The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
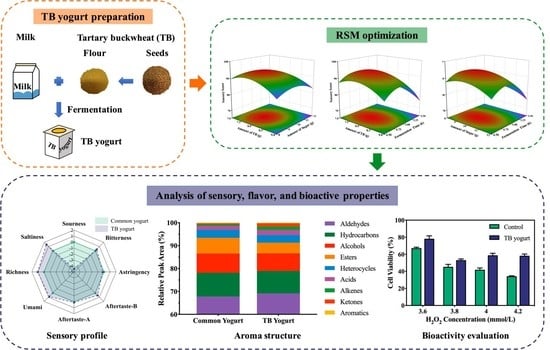
2.2. Yogurt Preparation
2.3. Physicochemical Determinations
2.4. Sensory Evaluation
2.5. Microbiological Determination
2.6. Experimental Design
2.7. Electronic Tongue Analysis
2.8. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.9. Bioactive Assay
2.10. Statistical Analysis
3. Results
3.1. Effects of TB on pH, Acidity, and Apparent Viscosity of Yogurt
3.2. The Viability of Acidogenic Bacteria
3.3. Effect on Yogurt Sensory Score
3.4. Fitness Verification of RSM Models and Optimization
3.5. Effect of TB Addition on Taste Attributes of Yogurt
3.6. Effect of TB on Volatile Flavor Profile of Yogurt
3.7. IPEC-J2 Cell Viability Affected by TB Yogurt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp. LWT Food Sci. Technol. 2022, 153, 112482. [Google Scholar] [CrossRef]
- Ilyasoğlu, H.; Yılmaz, F.; Burnaz, N.A.; Baltacı, C. Preliminary assessment of a yoghurt-like product manufactured from hazelnut slurry: Study using response surface methodology. LWT Food Sci. Technol. 2014, 62, 497–505. [Google Scholar] [CrossRef]
- Song, X.; Fu, H.; Chen, W. Effects of Flammulina velutipes polysaccharides on quality improvement of fermented milk and antihyperlipidemic on streptozotocin-induced mice. J. Funct. Foods 2021, 87, 104834. [Google Scholar] [CrossRef]
- Amadarshanie, D.B.T.; Gunathilaka, T.L.; Silva, R.M.; Navaratne, S.B.; Peiris, L.D.C. Functional and antiglycation properties of cow milk set yogurt enriched with Nyctanthes arbor-tristis L. flower extract. LWT Food Sci. Technol. 2022, 154, 112910. [Google Scholar] [CrossRef]
- Pereira, C.T.M.; Pereira, D.M.; de Medeiros, A.C.; Hiramatsu, E.Y.; Ventura, M.B.; Bolini, H.M.A. Skyr yogurt with mango pulp, fructooligosaccharide and natural sweeteners: Physical aspects and drivers of liking. LWT Food Sci. Technol. 2021, 150, 112054. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Shen, S.; Fang, Z. Cereal grain-based functional beverages: From cereal grain bioactive phytochemicals to beverage processing technologies, health benefits and product features. Crit. Rev. Food Sci. Nutr. 2020, 62, 2404–2431. [Google Scholar] [CrossRef]
- Hasani, S.; Khodadadi, I.; Heshmati, A. Viability of Lactobacillus acidophilus in rice bran-enriched stirred yoghurt and the physicochemical and sensory characteristics of product during refrigerated storage. Int. J. Food Sci. Technol. 2016, 51, 2485–2492. [Google Scholar] [CrossRef]
- Batista, A.L.D.; Silva, R.; Cappato, L.P.; Ferreira, M.V.S.; Nascimento, K.O.; Schmiele, M.; Esmerino, E.A.; Balthazar, C.F.; Silva, H.L.A.; Moraes, J.; et al. Developing a synbiotic fermented milk using probiotic bacteria and organic green banana flour. J. Funct. Foods 2017, 38, 242–250. [Google Scholar] [CrossRef]
- Do Espírito Santo, A.P.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.M.; Gioielli, L.A.; Perego, P.; Converti, A.; Oliveira, M.N. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef]
- Do Espírito Santo, A.P.; Perego, P.; Converti, A.; Oliveira, M.N. Influence of milk type and addition of passion fruit peel powder on fermentation kinetics, texture profile and bacterial viability in probiotic yoghurts. LWT Food Sci. Technol. 2012, 47, 393–399. [Google Scholar] [CrossRef]
