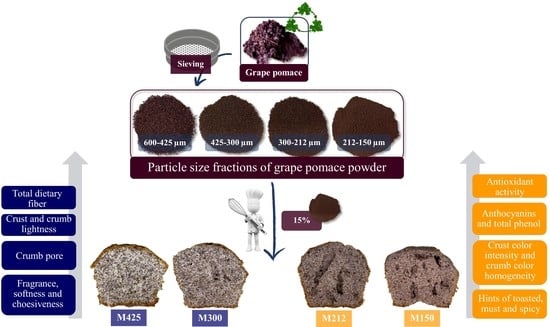
Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Pomace Preparation
2.3. Muffin Preparation
2.4. Extraction and Determination of Phenolic Compounds
2.5. Extraction and Determination of Anthocyanins
2.6. Antioxidant Activity Evaluation
2.7. Proximate Composition
2.8. Textural Profile Analysis (TPA)
2.9. Color and Image Analysis
2.10. Specific Volume Determination
2.11. Sensory Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Grape Pomace Powders
3.2. Characterization of Experimental Muffins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heo, Y.; Kim, M.J.; Lee, J.W.; Moon, B.K. Muffins Enriched with Dietary Fiber from Kimchi By-Product: Baking Properties, Physical–Chemical Properties, and Consumer Acceptance. Food Sci. Nutr. 2019, 7, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Heras, M.; Gómez, I.; de Pablos-Alcalde, S.; González-Sanjosé, M.L. Application of the Just-About-Right Scales in the Development of New Healthy Whole-Wheat Muffins by the Addition of a Product Obtained from White and Red Grape Pomace. Foods 2019, 8, 419. [Google Scholar]
- Global Muffin Market Report. 2021. Available online: https://www.absolutereports.com/global-muffins-market-19834098 (accessed on 10 January 2022).
- Shih, Y.T.; Wang, W.; Hasenbeck, A.; Stone, D.; Zhao, Y. Investigation of Physicochemical, Nutritional, and Sensory Qualities of Muffins Incorporated with Dried Brewer’s Spent Grain Flours as a Source of Dietary Fiber and Protein. J. Food Sci. 2020, 85, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D. Nutraceutical and Functional Food Regulations in the United States and around the World, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Bordiga, M. Wine Making By-Products. In Valorization of Wine Making By-Products; Academic Press: Cambridge, MA, USA, 2016; pp. 73–116. [Google Scholar]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of Grape Pomace Powder Addition on Chemical, Nutritional and Technological Properties of Cakes. LWT 2020, 134, 109950. [Google Scholar]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 12–14. [Google Scholar]
- Bhatt, S.; Ye, H.; Deutsch, J.; Jeong, H.; Zhang, J.; Suri, R. Food Waste and Upcycled Foods: Can a Logo Increase Acceptance of Upcycled Foods? J. Food Prod. Mark. 2021, 27, 188–203. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar]
- AACC. The Definition of Dietary Fiber. Report of the Dietary Fiber Definition Committee to the Board of Directors of the American Association of Cereal Chemists. Cereal Foods World 2001, 46, 112–126. [Google Scholar]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the Bioactivity and Potential Applications of Dietary Fibre from Grape Pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Marlett, G.N.; McBurney, M.I.; Slavin, J.L. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2002, 102, 993–1000. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, And Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Baldán, Y.; Riveros, M.; Fabani, M.P.; Rodriguez, R. Grape Pomace Powder Valorization: A Novel Ingredient to Improve the Nutritional Quality of Gluten-Free Muffins. Biomass Convers. Biorefin. 2021, 1–13. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Salvador, P.R.; Loureiro, B.B.; Lovatto, N.M.; Goulart, F.R.; Lovatto, M.T.; Miranda, M.Z.; Silva, L.P.; Penna, N.G. Grape Pomace Skins and the Effects of Its Inclusion in the Technological Properties of Muffins. J. Culin. Sci. Technol. 2017, 15, 143–157. [Google Scholar] [CrossRef]
