In Silico and In Vitro Analyses of Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Enzymatic Protein Hydrolysates from Taiwan Mackerel (Scomber australasicus) Steaming Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis of Mackerel Steaming Juice (MSJ)
2.3. Determination of pH and Salinity Value
2.4. Preparation of MSJ Powder
2.5. Preparation of Protein Isolates
2.6. Determination of Protein Content, Yield and Protein Recovery
2.7. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
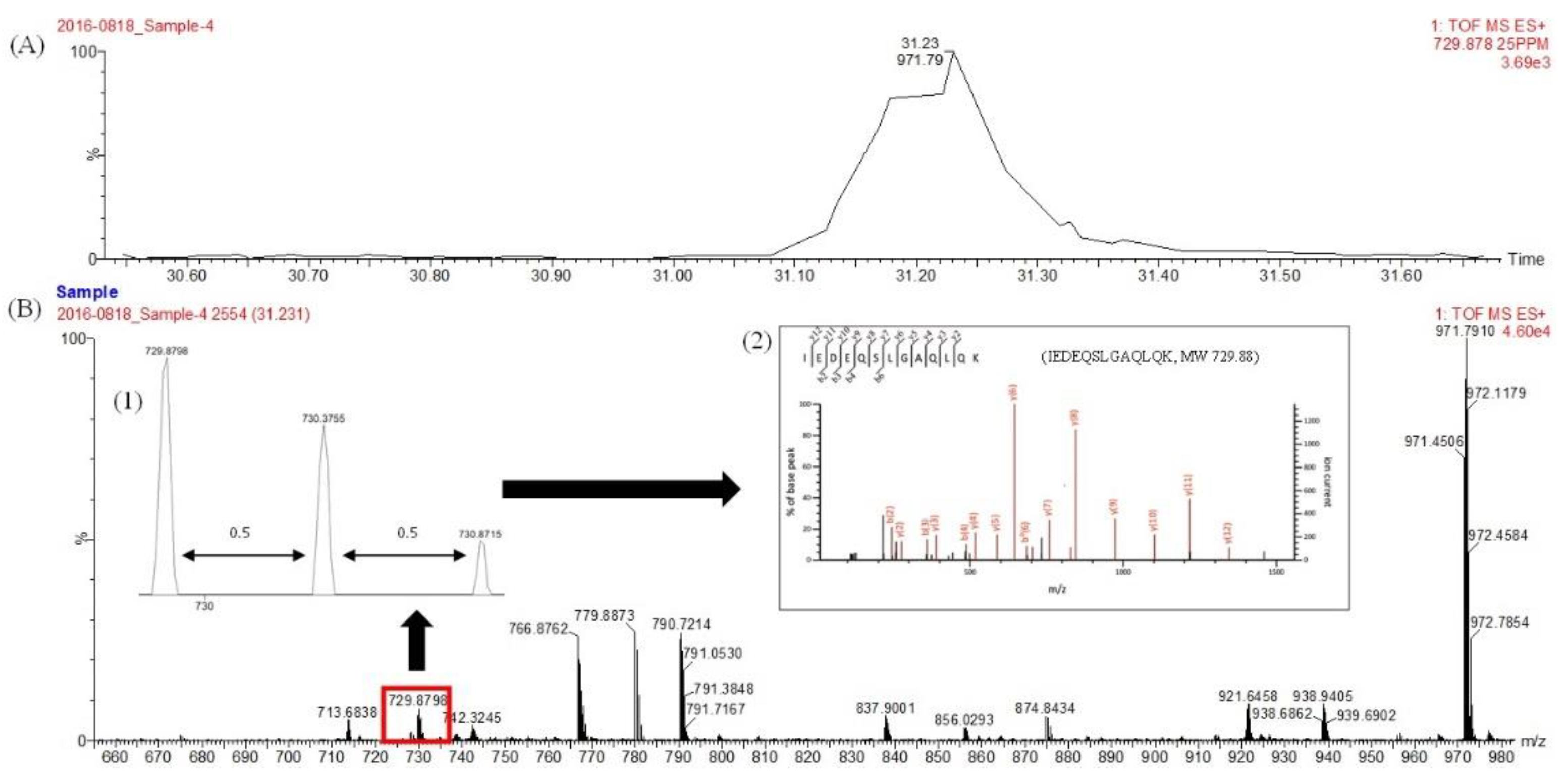
2.8. Proteomics Technique
2.9. BIOPEP-UWM Database Analysis of Bioactive Peptides and Enzyme Cleavages
2.10. Preparation of Enzymatic Hydrolysate
2.11. Degree of Hydrolysis (DH)
2.12. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.13. Determination of Fe2+ Chelating Activity
2.14. Reducing Power Assay
2.15. Angiotensin-I Converting Enzyme (ACE-I) Inhibitory Assay
