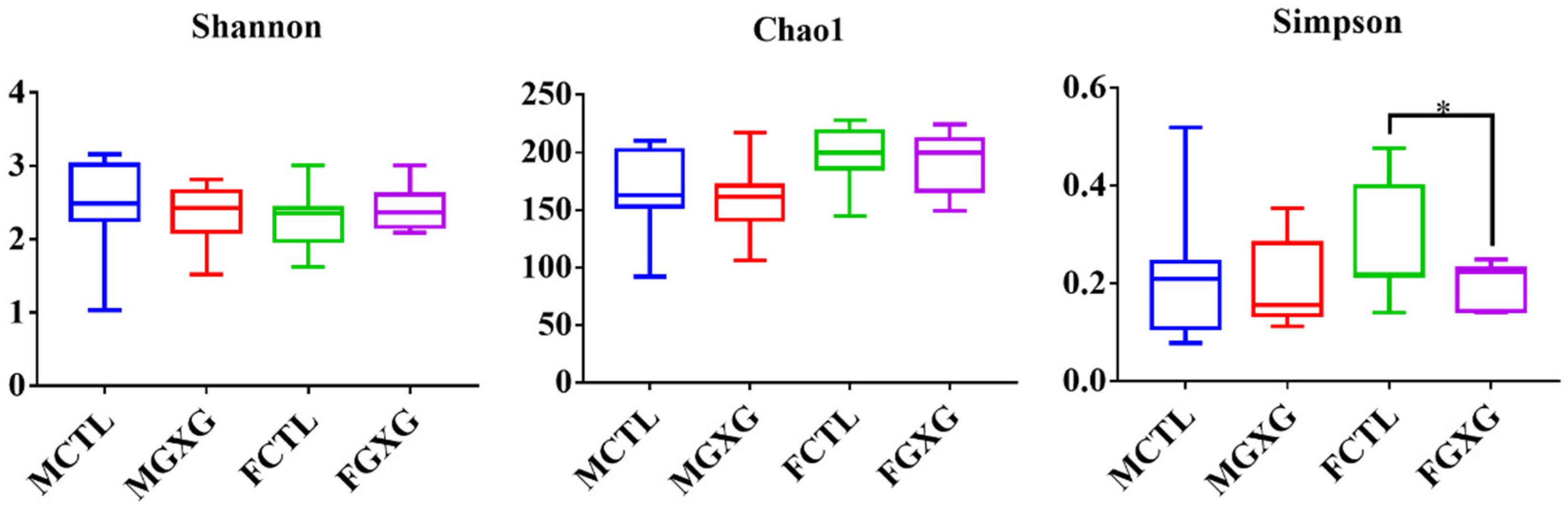
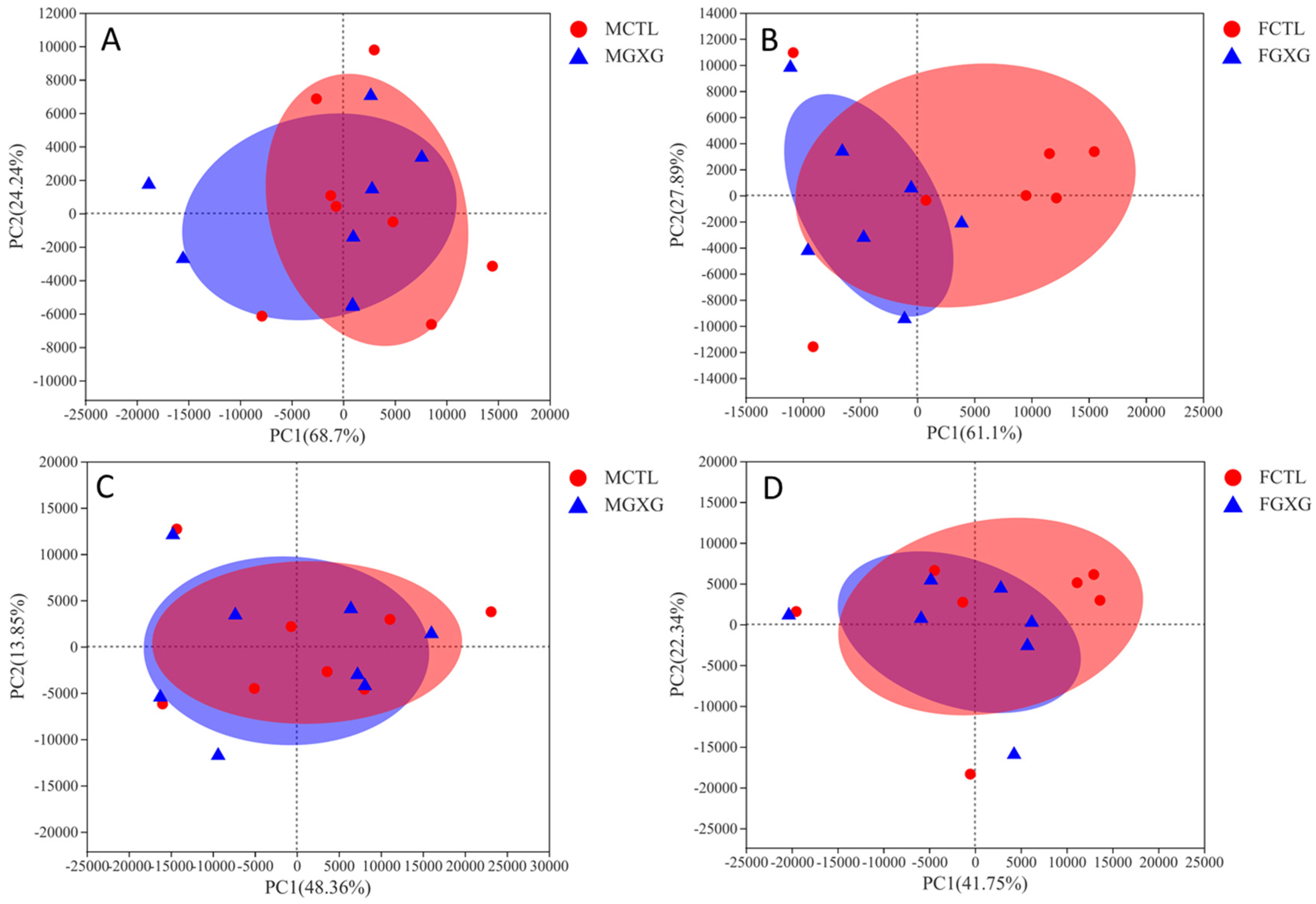
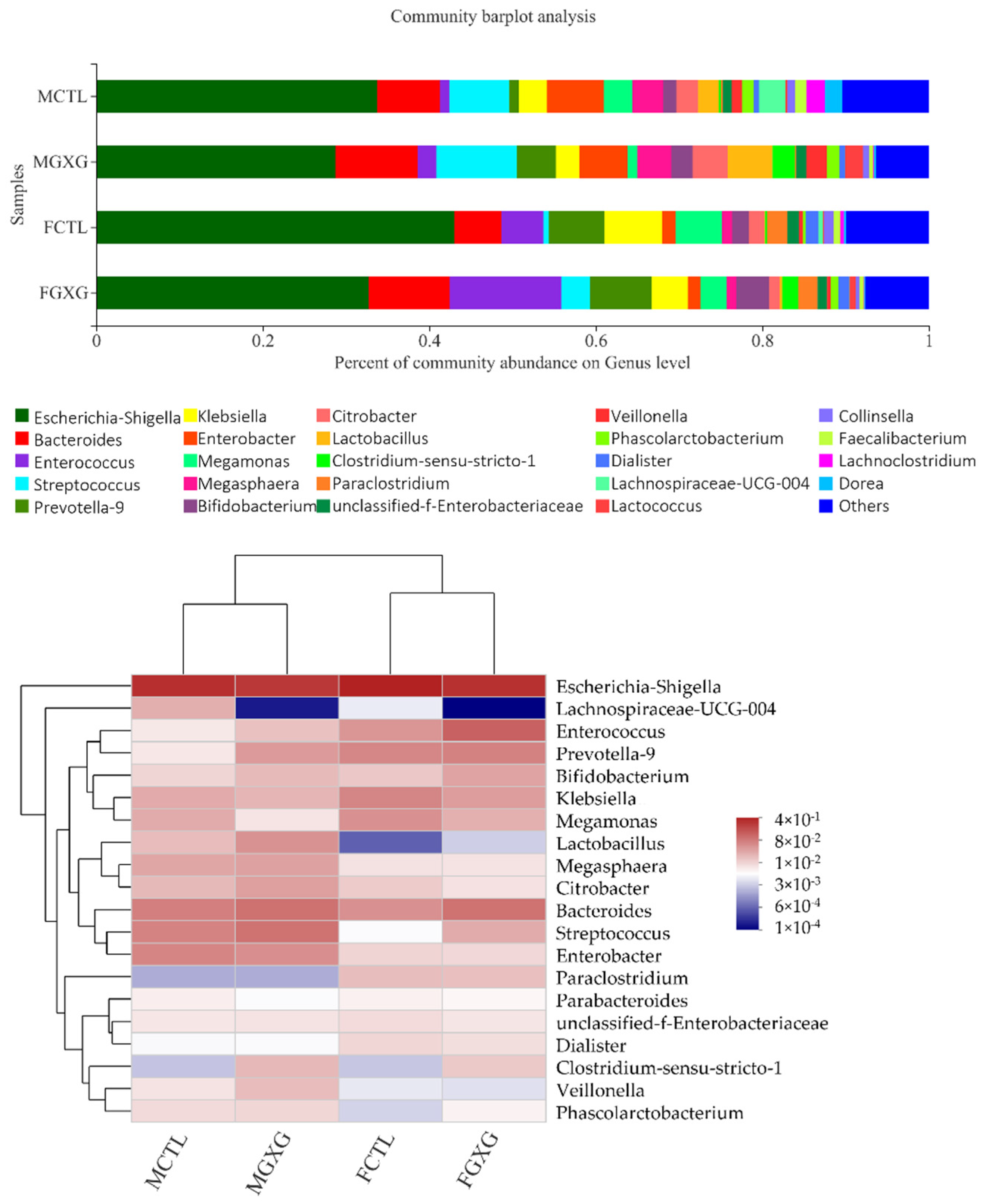
3.2. The Regulatory Effect of DOP on the Microbiota
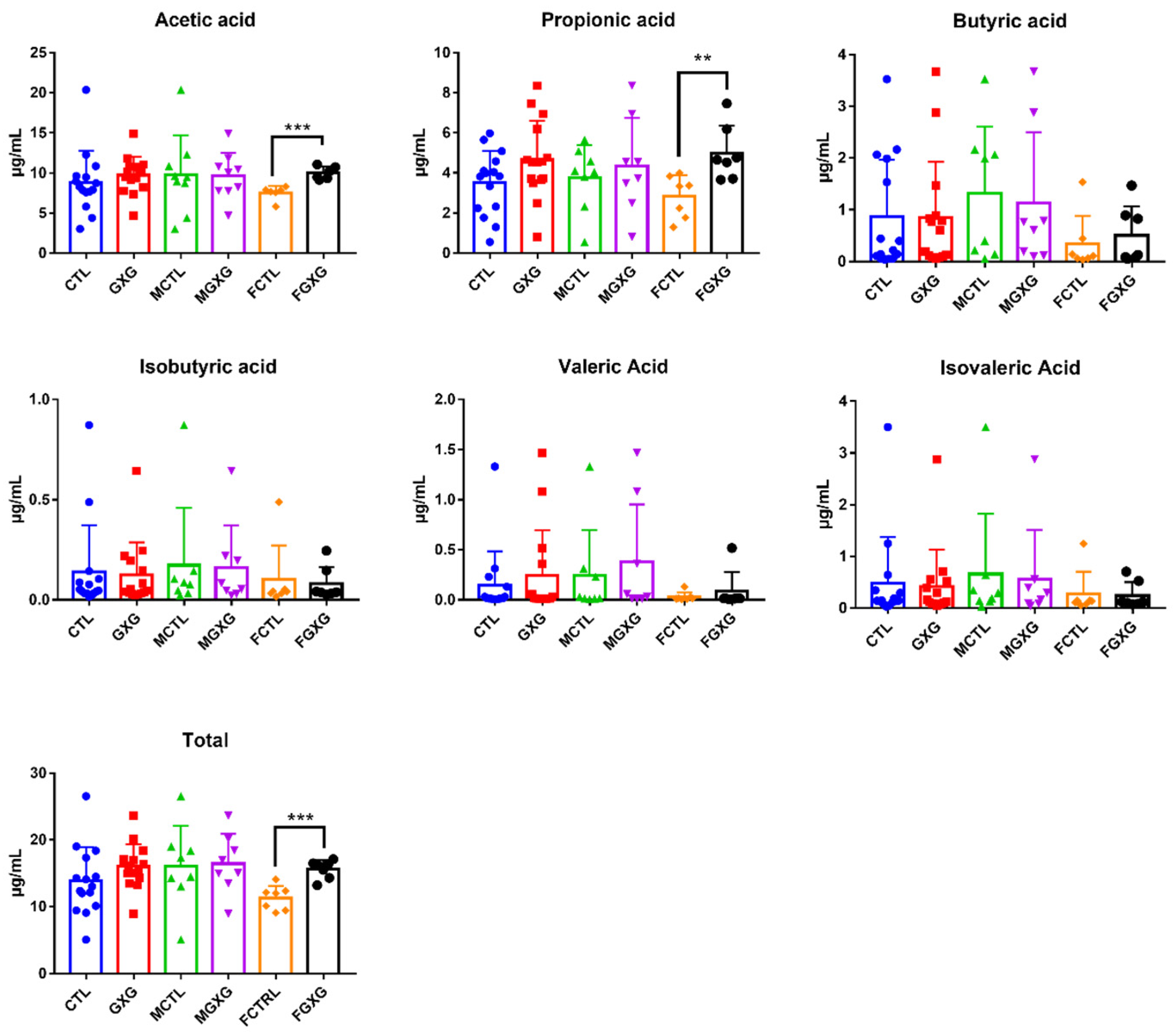
3.3. Effect of DOP on the Production of Short-Chain Fatty Acids
3.4. Correlation between SCFA, DOP, and Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Yue, H.; Wang, Y.; Guo, C.; Du, Z.; Jin, C.; Ding, K. Intestinal microbes derived butyrate is related to the immunomodulatory activities of Dendrobium officinale polysaccharide. Int. J. Biol. Macromol. 2020, 149, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Feng, S.; Farag, M.A.; Sun, P.; Shao, P. Synergistic cytotoxicity of erianin, a bisbenzyl in the dietetic Chinese herb Dendrobium against breast cancer cells. Food Chem. Toxicol. 2021, 149, 111960. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, D.; Cao, M.; Yu, Z.; Wu, M.; Liu, Y.; Zhou, J.; Yan, S.; Chen, J.; Huang, M.; et al. Effects of choline supplementation on liver biology, gut microbiota, and inflammation in Helicobacter pylori-infected mice. Life Sci. 2020, 259, 118200. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, H.; Chen, J.; He, L.; Du, X.; Zhou, L.; Xiong, Q.; Lai, X.; Yang, Y.; Huang, S.; et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol. Res. 2019, 148, 104417. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, T.; Sheng, Y.; Zheng, T.; Fu, L.; Zhang, Y. Dendrobium officinale Kimura et Migo: A review on its ethnopharmacology, phytochemistry, pharmacology, and industrialization. Evid. Based Complement. Altern. Med. 2017, 2017, 7436259. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Qiu, J.; Ding, L.; Huang, M.; Huang, S.; Yang, G.; Yin, J. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef]
- Yue, H.; Zeng, H.; Ding, K. A review of isolation methods, structure features and bioactivities of polysaccharides from Dendrobium species. Chin. J. Nat. Med. 2020, 18, 1–27. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Bergen, M.V.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [Green Version]
- Fransen, F.; Van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; Van Der Gaast-De Jongh, C.; Savelkoul, H.F.; De Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, H.; Li, X.; Zhang, Q.; Wu, X.; Wong, T.; Zheng, H.; Fung, H.; Yang, B.; Ma, D.; et al. Destiny of dendrobium officinale polysaccharide after oral administration: Indigestible and nonabsorbing, ends in modulating gut microbiota. J. Agric. Food Chem. 2019, 67, 5968–5977. [Google Scholar] [CrossRef] [PubMed]
- Bernet-Camard, M.F.; Coconnier, M.H.; Hudault, S.; Servin, A.L. Differentiation-associated antimicrobial functions in human colon adenocarcinoma cell lines. Exp. Cell Res. 1996, 226, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Cai, G.L. The Structure of Exopolysaccharide from Bacillus Amyloliquefaciens and Its Probiotic Mechanism. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2020. [Google Scholar]
- Lo, T.C.-T.; Chang, C.A.; Chiu, K.-H.; Tsay, P.-K.; Jen, J.-F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Liu, Y.; Wu, D.; Cai, P.; Pan, Y. Structural characterization and immunomodulatory activity of a water soluble polysaccharide isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Wu, J.J.; Li, X.F.; Lu, J.M.; Wu, W.; Sun, Y.Q.; Zhu, B.; Qin, L.P. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zeng, Y.; Wang, H.; Lou, W. Extraction, purification and antioxidant activity of novel polysaccharides from Dendrobium officinale by deep eutectic solvents. Nat. Prod. Res. 2019, 33, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-P.; Cheng, J.; Liu, Q.; Xu, G.-H.; Li, C.-F.; Yi, L.