- Kuchtová, V.; Kohajdová, Z.; Karovičová, J.; Lauková, M. Physical, Textural and Sensory Properties of Cookies Incorporated with Grape Skin and Seed Preparations. Pol. J. Food Nutr. Sci. 2018, 68, 309–317. [Google Scholar]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F. Physico-Chemical, Sensory and Volatile Profiles of Biscuits Enriched with Grape Marc Extract. Food Res. Int. 2014, 65, 385–393. [Google Scholar] [CrossRef]
- Bressiani, J.; Oro, T.; Santetti, G.S.; Almeida, J.L.; Bertolin, T.E.; Gómez, M.; Gutkoski, L.C. Properties of Whole Grain Wheat Flour and Performance in Bakery Products as a Function of Particle Size. J. Cereal Sci. 2017, 75, 269–277. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of Superfine Grinding on the Physicochemical Properties and Antioxidant Activity of Red Grape Pomace Powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Beres, C.; Simas-Tosin, F.F.; Cabezudo, I.; Freitas, S.P.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M.C. Antioxidant Dietary Fibre Recovery from Brazilian Pinot Noir Grape Pomace. Food Chem. 2016, 201, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironeasa, S.; Iuga, M.; Zaharia, D.; Mironeasa, C. Rheological Analysis of Wheat Flour Dough as Influenced by Grape Peels of Different Particle Sizes and Addition Levels. Food Bioprocess Technol. 2019, 12, 228–245. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green Extracts from Coratina Olive Cultivar Leaves: Antioxidant Characterization and Biological Activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Difonzo, G.; Aresta, A.; Cotugno, P.; Ragni, R.; Squeo, G.; Summo, C.; Massari, F.; Pasqualone, A.; Faccia, M.; Zambonin, C.; et al. Supercritical Co2 Extraction of Phytocompounds from Olive Pomace Subjected to Different Drying Methods. Molecules 2021, 26, 598. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.S.; Zhang, X.K.; He, F.; Duan, C.Q. An Effective Method for the Semi-Preparative Isolation of High-Purity Anthocyanin Monomers from Grape Pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and Characterization of Functional Biscuits Obtained from Purple Wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Tarricone, L.; Faccia, M.; Masi, G.; Gambacorta, G. The Impact of Early Basal Leaf Removal at Different Sides of the Canopy on Aglianico Grape Quality. Agriculture 2020, 10, 630. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Dingeo, C.; Difonzo, G.; Paradiso, V.M.; Rizzello, C.G.; Pontonio, E. Teff Type-i Sourdough to Produce Gluten-Free Muffin. Microorganisms 2020, 8, 1149. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the American Association of Cereal Chemists; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of Polyphenols Content and Antioxidant Activity of Some Red Wines by Differential Pulse Voltammetry, HPLC and Spectrophotometric Methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- De Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Roginsky, V.; Barsukova, T.; Adams, D.O.; Waterhouse, A.L. Phenolics: A Comparison of Diverse Analytical Methods. Am. J. Enol. Viticult. 2004, 55, 389–400. [Google Scholar]