2.16. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition, pH and Salinity Value of Mackerel Steaming Juice (MSJ)
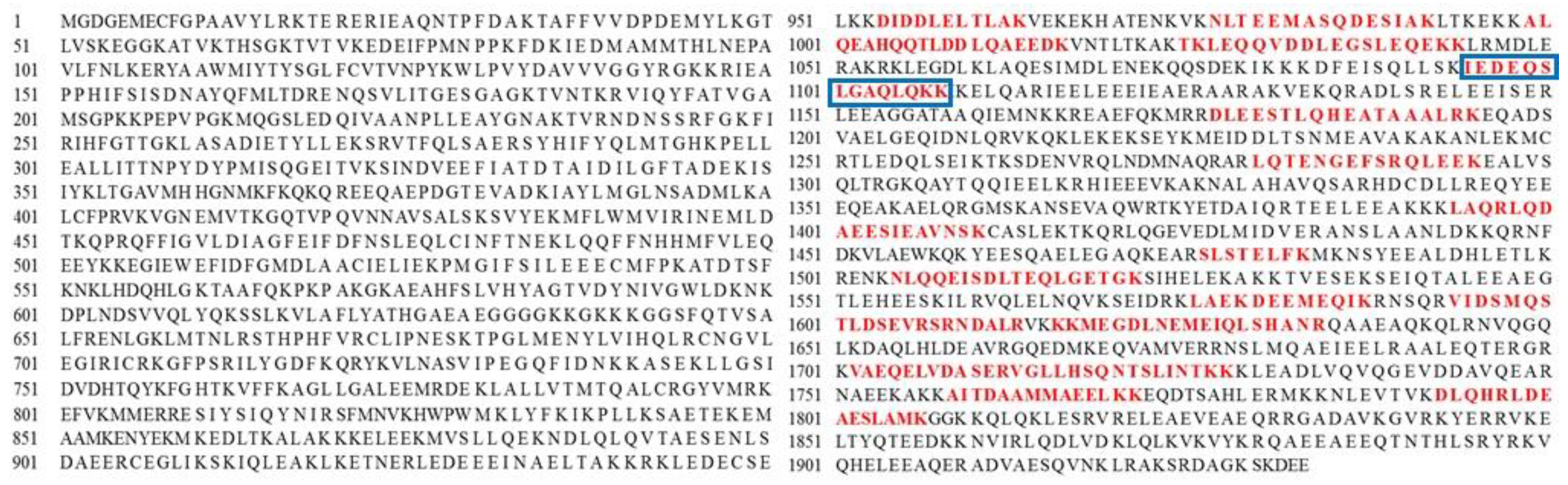
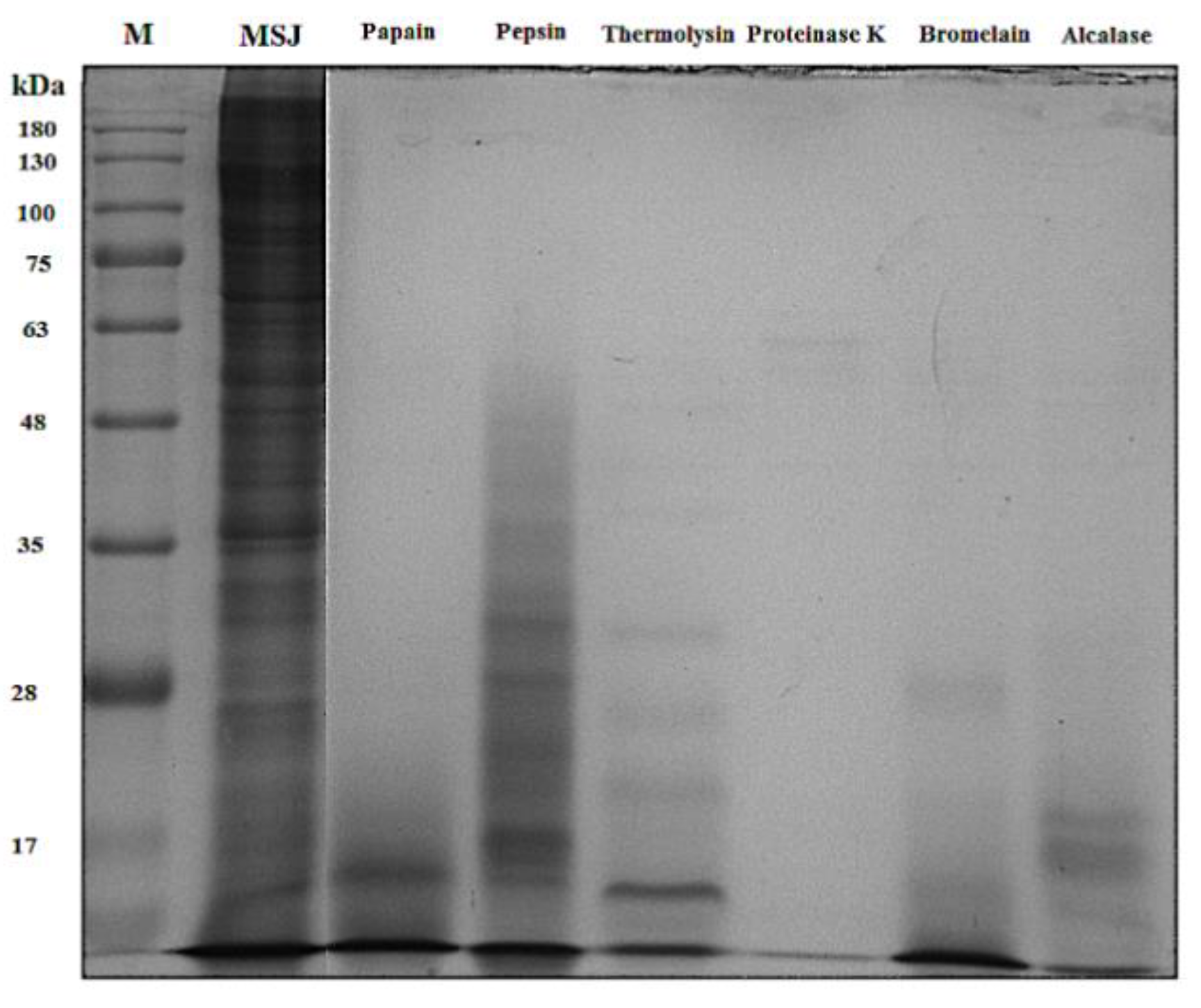
3.2. Identification of Protein in Mackerel Steaming Juice (MSJ)
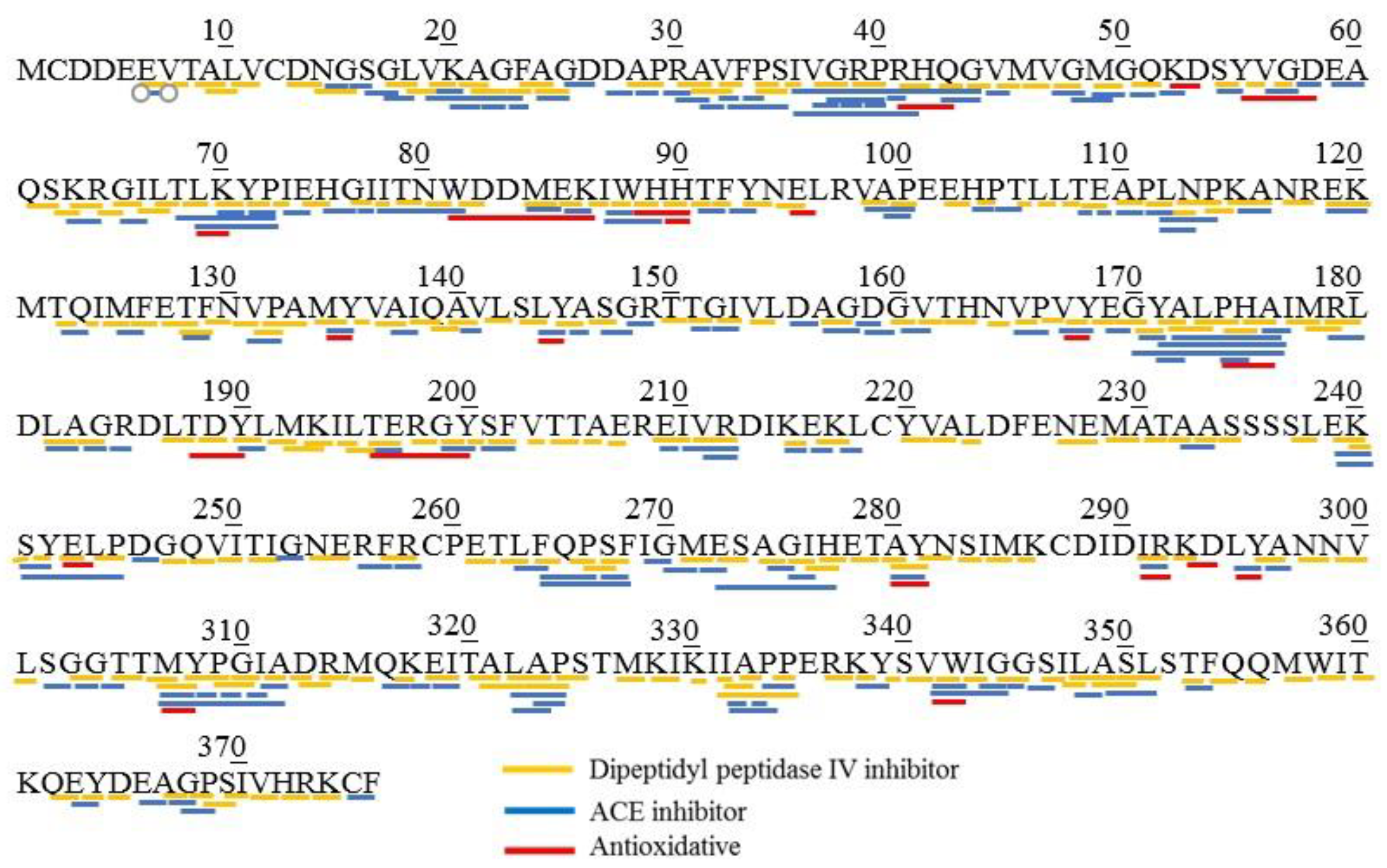
3.3. BIOPEP-UWM Databse Analysis of Mackerel Steaming Juice (MSJ)
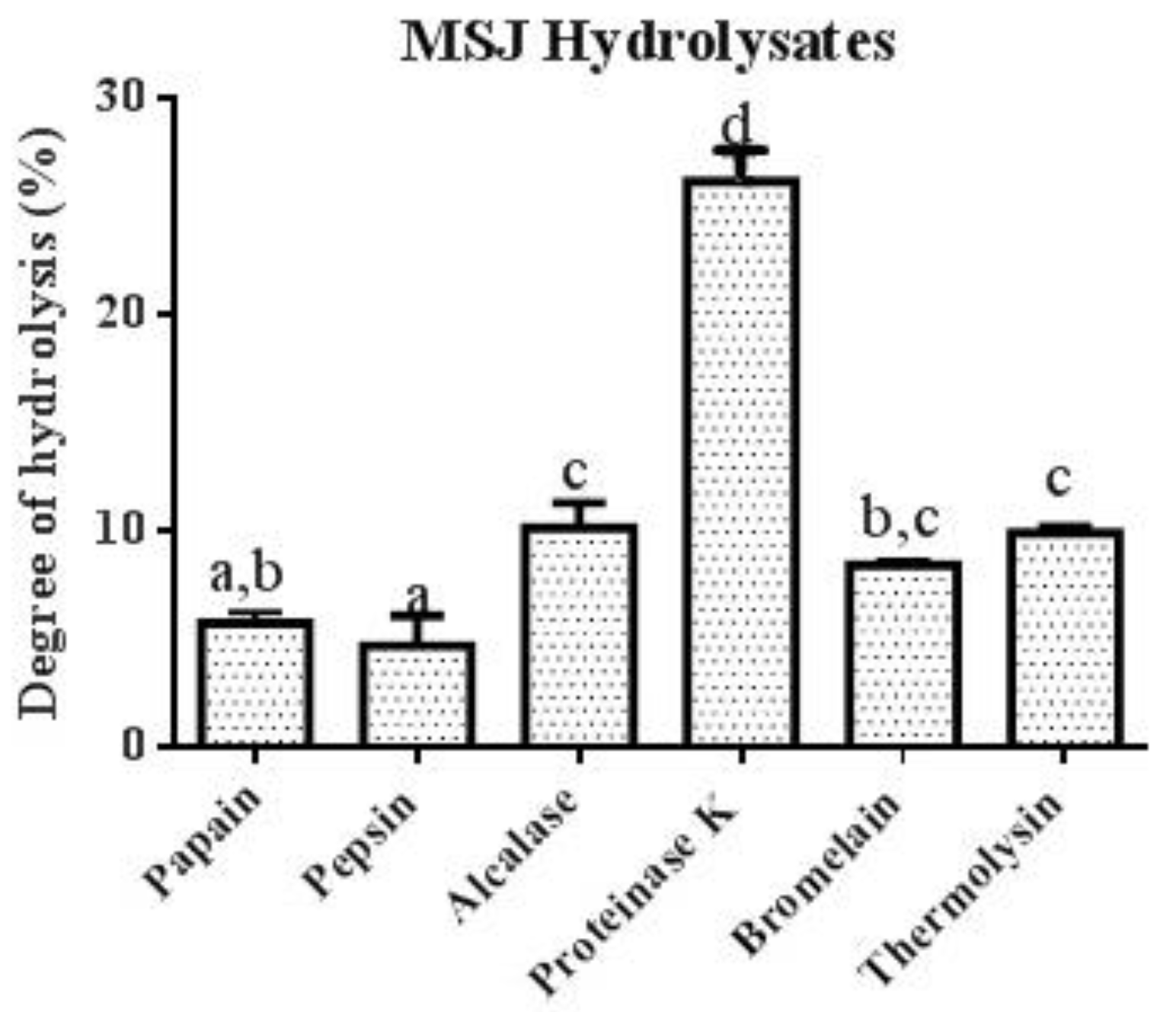
3.4. Peptide Profile of MSJ Protein Hydrolysates
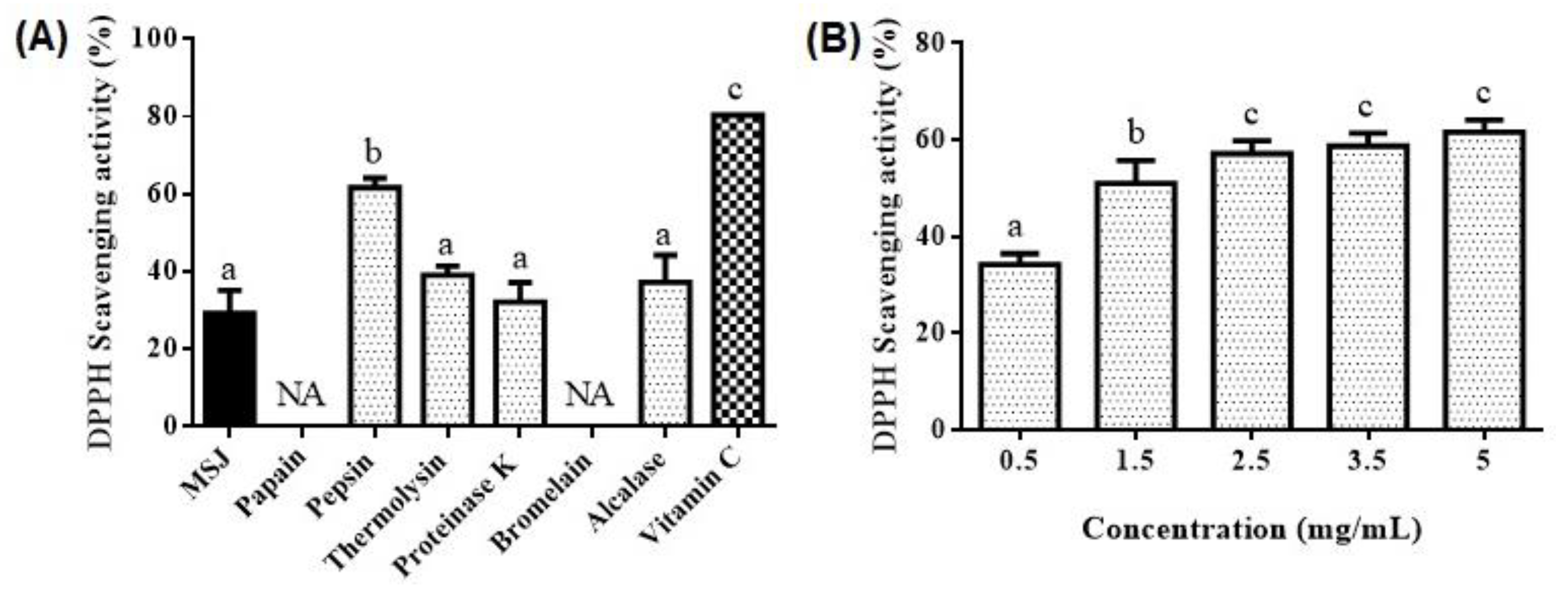
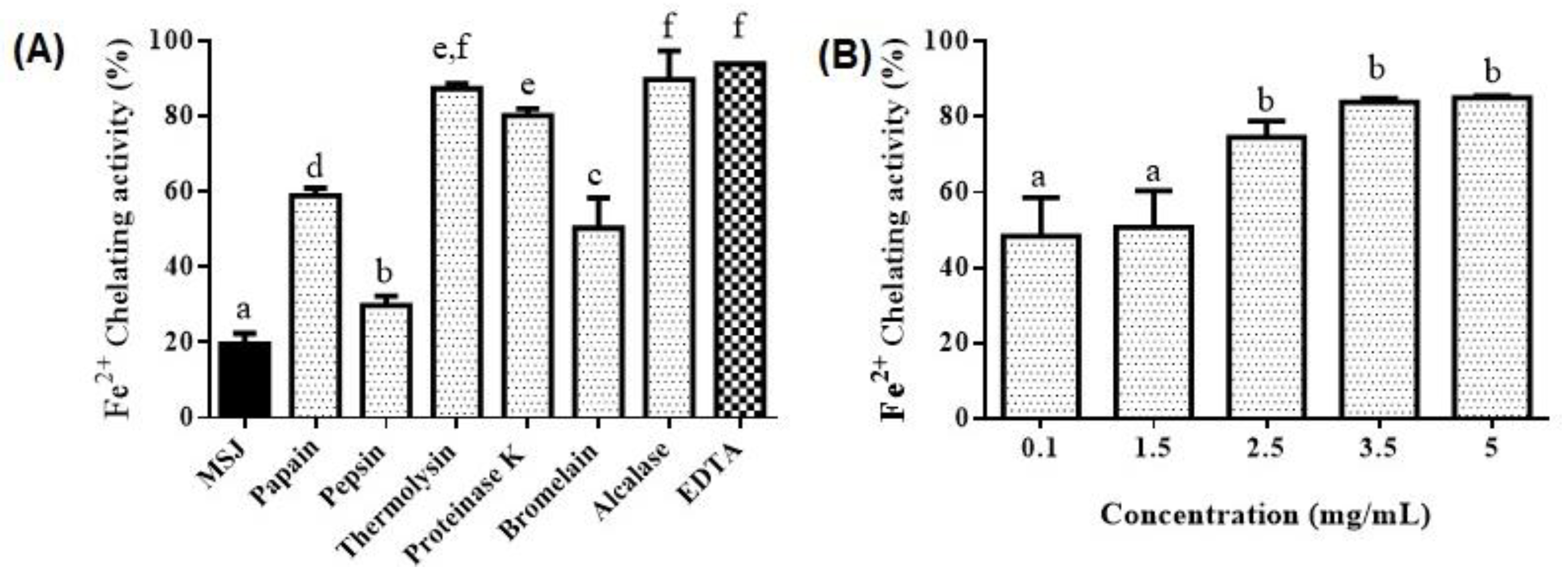
3.5. Antioxidant Properties
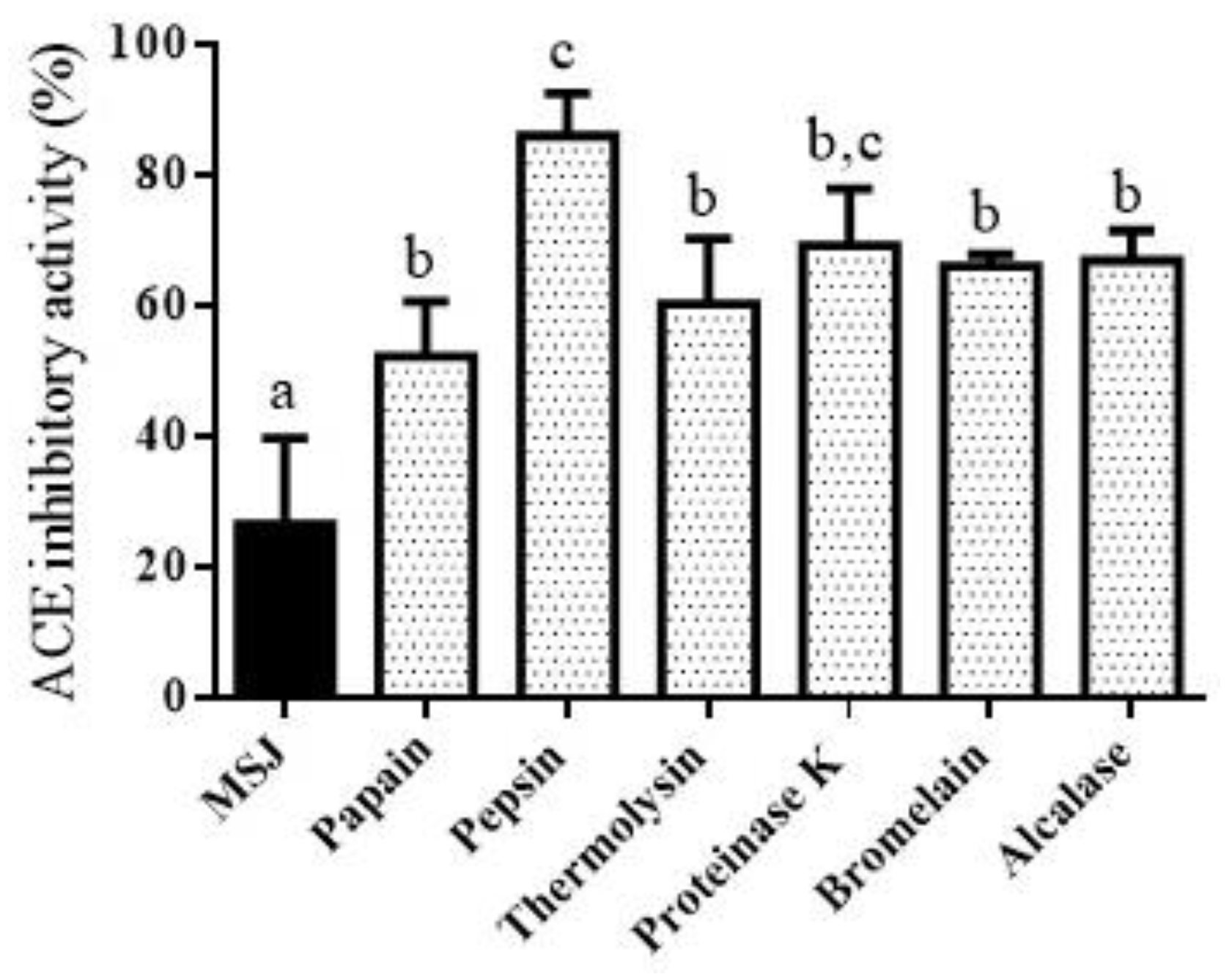
3.6. Angiotensin Converting Enzyme I (ACE-I) Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries Agency of the Republic of China (Taiwan). Fisheries Statistical Yearbook: Taiwan, Kinmen and Matsu Area; Fisheries Agency: Taipei, Taiwan, 2014; Volume 2022. [Google Scholar]
