Preparation and Properties of Blended Composite Film Manufactured Using Walnut-Peptide–Chitosan–Sodium Alginate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
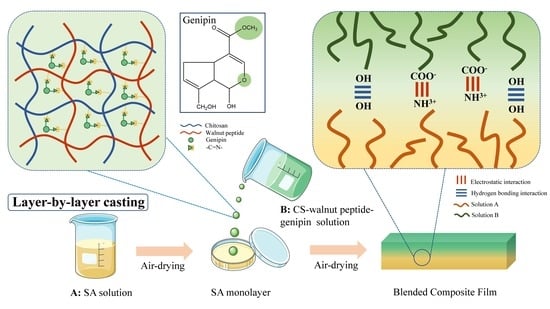
2.2. Preparation of Film Solutions and Film Formation
2.2.1. Sodium Alginate Monolayer Film Production
2.2.2. Chitosan–Walnut-Peptide Covalent Complex Production
2.2.3. Walnut-Peptide–Chitosan–Sodium Alginate Composite Edible Film Production
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. Mechanical Test
2.5. Chromatic Properties
2.6. Transmittance
2.7. Water Vapor Permeability (WVP)
2.8. Oil Absorption
2.9. Antioxidant Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Mechanical Properties
3.3. Chromatic Properties
3.4. Transmittance Properties
3.5. Water Vapor Transmission Rate
3.6. Oil Absorbency Capacity
3.7. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Z.-H.; Mou, J.-H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.-y.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, K.; Sangwan, K.S.; Goyal, D. Environment and economic impacts assessment of PET waste recycling with conventional and renewable sources of energy. Procedia CIRP 2019, 80, 422–427. [Google Scholar] [CrossRef]
- Fenton, R.L.; Duplan, A.; Hall, B.L. Modifying an Enzyme to Improve PET Plastic Recycling. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Rusdi, S.; Destian, R.A.; Rahman, F.; Chafidz, A. Preparation and Characterization of Bio-Degradable Plastic from Banana Kepok Peel Waste. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2020; Volume 981, pp. 132–137. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Milani, J.M.; Nemati, A. Lipid-Based Edible Films and Coatings: A Review of Recent Advances and Applications. J. Packag. Technol. Res. 2022, 6, 11–22. [Google Scholar] [CrossRef]
- Brzoska, N.; Mueller, M.; Nasui, L.; Schmid, M. Effects of film constituents on packaging-relevant properties of sodium caseinate-based emulsion films. Prog. Org. Coat. 2018, 114, 250–258. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of edible coatings on meats, poultry and seafoods: A review. LWT-Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.S.; Punotai, K.; Olson, D.; Williams, T.; Wood, D.; Rodov, V.; Poverenov, E.; McHugh, T. Layer-by-layer alginate and fungal chitosan based edible coatings applied to fruit bars. J. Food Sci. 2018, 83, 1880–1887. [Google Scholar] [CrossRef]
- Rahmani, B.; Hosseini, H.; Khani, M.; Farhoodi, M.; Honarvar, Z.; Feizollahi, E.; Shokri, B.; Shojaee-Aliabadi, S. Development and characterisation of chitosan or alginate-coated low density polyethylene films containing Satureja hortensis extract. Int. J. Biol. Macromol. 2017, 105, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; García, J.R.; Reis, R.L.; García, A.J.; Mano, J.F. Tuning cell adhesive properties via layer-by-layer assembly of chitosan and alginate. Acta Biomater. 2017, 51, 279–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, B.; Wang, H.; Zhang, Y.; Rao, C.; Wang, H.; Gao, X.; Li, W.; Niu, B. Effect of sodium alginate/phosphate-stabilized amorphous calcium carbonate nanoparticles on chitosan membranes. Food Biosci. 2022, 46, 101570. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef] [Green Version]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Jo, M.J.; Jeong, S.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Na, Y.J.; Park, S.H.; Jeong, Y.A.; Kim, B.G. Genipin induces mitochondrial dysfunction and apoptosis via downregulation of Stat3/mcl-1 pathway in gastric cancer. BMC Cancer 2019, 19, 739. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; Silva, J.A.L.d.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of genipin crosslinking on the properties of chitosan-based films. Polymers 2020, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Iahnke, A.O.e.S.; Stoll, L.; Bellé, A.S.; Hertz, P.F.; Rios, A.d.O.; Rahier, H.; Flôres, S.H. Gelatin capsule residue-based films crosslinked with the natural agent genipin. Packag. Technol. Sci. 2020, 33, 15–26. [Google Scholar] [CrossRef]
