Whey Protein Peptides Have Dual Functions: Bioactivity and Emulsifiers in Oil-In-Water Nanoemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPI Bioactive Peptide Fractions
2.3. Bioactivity and Hydrophobicity Measurement
2.4. Formation of WPI Bioactive Peptide-Stabilized Nanoemulsions
2.5. Droplet Size, Size Distribution, and Zeta (ζ) Potential Measurement
2.6. Storage Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Bioactivity of WPI Peptide Fractions
3.2. Surface Hydrophobicity of WPI Peptide Fractions
3.3. Effect of WPI Peptide Size on Nanoemulsion Formation
3.3.1. Less than 10 kDa (1–3, 3–5, 5–10 kDa) Fractions
3.3.2. Greater than 10 kDa (UC–10F and UP–10F) Fractions
3.4. Effect of WPI Bioactive Peptide Concentration on (Nano)Emulsion Formation
3.5. Effects of Storage Temperature and Time on (Nano)Emulsion Properties
3.5.1. Droplet Size and ζ-Potential
3.5.2. Creaming Stability
3.6. Effect of Peptide Fractionation on Nanoemulsion Formation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Formation of whey protein isolate hydrolysate stabilised nanoemulsion. Food Hydrocoll. 2014, 41, 169–177. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016, 61, 801–811. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Whey protein peptides as components of nanoemulsions: A review of emulsifying and biological functionalities. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Sun, X.; Sun, J.; Liu, C.; Liu, C.; Xu, J. Physicochemical properties and storage stability of food protein-stabilized nanoemulsions. Nanomaterials 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Madureira, A.; Tavares, T.; Gomes, A.M.P.; Pintado, M.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [Green Version]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; González-Córdova, A.F. Food-derived immunomodulatory peptides. J. Sci. Food Agric. 2016, 96, 3631–3641. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Lv, S.; Gu, J.; Zhang, R.; Zhang, Y.; Tan, H.; McClements, D.J. Vitamin E encapsulation in plant-based nanoemulsions fabricated using dual-channel microfluidization: Formation, stability, and bioaccessibility. J. Agri. Food Chem. 2018, 66, 10532–10542. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Kumar, D.L.; Sarkar, P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Komaiko, J.; McClements, D.J. Food-grade nanoemulsion filled hydrogels formed by spontaneous emulsification and gelation: Optical properties, rheology, and stability. Food Hydrocoll. 2015, 46, 67–75. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.-C.; Huang, D.-W.; Wang, C.-C.; Yeh, C.-H.; Tsai, J.-C.; Huang, Y.-T.; Li, P.-H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Schröder, A.; Berton-Carabin, C.; Venema, P.; Cornacchia, L. Interfacial properties of whey protein and whey protein hydrolysates and their influence on O/W emulsion stability. Food Hydrocoll. 2017, 73, 129–140. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Tamm, F.; Drusch, S. Impact of enzymatic hydrolysis on the interfacial rheology of whey protein/pectin interfacial layers at the oil/water-interface. Food Hydrocoll. 2017, 63, 8–18. [Google Scholar] [CrossRef]