- Jao, C.-L.; Ko, W.-C. Utilization of cooking juice of young tuna processed into canned tuna as condiments: Effect of enzymatic hydrolysis and membrane treatment. Fish. Sci. 2002, 68, 1344–1351. [Google Scholar] [CrossRef]
- Tom, B.; Dendorfer, A.; Danser, A.J. Bradykinin, angiotensin-(1–7), and ACE inhibitors: How do they interact? Int. J. Biochem. Cell Biol. 2003, 35, 792–801. [Google Scholar] [CrossRef]
- Riordan, J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antonios, T.F.; MacGregor, G.A. Angiotensin converting enzyme inhibitors in hypertension: Potential problems. J. Hypertens. 1995, 13, S11–S16. [Google Scholar] [CrossRef]
- Itou, K.; Nagahashi, R.; Saitou, M.; Akahane, Y. Antihypertensive effect of narezushi, a fermented mackerel product, on spontaneously hypertensive rats. Fish. Sci. 2007, 73, 1344–1352. [Google Scholar]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Autolysis-assisted production of fish protein hydrolysates with antioxidant properties from Pacific hake (Merluccius productus). Food Chem. 2008, 107, 768–776. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Choi, J.-I.; Sung, N.-Y.; Lee, J.-W. Radiation hydrolysate of tuna cooking juice with enhanced antioxidant properties. Radiat. Phys. Chem. 2012, 81, 1088–1090. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Lu, G.-H.; Jao, C.-L. Antioxidative properties of peptides prepared from tuna cooking juice hydrolysates with orientase (Bacillus subtilis). Food Res. Int. 2009, 42, 647–652. [Google Scholar] [CrossRef]
- Huang, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 2012, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-I.; Kim, J.-H.; Lee, J.-W. Physiological properties of tuna cooking drip hydrolysate prepared with gamma irradiation. Process Biochem. 2011, 46, 1875–1878. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Cheng, M.-L.; Hwang, J.