- Jahanbani, R.; Ghaffari, S.M.; Salami, M.; Vahdati, K.; Sepehri, H.; Sarvestani, N.N.; Sheibani, N.; Moosavi-Movahedi, A.A. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum. Nutr. 2016, 71, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Hardman, W.E.; Primerano, D.A.; Legenza, M.T.; Morgan, J.; Fan, J.; Denvir, J. mRNA expression data in breast cancers before and after consumption of walnut by women. Data Brief 2019, 25, 104050. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Jiang, Y.; Zhong, Y.; Zhao, Y.; Deng, Y.; Yue, J.; Wang, D.; Jiao, S.; Gao, H.; Chen, H. Development and characterization of nano-bilayer films composed of polyvinyl alcohol, chitosan and alginate. Food Control 2018, 86, 191–199. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.; Duan, X. Improving physicochemical properties of edible wheat gluten protein films with proteins, polysaccharides and organic acid. LWT 2022, 154, 112868. [Google Scholar] [CrossRef]
- Zareie, Z.; Yazdi, F.T.; Mortazavi, S.A. Development and characterization of antioxidant and antimicrobial edible films based on chitosan and gamma-aminobutyric acid-rich fermented soy protein. Carbohydr. Polym. 2020, 244, 116491. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.-C.; Liang, H.-C.; Sung, H.-W. Reconstruction of the right ventricular outflow tract with a bovine jugular vein graft fixed with a naturally occurring crosslinking agent (genipin) in a canine model. J. Thorac. Cardiovasc. Surg. 2001, 122, 1208–1218. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197. [Google Scholar] [CrossRef] [PubMed]
- Sheela, T.; Bhajantri, R.; Nambissan, P.; Ravindrachary, V.; Lobo, B.; Naik, J.; Rathod, S.G. Ionic conductivity and free volume related microstructural properties of LiClO4/PVA/NaAlg polymer composites: Positron annihilation spectroscopic studies. J. Non-Cryst. Solids 2016, 454, 19–30. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Ferreira, M. Chitosan as a smart coating for corrosion protection of aluminum alloy 2024: A review. Prog. Org. Coat. 2015, 89, 348–356. [Google Scholar] [CrossRef]
- Hisham, S.F.; Kasim, S.H.; Abu Bakar, S.; Mohd Sabri, S.N.; Mastor, A.; Abdul Manaf, A.Y.; Abu, N.; Noorsal, K.; Abdul Rashid, A.H. Cross-linked effects by genipin on physicochemical properties of chitosan film. In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2016; Volume 1133, pp. 108–112. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochim. Acta 2003, 48, 993–999. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, J.; Chen, S.; He, J.; Huang, Y. Characterization of calcium alginate/deacetylated konjac glucomannan blend films prepared by Ca2+ crosslinking and deacetylation. Food Hydrocoll. 2018, 82, 363–369. [Google Scholar] [CrossRef]
- Gholizadeh, B.S.; Buazar, F.; Hosseini, S.M.; Mousavi, S.M. Enhanced antibacterial activity, mechanical and physical properties of alginate/hydroxyapatite bionanocomposite film. Int. J. Biol. Macromol. 2018, 116, 786–792. [Google Scholar] [CrossRef]
- Aldana, A.A.; González, A.; Strumia, M.C.; Martinelli, M. Preparation and characterization of chitosan/genipin/poly (N-vinyl-2-pyrrolidone) films for controlled release drugs. Mater. Chem. Phys. 2012, 134, 317–324. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.; Rodrigues, R.C.; Costa, T.M.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; Zhang, Y.; Li, Y.; McHugh, T.H.; Zhong, F. Study of combined effects of glycerol and transglutaminase on properties of gelatin films. Food Hydrocoll. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; Mudai, A.; Roy, B.