- Fernandes, A.; Oliveira, J.; Fonseca, F.; Silva, F.; Mateus, N.; Vincken, J.-P.; Freitas, V. Molecular binding between anthocyanins and pectic polysaccharides—Unveiling the role of pectic polysaccharides structure. Food Hydrocoll. 2019, 102, 105625. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: A controlled trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef] [Green Version]
- Hayta, M.; Özuǧur, G.; Etgü, H.; Şeker, I.T. Effect of Grape (Vitis Vinifera L.) Pomace on the Quality, Total Phenolic Content and Anti-Radical Activity of Bread. J. Food Processing Preserv. 2014, 38, 980–986. [Google Scholar] [CrossRef]
- Wang, S.; Chang, T.; Wang, C.; Shi, L.; Wang, W.; Yang, H.; Cui, M. Effect of Particle Sizes of Soy Okara on Textural, Color, Sensory and Rheological Properties of Pork Meat Gels. J. Food Qual. 2015, 38, 248–255. [Google Scholar] [CrossRef]
- Majzoobi, M.; Poor, Z.V.; Jamalian, J.; Farahnaky, A. Improvement of the Quality of Gluten-Free Sponge Cake Using Different Levels and Particle Sizes of Carrot Pomace Powder. Int. J. Food Sci. Technol. 2016, 51, 1369–1377. [Google Scholar] [CrossRef]
- Dhen, N.; Román, L.; Ben Rejeb, I.; Martínez, M.M.; Garogouri, M.; Gómez, M. Particle Size Distribution of Soy Flour Affecting the Quality of Enriched Gluten-Free Cakes. LWT 2016, 66, 179–185. [Google Scholar] [CrossRef]
- de la Hera, E.; Martinez, M.; Gómez, M. Influence of Flour Particle Size on Quality of Gluten-Free Rice Bread. LWT-Food Sci. Technol. 2013, 54, 199–206. [Google Scholar] [CrossRef]
- Sert, D.; Mercan, E.; Tongur, A.; Kara, Ü. Production of Bread from Doughs Composed of High-Pressure Homogenisation Treated Flour Slurries: Effects on Physicochemical, Crumb Grain and Textural Characteristics. J. Food Meas. Charact. 2021, 15, 3052–3059. [Google Scholar] [CrossRef]
- Lazaridou, A.; Marinopoulou, A.; Biliaderis, C.G. Impact of Flour Particle Size and Hydrothermal Treatment on Dough Rheology and Quality of Barley Rusks. Food Hydrocoll. 2019, 87, 561–569. [Google Scholar] [CrossRef]
- Qin, W.; Lin, Z.; Wang, A.; Chen, Z.; He, Y.; Wang, L.; Liu, L.; Wang, F.; Tong, L.T. Influence of Particle Size on the Properties of Rice Flour and Quality of Gluten-Free Rice Bread. LWT 2021, 151, 112236. [Google Scholar] [CrossRef]
- Bartolozzo, J.; Borneo, R.; Aguirre, A. Effect of Triticale-Based Edible Coating on Muffin Quality Maintenance during Storage. J. Food Meas. Charact. 2016, 10, 88–95. [Google Scholar] [CrossRef]
- Torri, L.; Piochi, M.; Lavelli, V.; Monteleone, E. Descriptive sensory analysis and consumers’ preference for dietary fibre-and polyphenol-enriched tomato purees obtained using winery by-products. LWT-Food Sci. Technol. 2015, 62, 294–300. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of Grape Pomace and Grape Seed Flours on Cookie Quality. Qual. Assur. Saf. Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]





| Parameters | 600–425 µm | 425–300 µm | 300–212 µm | 212–150 µm |
|---|---|---|---|---|