-S. Hydrolysates from tuna cooking juice as an anti-hypertensive agent. J. Food Drug Anal. 2007, 15, 169–173. [Google Scholar] [CrossRef]
- Hwang, J.-S. Impact of processing on stability of angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from tuna cooking juice. Food Res. Int. 2010, 43, 902–906. [Google Scholar] [CrossRef]
- Ko, W.-C.; Cheng, M.-L.; Hsu, K.-C.; Hwang, J.-S. Absorption-enhancing Treatments for Antihypertensive Activity of Oligopeptides from Tuna Cooking Juice: In Vivo Evaluation in Spontaneously Hypertensive Rats. J. Food Sci. 2006, 71, S13–S17. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing by-products using commercial enzymes. Food Bioprod. Process. 2015, 96, 145–153. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef]
- Kumar, N.S.; Nazeer, R.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2003. [Google Scholar]
- Pazos, M.; Méndez, L.; Gallardo, J.M.; Aubourg, S.P. Selective-targeted effect of high-pressure processing on proteins related to quality: A proteomics evidence in Atlantic mackerel (Scomber scombrus). Food Bioprocess Technol. 2014, 7, 2342–2353. [Google Scholar] [CrossRef]
- Huang, B.-B.; Lin, H.-C.; Chang, Y.-W. Analysis of proteins and potential bioactive peptides from tilapia (Oreochromis spp.) processing co-products using proteomic techniques coupled with BIOPEP database. J. Funct. Foods 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Ostasiewicz, P.; Zielinska, D.F.; Mann, M.; Wiśniewski, J.R. Proteome, phosphoproteome, and N-glycoproteome are quantitatively preserved in formalin-fixed paraffin-embedded tissue and analyzable by high-resolution mass spectrometry. J. Proteome Res. 2010, 9, 3688–3700. [Google Scholar] [CrossRef]
- BIOPEP-UWM Database. Available online: https://biochemia.uwm.edu.pl/en/biopep-uwm-2/ (accessed on 20 April 2022).