- Mann, B.; Athira, S.; Sharma, R.; Kumar, R.; Sarkar, P. Bioactive peptides from whey proteins. In Whey Proteins; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 519–547. [Google Scholar] [CrossRef]
- da Costa, E.L.; da Rocha Gontijo, J.A.; Netto, F.M. Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates. Int. Dairy J. 2007, 17, 632–640. [Google Scholar] [CrossRef]
- Pan, D.; Cao, J.; Guo, H.; Zhao, B. Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate. Food Chem. 2012, 130, 121–126. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure–function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hettiarachchy, N.; Qi, M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J. Am. Oil Chem. Soc. 1998, 75, 845–850. [Google Scholar] [CrossRef]
- Euston, S.R.; Finnigan, S.R.; Hirst, R.L. Heat-induced destabilization of oil-in-water emulsions formed from hydrolyzed whey protein. J. Agric. Food Chem. 2001, 49, 5576–5583. [Google Scholar] [CrossRef] [PubMed]
- Agboola, S.O.; Singh, H.; Munro, P.A.; Dalgleish, D.G.; Singh, A.M. Destabilization of oil-in-water emulsions formed using highly hydrolyzed whey proteins. J. Agric. Food Chem. 1998, 46, 84–90. [Google Scholar] [CrossRef]