- He, Q.; Guo, Z.; Cao, Y.; Yang, M.; Yao, S. Selective separation of main flavonoids by combinational use of ionic liquid-loaded microcapsules from crude extract of Tartary buckwheat. Food Chem. 2021, 362, 130255. [Google Scholar] [CrossRef]
- Yan, H.; Liu, C.; Zhao, J.; Ye, X.; Wu, Q.; Yao, T.; Peng, L.; Zou, L.; Zhao, G. Genome-wide analysis of the NF-Y gene family and their roles in relation to fruit development in Tartary buckwheat (Fagopyrum tataricum). Int. J. Biol. Macromol. 2021, 190, 487–498. [Google Scholar] [CrossRef]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: Identification, inhibitory activity, and action mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef]
- Staffolo, M.D.; Bertola, N.; Martino, M.; Bevilacqua, y.A. Influence of dietary fiber addition on sensory and rheological properties of yogurt. Int. Dairy J. 2004, 14, 263–268. [Google Scholar] [CrossRef]
- Ge, X.; Tang, N.; Huang, Y.; Chen, X.; Dong, M.; Rui, X.; Zhang, Q.; Li, W. Fermentative and physicochemical properties of fermented milk supplemented with sea buckthorn (Hippophae eleagnaceae L.). LWT Food Sci. Technol. 2022, 153, 112484. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT Food Sci. Technol. 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, R.; Ren, F.; Mao, X. Addition of buttermilk improves the flavor and volatile compound profiles of low-fat yogurt. LWT Food Sci. Technol. 2018, 98, 9–17. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Lunardello, K.A.; Yamashita, F.; De Toledo Benassi, M.; De Rensis, C.M.V.B. The physicochemical characteristics of nonfat set yoghurt containing some hydrocolloids. Int. J. Dairy Technol. 2012, 65, 260–267. [Google Scholar] [CrossRef]
- Hernández-Morales, C.; Hernández-Montes, A.; Villegas-de Gante, A. Effect of the partial substitution of sucrose by neotame on the sensory and consistency characteristics of plain yogurt. Rev. Mex. Ing. Química 2007, 6, 203–209. [Google Scholar] [CrossRef]
- Zomorodi, S.; Aberun, N.; Khosro shahi asl, A. Increase the survival of Lactobacillus acidophilus and improved quality properties of senbiotic yogurt using apple and wheat fibers. J. Food Sci. Technol. 2015, 12, 203–214. [Google Scholar]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT Food Sci. Technol. 2018, 101, 276–284. [Google Scholar] [CrossRef]
- Varnaite, L.; Kersiene, M.; Sipailiene, A.; Kazernaviciute, R.; Venskutonis, P.R.; Leskauskaite, D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods 2022, 11, 758. [Google Scholar] [CrossRef]
- Shah, N.P.; Ravula, R.R. Influence of water activity on fermentation, organic acids production and viability of yogurt and probiotic bacteria. Aust. J. Dairy Technol. 2000, 55, 127–131. [Google Scholar] [CrossRef]
- Michael, M.; Phebus, R.K.; Schmidt, K.A. Impact of a plant extract on the viability of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus in nonfat yogurt. Int. Dairy J. 2010, 20, 665–672. [Google Scholar] [CrossRef]
- Karimi, R.; Mortazavian, A.M.; Da Cruz, A.G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Technol. 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. The influence of Allium sativum or Cinnamomum verum on cow-and camel-milk yogurts: Proteolytic and angiotensin-I converting enzyme-inhibitory activities. Adv. Mater. Res. 2014, 832, 639–643. [Google Scholar] [CrossRef]