-T. Dendrobium officinale polysaccharides alleviate depression-like symptoms via regulating gut microbiota-neuroinflammation in perimenopausal mice. J. Funct. Foods 2022, 88, 104912. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, D.; Aloisio, I.; Mazzola, G.; Biavati, B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants. Appl. Microbiol. Biotechnol. 2014, 98, 563–577. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Zhou, C.H.; Meng, Y.T.; Xu, J.J.; Fang, X.; Zhao, J.L.; Zhou, W.; Zhao, J.; Han, J.C.; Zhang, L.; Wang, K.X.; et al. Altered diversity and composition of gut microbiota in Chinese patients with chronic pancreatitis. Pancreatology 2020, 20, 16–24. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sanchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi (R)). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46, S12–S17. [Google Scholar] [CrossRef]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333. [Google Scholar]
- Fu, Y.; Zhang, J.; Chen, K.; Xiao, C.; Fan, L.; Zhang, B.; Ren, J.; Fang, B. An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. J. Funct. Foods 2019, 53, 44–53. [Google Scholar] [CrossRef]
- Hernandez, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, N.; Pluznick, J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol. Cell Physiol. 2014, 307, C979–C985. [Google Scholar] [CrossRef] [Green Version]


| Peak | Name | Retention Time (min) | Peak Area (nC×min) | mor% |
|---|---|---|---|---|
| 1 | Rhamnose | 7.35 | 0.30 | 0.59 |
| 2 | Galactose | 9.20 | 0.69 | 0.85 |
| 3 | Glucose | 10.54 | 23.33 | 36.92 |
| 4 | Mannose | 12.80 | 41.54 | 61.10 |
| 5 | Galacturonic acid | 21.61 | 0.19 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Liu, W.; Wang, M.; Zhou, W.; Xing, J.; Xu, J.; Pi, X.; Wang, X.; Lu, S.; Yang, Y. Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men. Foods 2022, 11, 1641. https://doi.org/10.3390/foods11111641
Tao W, Liu W, Wang M, Zhou W, Xing J, Xu J, Pi X, Wang X, Lu S, Yang Y. Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men. Foods. 2022; 11(11):1641. https://doi.org/10.3390/foods11111641
Chicago/Turabian StyleTao, Wenyang, Wei Liu, Mingzhe Wang, Wanyi Zhou, Jianrong Xing, Jing Xu, Xionge Pi, Xiaotong Wang, Shengmin Lu, and Ying Yang. 2022. "Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men" Foods 11, no. 11: 1641. https://doi.org/10.3390/foods11111641
APA StyleTao, W., Liu, W., Wang, M., Zhou, W., Xing, J., Xu, J., Pi, X., Wang, X., Lu, S., & Yang, Y. (2022). Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men. Foods, 11(11), 1641. https://doi.org/10.3390/foods11111641