- Mutilangi, W.; Panyam, D.; Kilara, A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J. Food Sci. 1996, 61, 270–275. [Google Scholar] [CrossRef]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Jafari, S.M.; Beheshti, P.; Assadpoor, E. Rheological behavior and stability of d-limonene emulsions made by a novel hydrocolloid (Angum gum) compared with Arabic gum. J. Food Eng. 2012, 109, 1–8. [Google Scholar] [CrossRef]
- Tirok, S.; Scherze, I.; Muschiolik, G. Behaviour of formula emulsions containing hydrolysed whey protein and various lecithins. Colloids Surf. B Biointerfaces 2001, 21, 149–162. [Google Scholar] [CrossRef]
- Tamm, F.; Herbst, S.; Brodkorb, A.; Drusch, S. Functional properties of pea protein hydrolysates in emulsions and spray-dried microcapsules. Food Hydrocoll. 2016, 58, 204–214. [Google Scholar] [CrossRef]
- Tesch, S.; Schubert, H. Influence of increasing viscosity of the aqueous phase on the short-term stability of protein stabilized emulsions. J. Food Eng. 2002, 52, 305–312. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, S.J.; Li, Y.; Decker, E.A.; McClements, D.J. Protein-stabilized nanoemulsions and emulsions: Comparison of physicochemical stability, lipid oxidation, and lipase digestibility. J. Agric. Food Chem. 2011, 59, 415–427. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Le Potier, I.; de Kerdaniel Tdu, F.; Grossiord, J.L.; Rosilio, V.; Agnely, F. Stabilization mechanism of oil-in-water emulsions by beta-lactoglobulin and gum arabic. J. Colloid Interface Sci. 2011, 354, 467–477. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides, and proteins. In Fennema’s Food Chemistry, 5th ed.; Damodaran, S., Parkin, L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 235–356. [Google Scholar]