| Total phenols (mg/g) | 10.39 ± 0.14 a | 12.39 ± 0.77 a | 11.69 ± 0.09 a | 11.18 ± 0.74 a |
| Total anthocyanins (mg/g) | 1.52 ± 0.02 c | 1.71 ± 0.03 b | 1.78 ± 0.01 a | 1.76 ± 0.01 a |
| ABTS (µmol TE/g) | 40.12 ± 1.46 b | 50.79 ± 2.10 a | 52.01 ± 2.25 a | 52.54 ± 1.69 a |
| DPPH (µmol TE/g) | 38.16 ± 0.90 b | 45.43 ± 0.77 a | 44.51 ± 0.24 a | 46.21 ± 0.52 a |
| Phenolic Profile | 600–425 µm | 425–300 µm | 300–212 µm | 212–150 µm |
|---|---|---|---|---|
| Phenolic acids | ||||
| Gallic acid | 442.23 ± 3.23 c | 565.80 ± 4.29 a | 459.11 ± 7.20 b | 455.20 ± 3.90 b |
| Syringic acid | 205.71 ± 2.46 c | 217.60 ± 1.51 b | 237.65 ± 4.27 a | 176.29 ± 7.26 d |
| Flavonols | ||||
| Quercetin | 240.23 ± 22.23 a | 240.10 ± 0.62 a | 223.57 ± 2.73 b | 191.27 ± 2.22 c |
| Quercetin-3-O-glucoside | 485.60 ± 11.48 a | 474.96 ± 0.78 ab | 462.55 ± 4.22 b | 390.84 ± 2.38 c |
| Myricetin | 27.83 ± 0.92 a | 26.50 ± 0.32 a | 26.98 ± 0.74 a | 22.03 ± 0.78 b |
| Rutin | 485.29 ± 3.10 ab | 490.86 ± 2.46 a | 471.09 ± 9.90 b | 389.41 ± 3.61 c |
| Kaempferol | 39.89 ± 0.54 a | 37.25 ± 1.69 b | 36.31 ± 0.40 b | 28.06 ± 0.80 c |
| Flavanols | ||||
| Catechin | 785.19 ± 5.60 a | 770.64 ± 16.43 a | 767.55 ± 27.09 a | 718.20 ± 34.76 b |
| Stilbenes | ||||
| trans-Resveratrol | 27.47 ± 1.32 ab | 30.50 ± 1.20 a | 27.33 ± 1.19 b | 26.94 ± 1.01 b |
| ε-Viniferin | 32.00 ± 0.22 a | 29.38 ± 0.41 b | 25.12 ± 0.39 c | 19.17 ± 0.84 d |
| Anthocyanins | ||||
| Delphinidin-3-glucoside | 50.21 ± 0.89 c | 58.79 ± 0.84 a | 60.59 ± 1.44 a | 53.95 ± 1.89 b |
| Cyanidin-3-glucoside | 33.00 ± 1.59 c | 42.64 ± 1.73 a | 38.95 ± 0.51 b | 35.86 ± 1.45 bc |
| Petunidin-3-glucoside | 121.05 ± 2.86 b | 147.53 ± 1.55 a | 143.86 ± 3.15 a | 125.33 ± 0.35 b |
| Peonidin-3-glucoside (mg/kg) | 117.13 ± 4.18 b | 147.17 ± 3.34 a | 144.02 ± 6.23 a | 128.46 ± 5.02 b |
| Malvidin-3-glucoside | 811.68 ± 15.04 c | 975.32 ± 16.65 a | 952.16 ± 1.95 ab | 927.15 ± 9.05 b |
| cis-Malvidin-3-coumaryl-glucoside | 31.79 ± 1.03 b | 35.80 ± 1.20 a | 37.83 ± 1.37 a | 38.37 ± 1.33 a |
| Malvidin-3-caffeoyl-glucoside | 49.51 ± 0.28 c | 64.49 ± 1.79 b | 69.91 ± 3.01 ab | 73.44 ± 2.35 a |
| Peonidin-3-coumaroyl-glucoside | 128.26 ± 4.80 b | 179.08 ± 3.24 a | 186.07 ± 4.27 a | 188.59 ± 8.72 a |
| Petunidin-3-coumaroyl-glucoside | 95.66 ± 0.06 c | 121.85 ± 1.50 b | 133.09 ± 1.09 a | 135.00 ± 1.31 a |
| trans-Malvidin-3-coumaroyl-glucoside | 1789.30 ± 41.24 c | 2080.86 ± 40.96 b | 2175.09 ± 59.49 ab | 2278.61 ± 12.53 a |
| Parameters | M425 | M300 | M212 | M150 |
|---|---|---|---|---|
| Moisture (g/100 g) | 23.34 ± 2.70 a | 22.31 ± 0.54 a | 21.90 ± 0.58 a | 24.90 ± 1.07 a |
| Lipids (g/100 g) | 22.70 ± 0.57 b | 24.90 ± 0.77 a | 25.45 ± 0.73 a | 25.88 ± 0.71 a |
| Proteins (g/100 g) | 9.72 ± 0.26 a | 9.03 ± 0.17 b | 8.76 ± 0.14 b | 8.81 ± 0.10 b |
| Ashes (g/100 g) | 1.80 ± 0.18 b | 1.83 ± 0.05 b | 2.12 ± 0.05 a | 2.09 ± 0.01 a |
| Carbohydrates (g/100 g) | 40.04 ± 0.81 a | 38.01 ± 0.96 a | 38.67 ± 0.98 a | 35.26 ± 0.82 b |
| Total dietary fiber (g/100 g) | 3.40 ± 0.25 ab | 3.92 ± 0.24 a | 3.10 ± 0.21 b | 3.06 ± 0.16 b |
| Total polyphenols (mg/g) | 0.64 ± 0.01 b | 0.65 ± 0.01 ab | 0.68 ± 0.01 ab | 0.69 ± 0.01 a |