- Oliveira, A.A.A.; Andrade, A.C.; Bastos, S.C.; Pinheiro, A.C.M.; Condino, J.P.F.; Curzi Júnior, A. Use of strawberry and vanilla natural flavors for sugar reduction: A dynamic sensory study with yogurt. Food Res. Int. 2020, 139, 109972. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT Food Sci. Technol. 2021, 140, 110764. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Janeš, D.; Prosen, H.; Kreft, S. Identification and quantification of aroma compounds of tartary buckwheat (Fagopyrum tataricum Gaertn.) and some of its milling fractions. J. Food Sci. 2012, 77, C746–C751. [Google Scholar] [CrossRef]
- Bezerril, F.F.; Pimentel, T.C.; Marília da Silva Sant’Ana, A.; de Fátima Vanderlei de Souza, M.; Lucena de Medeiros, L.; Galvão, M.; Madruga, M.S.; de Cássia Ramos do Egypto Queiroga, R.; Magnani, M. Lacticaseibacillus casei 01 improves the sensory characteristics in goat milk yogurt added with xique-xique (Pilosocereus gounellei) jam through changes in volatiles concentration. LWT Food Sci. Technol. 2022, 154, 112598. [Google Scholar] [CrossRef]
- Costa, M.F.; Pimentel, T.C.; Guimaraes, J.T.; Balthazar, C.F.; Rocha, R.S.; Cavalcanti, R.N.; Esmerino, E.A.; Freitas, M.Q.; Raices, R.S.L.; Silva, M.C.; et al. Impact of prebiotics on the rheological characteristics and volatile compounds of Greek yogurt. LWT Food Sci. Technol. 2019, 105, 371–376. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Jin, R.L.; Zhang, H.P.; Zhou, T.T.; Sun, T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J. Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef] [Green Version]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yang, P.; Wang, H.; Song, H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. LWT Food Sci. Technol. 2022, 154, 112689. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef]
- Moineau-Jean, A.; Raymond, Y.; Sabik, H.; Graveline, N.; Champagne, C.P.; Roy, D.; LaPointe, G. Effect of manufacturing processes and storage on aroma compounds and sensory properties of yoghurt. Int. Dairy J. 2020, 105, 104662. [Google Scholar] [CrossRef]







| Runs | A: Amount of TB * (%) | B: Amount of Sugar (%) | C: Fermentation Time (h) | Sensory Score |
|---|---|---|---|---|
| 1 | 8 | 12 | 4.5 | 83 |
| 2 | 9 | 10 | 4.5 | 85 |
| 3 | 8 | 10 | 5.0 | 93 |
| 4 | 9 | 8 | 5.0 | 85 |
| 5 | 7 | 10 | 4.5 | 89 |
| 6 | 8 | 8 | 5.5 | 80 |
| 7 | 7 | 12 | 5.0 | 84 |
| 8 | 8 | 10 | 5.0 | 95 |
| 9 | 9 | 12 | 5.0 | 82 |
| 10 | 9 | 10 | 5.5 | 73 |
| 11 | 8 | 10 | 5.0 | 96 |
| 12 | 7 | 8 | 5.0 | 90 |
| 13 | 7 | 10 | 5.5 | 76 |
| 14 | 8 | 12 | 5.5 | 78 |
| 15 | 8 | 10 | 5.0 | 92 |
| 16 | 8 | 8 | 4.5 | 85 |
| 17 | 8 | 10 | 5.0 | 94 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 714.51 | 9 | 79.39 | 13.47 | 0.0012 | significant |
| A | 24.50 | 1 | 24.50 | 4.16 | 0.0808 | |
| B | 21.13 | 1 | 21.13 | 3.58 | 0.1002 | |
| C | 153.12 | 1 | 153.12 | 25.98 | 0.0014 | |
| AB | 2.25 | 1 | 2.25 | 0.38 | 0.5562 | |
| AC | 0.25 | 1 | 0.25 | 0.04 | 0.8427 | |
| BC | 0.00 | 1 | 0.00 | 0.00 | 1.0000 | |
| A2 | 95.00 | 1 | 95.00 | 16.12 | 0.0051 | |
| B2 | 67.37 | 1 | 67.37 | 11.43 | 0.0117 | |
| C2 | 304.21 | 1 | 304.21 | 51.62 | 0.0002 | |
| Residual | 41.25 | 7 | 5.89 | |||
| Lack-of-fit | 31.25 | 3 | 10.42 | 4.17 | 0.1008 | not significant |
| Pure error | 10.00 | 4 | 2.50 | |||
| Cor total | 755.76 | 16 | ||||
| R2 | 0.9454 | Std. Dev. | 2.43 | |||
| Adj R2 | 0.8752 | Mean | 85.88 | |||
| Pred R2 | 0.3177 | C.V. % | 2.83 | |||
| Adeq Precision | 10.2722 | PRESS | 515.63 |