- Turgeon, S.L.; Sanchez, C.; Gauthier, S.F.; Paquin, P. Stability and rheological properties of salad dressing containing peptidic fractions of whey proteins. Int. Dairy J. 1996, 6, 645–658. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of beta-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Kim, H.; Decker, E.; McClements, D. Influence of protein concentration and order of addition on thermal stability of β-lactoglobulin stabilized n-hexadecane oil-in-water emulsions at neutral pH. Langmuir 2005, 21, 134–139. [Google Scholar] [CrossRef]
- Singh, H. Aspects of milk-protein-stabilised emulsions. Food Hydrocoll. 2011, 25, 1938–1944. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M. Depletion flocculation of emulsions containing unadsorbed sodium caseinate. Food Hydrocoll. 1997, 11, 13–18. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y. Functional and biological properties of peptides obtained by enzymatic hydrolysis of whey proteins. J. Dairy Sci. 2003, 86, E78–E87. [Google Scholar] [CrossRef]
- van Aken, G.A. Coalescence mechanisms in protein-stabilized emulsions. In Food Emulsions, 4th ed.; Friberg, S., Larsson, K., Sjoblom, J., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 299–325. [Google Scholar]
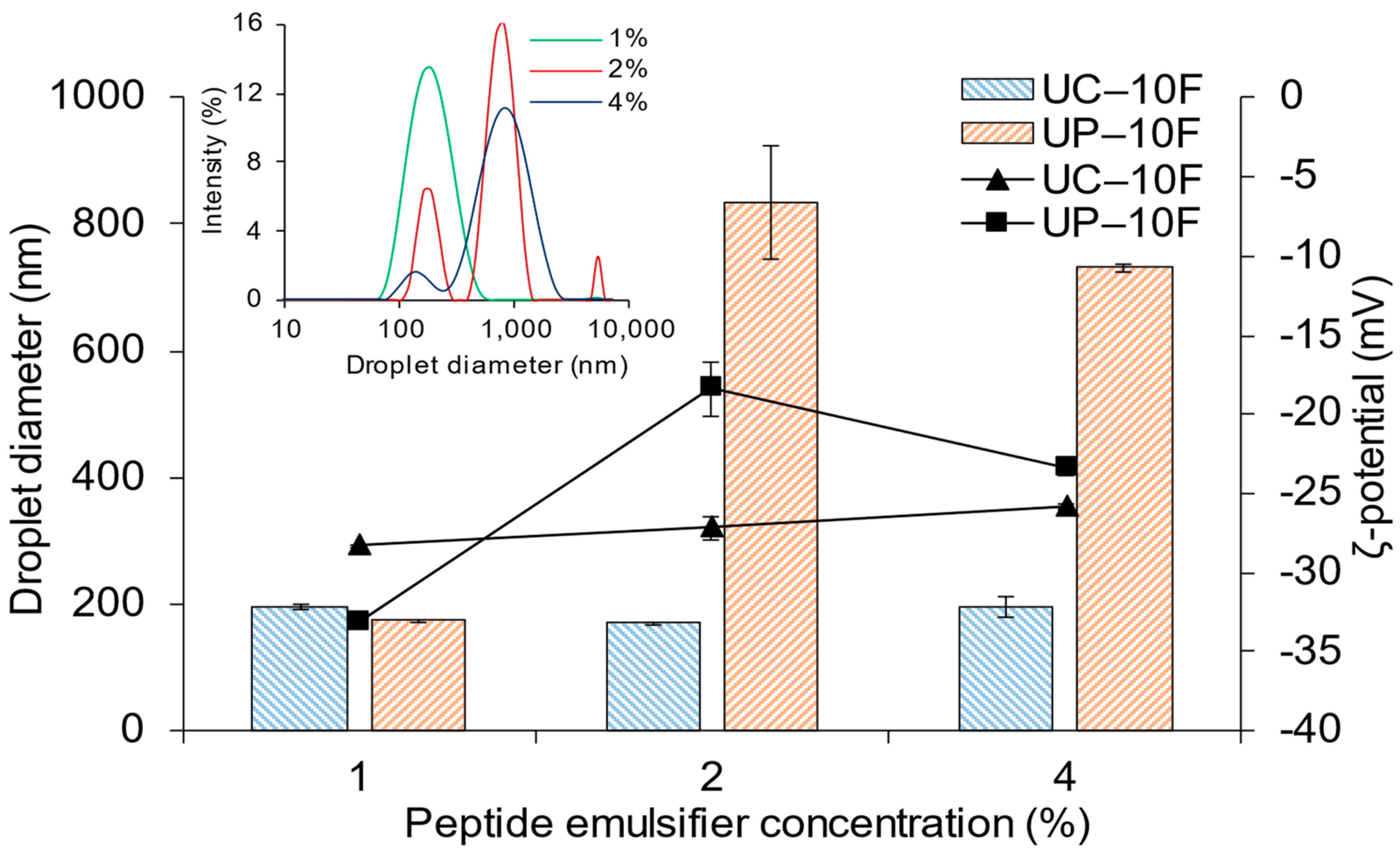

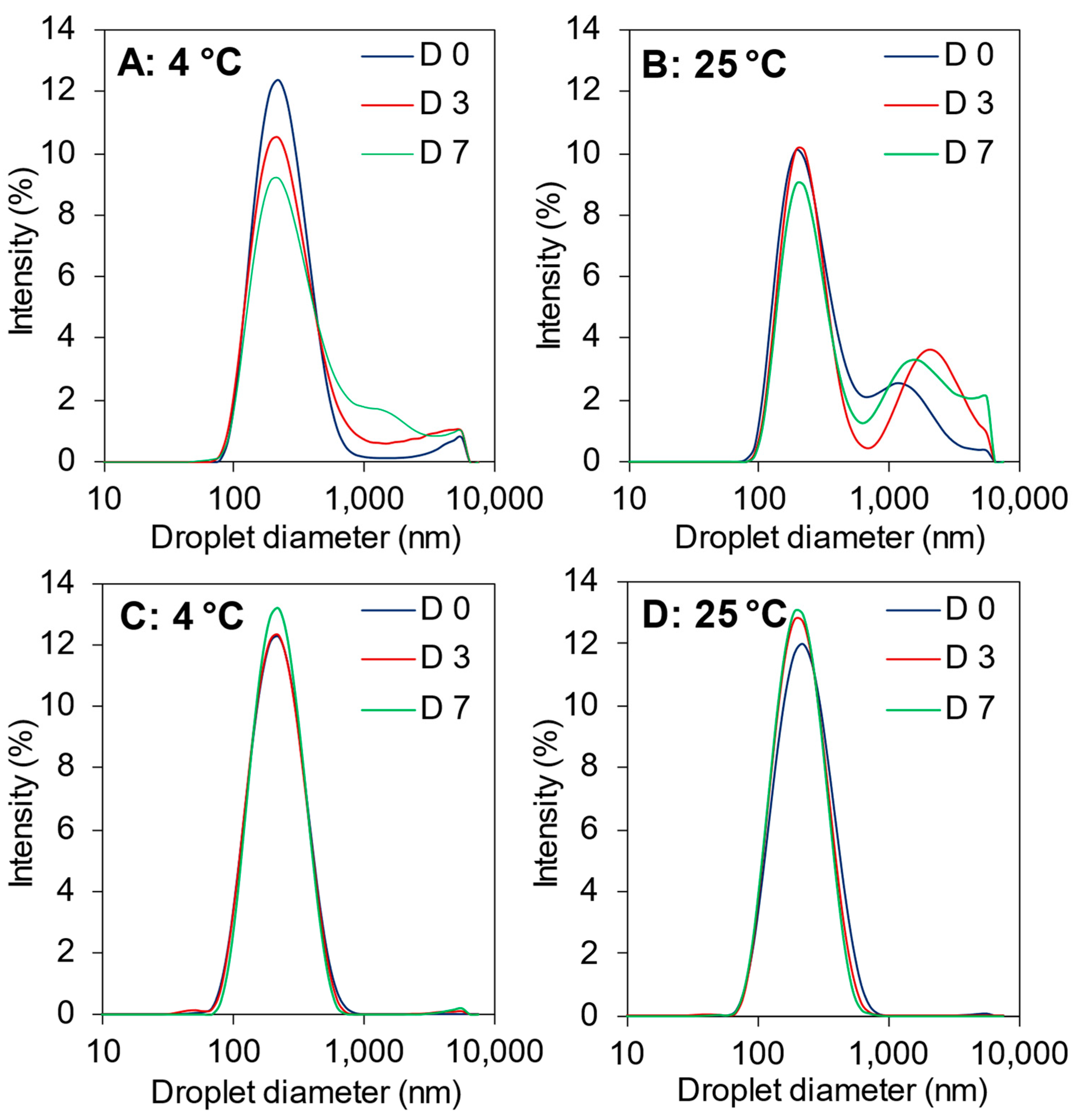
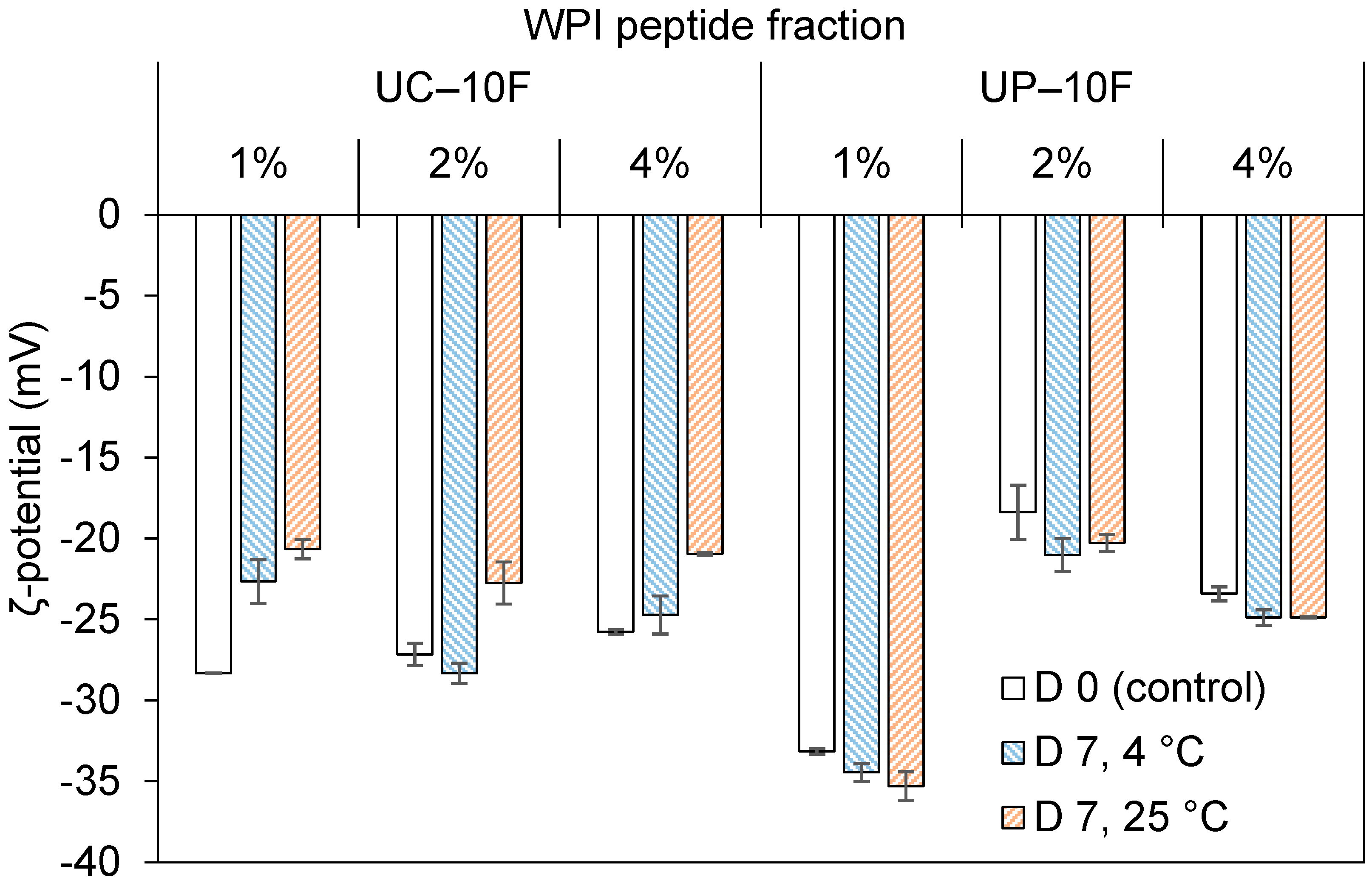
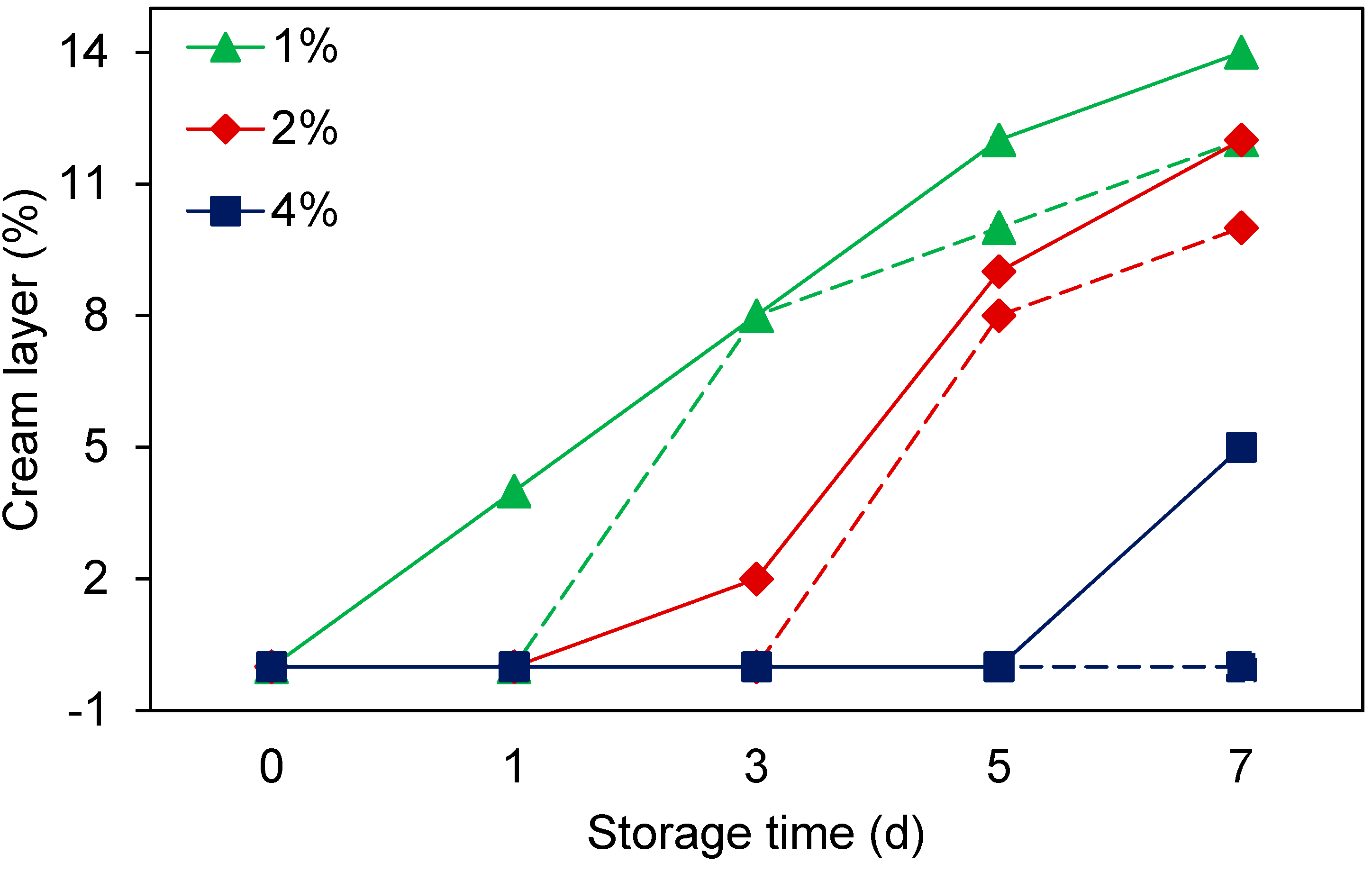

| Peptide Fractions | Antioxidant Activity (µmol TE/mg peptide) * | ACE-Inhibition (IC50, mg peptide/mL) * | Surface Hydrophobicity | |||
|---|---|---|---|---|---|---|
| Chymotrypsin | Pepsin | Chymotrypsin | Pepsin | Chymotrypsin | Pepsin | |
| 1–3 kDa | 0.30 ± 0.04 a | 0.25 ± 0.04 a | 0.565 ± 4.8 a | 0.38 ± 51.5 a | ≤44 | |
| 3–5 kDa | 0.28 ± 0.04 a | 0.21 ± 0.04 ab | 1.041 ± 72.4 c | 0.97 ± 90.7 b | ||
| 5–10 kDa | 0.26 ± 0.05 a | 0.21 ± 0.02 ab | 0.872 ± 75.3 b | 1.24 ± 42.1 c | ||
| >10 kDa | 0.24 ± 0.04 a | 0.14 ± 0.02 b | 1.119 ± 46.6 c | 1.90 ± 27.0 d | 1624.5 ± 4.0 b | 2088.5 ± 8.0 a |
| Emulsifer Type (Concentration) | 0 h | Storage Time (h) at 25 °C | Droplet Diameter (nm) | ζ-Potential (mV) | |
|---|---|---|---|---|---|
| Droplet Diameter (nm) | ζ-Potential (mV) | ||||
| Chymotrypsin WPI fractions | |||||
| 1–3 kDa (2%) | 218 ± 2.9 | −35.9 ± 1.49 | 12 | 3125 ± 0.71 | −32.8 ± 1.41 |
| 3–5 kDa (2%) | 290 ± 6.7 | −27.1 ± 1.61 | 12 | 1697 ± 236 | −24.2 ± 0.50 |
| 5–10 kDa (2%) | 199 ± 6.1 | −29.2 ± 1.54 | 12 | 2794 ± 64 | −19.4 ± 0.35 |
| UC–10F (1%) | 196 ± 0.8 | −28.3 ± 0.04 | 24 | 262 ± 4.8 | −25.2 ± 0.05 |
| Pepsin WPI fractions | |||||
| 1–3 kDa (2%) | 4122 ± 632 | −28.5 ± 0.39 | 12 | 290 ± 56 | −29.3 ± 0.57 |
| 3–5 kDa (2%) | 1233 ± 515 | −19.5 ± 0.35 | 12 | 560 ± 280 | −22.7 ± 0.53 |