- Charoenphun, N.; Cheirsilp, B.; Sirinupong, N.; Youravong, W. Calcium-binding peptides derived from tilapia (Oreochromis niloticus) protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 57–63. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chemists’ Soc. 2011, 88, 381–389. [Google Scholar] [CrossRef]
- Pihlanto, A.; Virtanen, T.; Korhonen, H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010, 20, 3–10. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Lin, Y.-S.; Hou, W.-C.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods 2009, 1, 199–207. [Google Scholar] [CrossRef]
- Chuo, Y.Y. Studies on Utilization of Canned Mackerel Cooking Juice as Powder Seasoning Ingredient and Fish Sauce; National Taiwan Ocean University: Keelung, Taiwan, 2005. [Google Scholar]
- Martínez-Montaño, E.; Osuna-Ruíz, I.; Benítez-García, I.; Osuna, C.O.; Pacheco-Aguilar, R.; Navarro-Peraza, R.S.; Sánchez, M.E.L.; Hernández, C.; Spanopoulos-Hernández, M.; Salazar-Leyva, J.A. Biochemical and antioxidant properties of recovered solids with pH shift from fishery effluents (sardine stickwater and tuna cooking water). Waste Biomass Valoriz. 2021, 12, 1901–1913. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.; Feng, L. Sensitivity enhancement of pH indicator and its application in the evaluation of fish freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Davy, A.; Thomsen, K.K.; Juliano, M.A.; Alves, L.C.; Svendsen, I.; Simpson, D.J. Purification and characterization of barley dipeptidyl peptidase IV. Plant Physiol. 2000, 122, 425–432. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure− activity relationship study of di-and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Kasiwut, J.; Youravong, W.; Adulyatham, P.; Sirinupong, N. Angiotensin I-converting enzyme inhibitory and C a-binding activities of peptides prepared from tuna cooking juice and spleen proteases. Int. J. Food Sci. Technol. 2015, 50, 389–395. [Google Scholar] [CrossRef]
- Hung, C.-C.; Yang, Y.-H.; Kuo, P.-F.; Hsu, K.-C. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J. Funct. Foods 2014, 11, 563–570. [Google Scholar] [CrossRef]
- Jao, C.-L.; Ko, W.-C. 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolyzates from tuna cooking juice. Fish. Sci. 2002, 68, 430–435. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]


| Sample | Moisture (%) | Crude Protein (%) | Crude Fat (%) | Ash (%) | NaCl (%) | pH |
|---|---|---|---|---|---|---|
| Mackerel steaming juice | 93.76 ± 0.67 | 5.30 ± 0.07 | 0.19 ± 0.01 | 0.84 ± 0.02 | 0.38 ± 0.17 | 5.93 ± 0.08 |
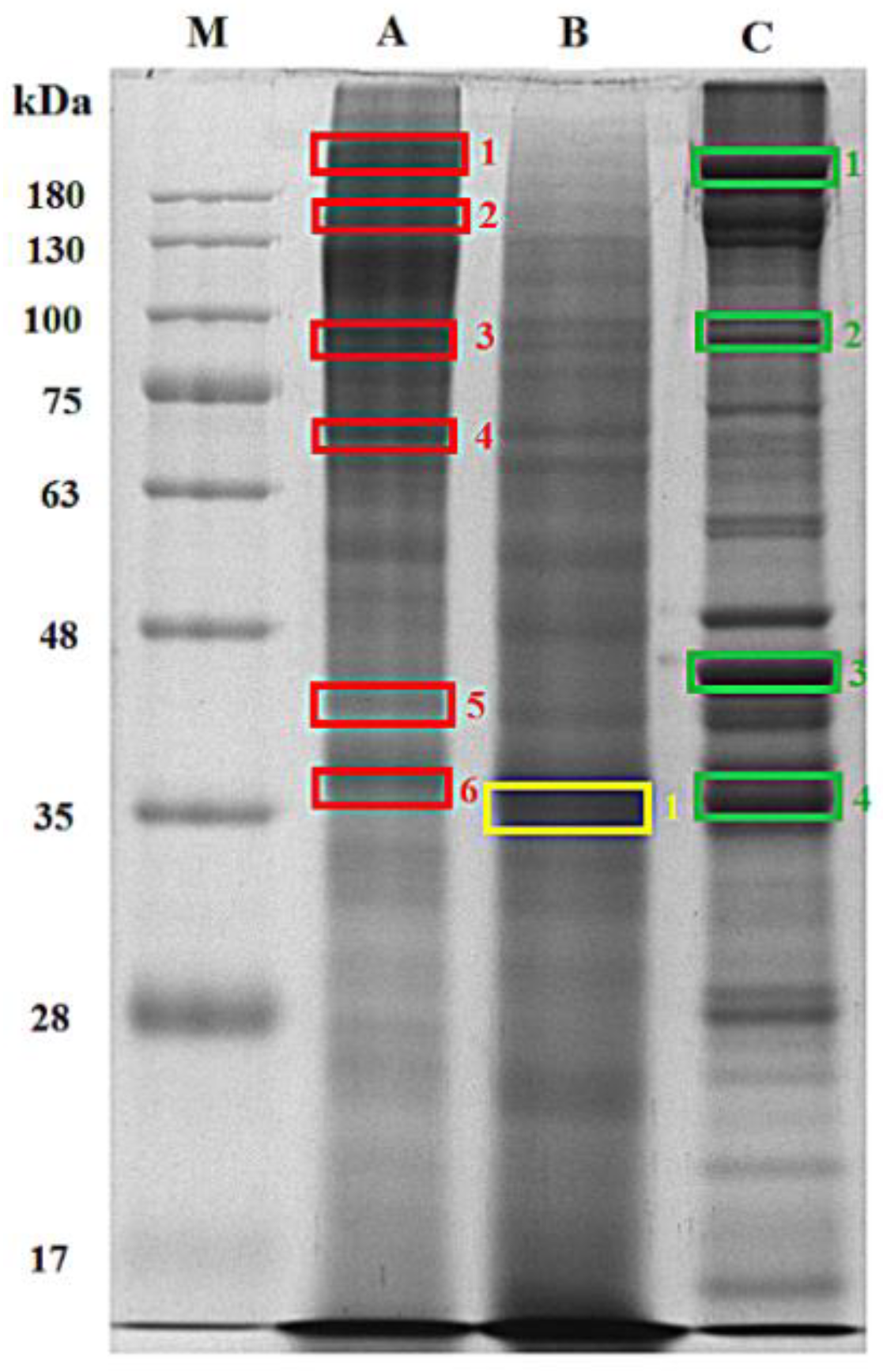
| Protein | Molecular (kDa) Weight from Database | Molecular Weight (kDa) Estimated from SDS-PAGE | ||
|---|---|---|---|---|
| MSJ | MSJSDS | MuscleSDS | ||
| Collagen alpha-2(I) chain | 126.908 | 241 (A1), 127 (A2), 42 (A5) | - | - |
| Myosin heavy chain, fast skeletal muscle | 221.462 | 91 (A3), 70 (A4),42 (A5), 37 (A6) | 37 (B1) | 37 (C4), 42 (C3), 242 (C1), 90 (C2) |
| Actin, alpha skeletal muscle | 41.944 | 42 (A5), 37 (A6) | 37 (B1) | 37 (C4), 42 (C3), 90 (C2), 242 (C1) |
| Actin, alpha skeletal muscle B | 41.950 | - | - | 42 (C3) |
| Actin, cytoplasmic 2 | 41.726 | - | - | 90 (C2), 42 (C3) |
| Actin, alpha anomalous | 41.952 | - | - | 42 (C3), 37 (C4) |
| Actin, cytoplasmic 3 | 41.756 | - | - | 42 (C3), 37 (C4) |
| Tropomyosin alpha-1 chain | 32.767 | 92 (A3), 70 (A4), 42 (A5), 37 (A6) | 37 (B1) | 37 (C4), 42 (C3), 242 (C1) |