| No. | Compound | Molecular Formula | Retention Time (min) | Relative Peak Areas (%) * | |
|---|---|---|---|---|---|
| Common Yogurt | TB Yogurt | ||||
| Aldehydes | |||||
| 1 | 3-Methylbutyraldehyde | C5H10O | 3.370 | 0.46 | 0.34 |
| 2 | Hexanal | C6H12O | 5.093 | 8.80 | 5.90 |
| 3 | Heptanal | C7H14O | 7.815 | 4.87 | 18.06 |
| 4 | (Z)-2-Heptenal | C7H12O | 9.548 | 2.25 | 2.67 |
| 5 | Octanal | C8H16O | 10.965 | 7.85 | 8.85 |
| 6 | (E)-2-Octenal | C8H14O | 12.640 | 2.54 | 2.50 |
| 7 | m-Tolualdehyde | C8H8O | 12.891 | 0.40 | 0.46 |
| 8 | Nonanal | C9H18O | 14.057 | 17.07 | 17.08 |
| 9 | Decanal | C10H20O | 17.031 | 5.37 | 5.22 |
| 10 | Undecanal | C11H22O | 19.860 | 3.41 | 3.18 |
| 11 | (E,E)-2,4-Decadienal | C10H16O | 20.128 | 1.39 | 1.19 |
| 12 | 2-Dodecenal | C12H22O | 21.374 | 6.45 | - |
| 13 | Dodecanal | C12H24O | 22.529 | 4.64 | 5.19 |
| 14 | Myristic aldehyde | C14H28O | 25.658 | 1.96 | 2.32 |
| 15 | Pentadecanal | C15H30O | 32.825 | - | 2.93 |
| 16 | 3,5-Di-tert-butyl-4-hydroxybenzaldehyde | C15H22O2 | 34.034 | 0.71 | 0.84 |
| 17 | Undecane | C11H24 | 13.929 | 0.87 | 0.96 |
| 18 | Dodecane | C12H26 | 16.876 | 1.04 | 1.10 |
| 19 | Tridecane | C13H28 | 19.657 | 1.30 | 1.52 |
| 20 | Cyclododecane | C12H24 | 22.096 | 0.35 | - |
| 21 | Tetradecane | C14H30 | 22.294 | 1.20 | 1.31 |
| 22 | Pentadecane | C15H32 | 25.273 | 1.24 | 1.51 |
| 23 | Hexadecane | C16H34 | 29.295 | 0.82 | 0.57 |
| 24 | 1,2-Epoxyoctadecane | C18H36O | 29.739 | 2.05 | 2.58 |
| 25 | Heptadecane | C17H36 | 32.413 | 0.98 | 1.37 |
| Alcohols | |||||
| 26 | (E)-2-Methylcyclopentanol | C6H12O | 5.221 | 0.54 | 0.29 |
| 27 | 2-Cyclohexenol | C6H10O | 18.609 | 7.38 | 7.39 |
| 28 | Nona-decanol | C19H40O | 32.221 | 0.51 | 0.80 |
| Esters | |||||
| 29 | Methyl hexa-decanoate | C17H34O2 | 37.495 | 1.58 | 1.12 |
| 30 | Methyl linoleate | C19H34O2 | 40.436 | 0.98 | 0.85 |
| 31 | Methyl oleate | C19H36O2 | 40.549 | 3.24 | 2.68 |
| 32 | Methyl stearate | C19H38O2 | 40.971 | 1.11 | 0.54 |
| Heterocycles | |||||
| 33 | 2-Pentylfuran | C9H14O | 10.596 | 3.51 | 3.71 |
| Acids | |||||
| 34 | 5-Hexenoic acid | C6H10O2 | 7.446 | 0.58 | - |
| 35 | Pentadecanoic acid | C15H30O2 | 13.297 | - | 0.61 |
| 36 | Palmitic acid | C16H32O2 | 38.297 | 1.21 | 1.86 |
| Alkenes | |||||
| 37 | 1-Dodecene | C12H24 | 16.630 | - | 0.35 |
| 38 | 1-Pentadecene | C15H30 | 25.000 | 0.62 | - |
| 39 | 1,19-Eicosadiene | C20H38 | 37.345 | - | 1.28 |
| Ketones | |||||
| 40 | 2-Nonanone | C9H18O | 13.715 | 0.44 | 1.27 |
| 41 | 2-Undecanone,6,10-dimethyl- | C13H26O | 16.667 | - | 0.32 |
| Aromatics | |||||
| 42 | Phenyl-pentane | C11H16 | 15.608 | 0.27 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Li, P.; Zhou, J.; He, J.; Cai, J. The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat. Foods 2022, 11, 1774. https://doi.org/10.3390/foods11121774
Ye Y, Li P, Zhou J, He J, Cai J. The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat. Foods. 2022; 11(12):1774. https://doi.org/10.3390/foods11121774
Chicago/Turabian StyleYe, Yuanyuan, Pei Li, Jiaojiao Zhou, Jiangling He, and Jie Cai. 2022. "The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat" Foods 11, no. 12: 1774. https://doi.org/10.3390/foods11121774
APA StyleYe, Y., Li, P., Zhou, J., He, J., & Cai, J. (2022). The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat. Foods, 11(12), 1774. https://doi.org/10.3390/foods11121774