| 5–10 kDa (2%) | 4085 ± 1258 | −15.4 ± 1.06 | 12 | 1741 ± 1122 | −17.8 ± 2.02 |
| UP–10F (1%) | 174 ± 2.3 | −33.2 ± 0.85 | 24 | 201 ± 9.4 | −32.7 ± 1.85 |
| Non-ionic surfactant | |||||
| Tween 40 (1%) | 166 ± 5.5 | −7.85 ± 0.20 | 24 | 173 ± 10.7 | −8.52 ± 0.70 |
| Storage Time (d) | 1 (%) | 2 (%) | ||
|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | |
| 0 | – | – | – | – |
| 1 | – | – | – | – |
| 3 | – | 8 | – | – |
| 5 | – | 10 | – | 8 |
| 7 | – | 12 | – | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjonu, R.; Doran, G.S.; Torley, P.; Sampson, G.O.; Agboola, S.O. Whey Protein Peptides Have Dual Functions: Bioactivity and Emulsifiers in Oil-In-Water Nanoemulsion. Foods 2022, 11, 1812. https://doi.org/10.3390/foods11121812
Adjonu R, Doran GS, Torley P, Sampson GO, Agboola SO. Whey Protein Peptides Have Dual Functions: Bioactivity and Emulsifiers in Oil-In-Water Nanoemulsion. Foods. 2022; 11(12):1812. https://doi.org/10.3390/foods11121812
Chicago/Turabian StyleAdjonu, Randy, Gregory S. Doran, Peter Torley, Gilbert O. Sampson, and Samson O. Agboola. 2022. "Whey Protein Peptides Have Dual Functions: Bioactivity and Emulsifiers in Oil-In-Water Nanoemulsion" Foods 11, no. 12: 1812. https://doi.org/10.3390/foods11121812
APA StyleAdjonu, R., Doran, G. S., Torley, P., Sampson, G. O., & Agboola, S. O. (2022). Whey Protein Peptides Have Dual Functions: Bioactivity and Emulsifiers in Oil-In-Water Nanoemulsion. Foods, 11(12), 1812. https://doi.org/10.3390/foods11121812