| Total anthocyanins (µg/g) | 25.20 ± 0.20 b | 24.50 ± 0.20 b | 28.03 ± 0.15 a | 28.03 ± 0.55 a |
| ABTS (µmol TE/g) | 2.23 ± 0.02 b | 2.21 ± 0.02 b | 2.50 ± 0.02 a | 2.49 ± 0.06 a |
| DPPH (µmol TE/g) | 1.72 ± 0.01 b | 1.75 ± 0.02 b | 1.74 ± 0.02 a | 1.78 ± 0.02 a |
| Crust | ||||
| L * | 43.85 ± 2.39 a | 40.82 ± 1.70 b | 37.76 ± 3.38 c | 36.73 ± 1.33 c |
| a * | 13.34 ± 1.42 a | 8.76 ± 2.23 b | 7.96 ± 1.74 b | 7.60 ± 2.09 b |
| b * | 26.81 ± 2.37 a | 20.90 ± 2.63 b | 17.10 ± 1.92 bc | 14.06 ± 3.96 c |
| Crumb | ||||
| L * | 61.64 ± 2.72 a | 54.60 ± 2.75 b | 44.18 ± 3.35 c | 46.34 ± 2.60 c |
| a * | 1.14 ± 0.15 d | 2.21 ± 0.25 c | 3.40 ± 0.36 b | 5.82 ± 0.30 a |
| b * | 9.17 ± 0.81 b | 9.50 ± 0.63 b | 9.90 ± 0.85 b | 11.53 ± 0.62 a |
| Range of Area (mm2) | Cell Area Distribution (%) | |||
|---|---|---|---|---|
| M425 | M300 | M212 | M150 | |
| 0.05–0.07 | 21.56 ± 0.93 c | 21.07 ± 0.76 c | 25.91 ± 0.62 a | 22.85 ± 0.03 b |
| 0.07–0.1 | 17.65 ± 0.27 b | 19.80 ± 1.79 b | 22.92 ± 0.54 a | 19.45 ± 0.66 b |
| 0.1–0.2 | 244.91 ± 0.62 a | 25.79 ± 0.28 a | 25.01 ± 1.06 a | 26.02 ± 0.84 a |
| 0.2–0.3 | 10.06 ± 0.37 a | 10.28 ± 0.06 a | 8.38 ± 0.48 b | 8.52 ± 0.38 b |
| 0.3–0.6 | 11.76 ± 0.36 a | 10.96 ± 0.65 ab | 7.66 ± 0.29 c | 9.94 ± 0.47 b |
| 0.6–2 | 10.50 ± 0.56 a | 8.35 ± 0.09 ab | 5.86 ± 0.17 c | 7.82 ± 0.66 ab |
| 2–8 | 3.55 ± 0.13 a | 3.52 ± 0.68 ab | 2.79 ± 0.26 b | 3.45 ± 0.61 ab |
| 8–18 | - | 0.23 ± 0.01 ab | 0.40 ± 0.03 a | 0.50 ± 0.09 a |
| 18–30 | - | - | 0.44 ± 0.06 a | 0.49 ± 0.04 a |
| 30–50 | - | - | 0.41 ± 0.06 a | 0.73 ± 0.09 a |
| 50–80 | - | - | 0.22 ± 0.03 a | 0.23 ± 0.01 a |
| Mean area (mm2) | 0.54 ± 0.01 b | 0.56 ± 0.13 b | 1.07 ± 0.15 a | 1.13 ± 0.10 a |
| Circularity | 0.47 ± 0.01 a | 0.47 ± 0.02 a | 0.37 ± 0.02 b | 0.34 ± 0.01 b |
| Solidity | 0.67 ± 0.01 a | 0.66 ± 0.01 a | 0.64 ± 0.01 b | 0.64 ± 0.01 b |
| Particle size | ABTS | DPPH | TPC | ACNs | TDF | L * Crust | a * Crust |
| −0.998 | −0.617 | −0.969 | −0.998 | 0.889 | 0.913 | 0.709 | |
| b * Crust | L * Crumb | a * Crumb | b * Crumb | Hardness | Springiness | Chewiness | |
| 0.869 | 0.927 | −0.843 | −0.761 | −0.419 | −0.482 | 0.429 | |
| Cohesiveness | Crust CI | Crumb CH | AH | Fragrance | Toasted | Must | |
| 0.849 | −0.911 | −0.976 | 0.894 | 0.482 | −0.713 | −0.728 | |
| Sweetness | Acid | Spicy | Astringent | Softness | Stickiness | OA | |
| −0.884 | −0.949 | −0.863 | −0.83 | 0.637 | −0.907 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troilo, M.; Difonzo, G.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties. Foods 2022, 11, 1799. https://doi.org/10.3390/foods11121799
Troilo M, Difonzo G, Paradiso VM, Pasqualone A, Caponio F. Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties. Foods. 2022; 11(12):1799. https://doi.org/10.3390/foods11121799
Chicago/Turabian StyleTroilo, Marica, Graziana Difonzo, Vito Michele Paradiso, Antonella Pasqualone, and Francesco Caponio. 2022. "Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties" Foods 11, no. 12: 1799. https://doi.org/10.3390/foods11121799
APA StyleTroilo, M., Difonzo, G., Paradiso, V. M., Pasqualone, A., & Caponio, F. (2022). Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties. Foods, 11(12), 1799. https://doi.org/10.3390/foods11121799