| Beta-enolase | 47.257 | 241 (A1), 70 (A4), 42 (A5) | 37 (B1) | 37 (C4), 42 (C3), 90 (C2), 242 (C1) |
| Fructose-bisphosphate aldolase A | 40.044 | 241 (A1), 42 (A5), 37 (A6) | 37 (B1) | 37 (C4), 42 (C3), 90 (C2), 242 (C1) |
| Glyceraldehyde-3-phosphate dehydrogenase | 35.761 | 241 (A1), 91 (A3),70 (A4), 42 (A5), 37 (A6) | 37 (B1) | 37 (C4), 42 (C3), 90 (C2), 242 (C1) |
| Myosin light chain-1, skeletal muscle isoform | 20.054 | 42 (A5) | 37 (B1) | 37 (C4), 42 (C3), 90 (C2), 242 (C1) |
| Protein | Number of Bioactive Peptides | ||||
|---|---|---|---|---|---|
| DPP-IV Inhibitor | ACE Inhibitor | Antithrombotic | Antioxidative | Antiamnestic | |
| Collagen alpha-2(I) chain | 1056 | 993 | 236 | 49 | 214 |
| Myosin heavy chain, fast skeletal muscle | 1174 | 657 | 8 | 118 | 4 |
| Actin, alpha skeletal muscle | 247 | 165 | 3 | 21 | 2 |
| Tropomyosin alpha-1 chain | 148 | 85 | 1 | 26 | 0 |
| Beta-enolase | 270 | 185 | 19 | 1 | 0 |
| Fructose-bisphosphate aldolase | 237 | 166 | 3 | 25 | 4 |
| Glyceraldehyde-3-phosphatedehydrogenase | 233 | 152 | 0 | 20 | 2 |
| Myosin light chain-1, skeletal muscle isoform | 122 | 89 | 0 | 3 | 0 |
| Total | 3487 | 2492 | 270 | 263 | 226 |
| Enzyme | Total number of Bioactive Peptides Released from Mackerel Steaming Juice (MSJ) Proteins * | ||||
|---|---|---|---|---|---|
| Dipeptidyl Peptidase IV Inhibitor | ACE Inhibitor | Antithrombotic | Antioxidative | Antiamnestic | |
| Ficain | 418 | 275 | 37 | 36 | 35 |
| Papain | 296 | 246 | 3 | 23 | 2 |
| Pepsin (pH > 2) | 351 | 276 | 31 | 29 | 31 |
| Chymotrypsin C | 245 | 188 | 32 | 20 | 32 |
| Proteinase K | 362 | 230 | 31 | 27 | 31 |
| Thermolysin | 225 | 171 | 0 | 41 | 0 |
| Pancreatic elastase | 274 | 184 | 35 | 25 | 35 |
| Cathepsin G | 110 | 78 | 1 | 16 | 0 |
| Bromelain | 248 | 194 | 24 | 22 | 23 |
| Sample | Peptide Contents (mg/g) | Yield (%) * |
|---|---|---|
| MSJ | 162.14 ± 0.001 b | - |
| MSJ papain hydrolysates | 195.21 ± 0.008 c | 88.29 |
| MSJ pepsin hydrolysates | 124.00 ± 0.003 a | 92.79 |
| MSJ thermolysin hydrolysates | 195.26 ± 0.008 c | 92.79 |
| MSJ proteinase K hydrolysates | 326.92 ± 0.007 e | 98.20 |
| MSJ bromelain hydrolysates | 187.45 ± 0.005 c | 89.19 |
| MSJ alcalase hydrolysates | 260.05 ± 0.005 d | 90.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panjaitan, F.C.A.; Chen, T.-Y.; Ku, H.-H.; Chang, Y.-W. In Silico and In Vitro Analyses of Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Enzymatic Protein Hydrolysates from Taiwan Mackerel (Scomber australasicus) Steaming Juice. Foods 2022, 11, 1785. https://doi.org/10.3390/foods11121785
Panjaitan FCA, Chen T-Y, Ku H-H, Chang Y-W. In Silico and In Vitro Analyses of Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Enzymatic Protein Hydrolysates from Taiwan Mackerel (Scomber australasicus) Steaming Juice. Foods. 2022; 11(12):1785. https://doi.org/10.3390/foods11121785
Chicago/Turabian StylePanjaitan, Fenny Crista A., Ting-Yi Chen, Hao-Hsiang Ku, and Yu-Wei Chang. 2022. "In Silico and In Vitro Analyses of Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Enzymatic Protein Hydrolysates from Taiwan Mackerel (Scomber australasicus) Steaming Juice" Foods 11, no. 12: 1785. https://doi.org/10.3390/foods11121785
APA StylePanjaitan, F. C. A., Chen, T.-Y., Ku, H.-H., & Chang, Y.-W. (2022). In Silico and In Vitro Analyses of Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Enzymatic Protein Hydrolysates from Taiwan Mackerel (Scomber australasicus) Steaming Juice. Foods, 11(12), 1785. https://doi.org/10.3390/foods11121785