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. Biodegradable hybrid nanocomposite of chitosan/gelatin and green synthesized zinc oxide nanoparticles for food packaging. Foods 2020, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Peltzer, M.A.; del Carmen Garrigós, M.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Scartazzini, L.; Tosati, J.; Cortez, D.; Rossi, M.; Flôres, S.; Hubinger, M.; Di Luccio, M.; Monteiro, A. Gelatin edible coatings with mint essential oil (Mentha arvensis): Film characterization and antifungal properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.-F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Nandane, A.S.; Jain, R. Study of mechanical properties of soy protein based edible film as affected by its composition and process parameters by using RSM. J. Food Sci. Technol. 2015, 52, 3645–3650. [Google Scholar] [CrossRef] [Green Version]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef]
- Kumari, N.; Bangar, S.P.; Petrů, M.; Ilyas, R.A.; Singh, A.; Kumar, P. Development and Characterization of Fenugreek Protein-Based Edible Film. Foods 2021, 10, 1976. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Touyama, R.; Takeda, Y.; Inoue, K.; Kawamura, I.; Yatsuzuka, M.; Ikumoto, T.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the Blue Pigments Produced from Genipin and Methylamine. I. Structures of the Brownish-Red Pigments, Intermediates Leading to the Blue Pigments. Chem. Pharm. Bull. 1994, 42, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly (butylene adipate-co-terephthalate) (PBAT), poly (butylene succinate) (PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control 2022, 132, 108541. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, S.; Regenstein, J.M.; Wang, F. Preparation, characterization and stability of nanoliposomes loaded with peptides from defatted walnut (Juglans regia L.) meal. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Jariteh, M.; Ebrahimzadeh, H.; Niknam, V.; Mirmasoumi, M.; Vahdati, K. Developmental changes of protein, proline and some antioxidant enzymes activities in somatic and zygotic embryos of Persian walnut (Juglans regia L.). Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 101–115. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine-liposomal suspension and organic solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef]
- Panda, P.; JenMing, Y.; YenHsiang, C.; WeiWen, S. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef] [PubMed]









| Sample Number | SA (w/v, %) | Glycerol (Lower Layer) (v/v, %) | CS (w/v, %) | Walnut Peptide (w/v, %) | Glycerol (Upper Layer) (v/v, %) | Genipin (w/v, %) |
|---|---|---|---|---|---|---|
| S-C0 | 1.5 | 2.0 | 1.0 | 1.0 | 2.0 | 0 |
| S-CG1 | 1.5 | 2.0 | 2.0 | 0 | 2.0 | 0.01 |
| S-CG2 | 1.5 | 2.0 | 1.5 | 0.5 | 2.0 | 0.01 |
| S-CG3 | 1.5 | 2.0 | 1.0 | 1.0 | 2.0 | 0.01 |
| S-CG4 | 1.5 | 2.0 | 0.5 | 1.5 | 2.0 | 0.01 |
| S-CG5 | 1.5 | 2.0 | 0 | 2.0 | 2.0 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Sun, H.; Liu, W.; Chen, H. Preparation and Properties of Blended Composite Film Manufactured Using Walnut-Peptide–Chitosan–Sodium Alginate. Foods 2022, 11, 1758. https://doi.org/10.3390/foods11121758
Yan W, Sun H, Liu W, Chen H. Preparation and Properties of Blended Composite Film Manufactured Using Walnut-Peptide–Chitosan–Sodium Alginate. Foods. 2022; 11(12):1758. https://doi.org/10.3390/foods11121758
Chicago/Turabian StyleYan, Wenqi, Haochen Sun, Wenxin Liu, and Hao Chen. 2022. "Preparation and Properties of Blended Composite Film Manufactured Using Walnut-Peptide–Chitosan–Sodium Alginate" Foods 11, no. 12: 1758. https://doi.org/10.3390/foods11121758
APA StyleYan, W., Sun, H., Liu, W., & Chen, H. (2022). Preparation and Properties of Blended Composite Film Manufactured Using Walnut-Peptide–Chitosan–Sodium Alginate. Foods, 11(12), 1758. https://doi.org/10.3390/foods11121758