Effect of Hydrothermal Cooking and Germination Treatment on Functional and Physicochemical Properties of Parkia timoriana Bean Flours: An Underexplored Legume Species of Parkia Genera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Raw Nitta Bean (NBR)
2.1.2. Germinated Nitta Bean (NBG)
2.1.3. Hydrothermal Cooking of Nitta Beans (NBHTC)
2.2. Physicochemical Parameters
2.2.1. Length, Width and Thickness
2.2.2. Equivalent Diameter
2.2.3. Sphericity
2.2.4. Aspect Ratio Is Calculated by Using Equation
2.2.5. Seed Volume (V) Is Calculated by Using the Formula
2.2.6. Surface Area Is Calculated by Following Equation
2.2.7. Seed Weight
2.2.8. Seed Volume
2.2.9. Husk Content
2.3. Field Emission–Scanning Electron Microscopic (FE-SEM) Analysis
2.4. Proximate Composition
2.5. Functional Properties
2.5.1. Color Measurement
2.5.2. Least Gelation Concentration
2.5.3. Emulsion Capacity (EC) and Emulsion Stability (ES)
2.5.4. Swelling Capacity
2.5.5. Hydration Capacity
2.5.6. Water Holding and Oil Holding Capacity
2.6. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.7. Thermal Properties
2.8. Antioxidant Activity
2.8.1. Total Polyphenol Content
2.8.2. DPPH Radical Scavenging Activity
2.8.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Proximate Composition
3.3. Functional Properties
3.3.1. Hunter Color Properties
3.3.2. Least Gelation Concentration
3.3.3. Emulsion Properties, Swelling Properties, Hydration Properties, and Water and Oil Holding Capacities
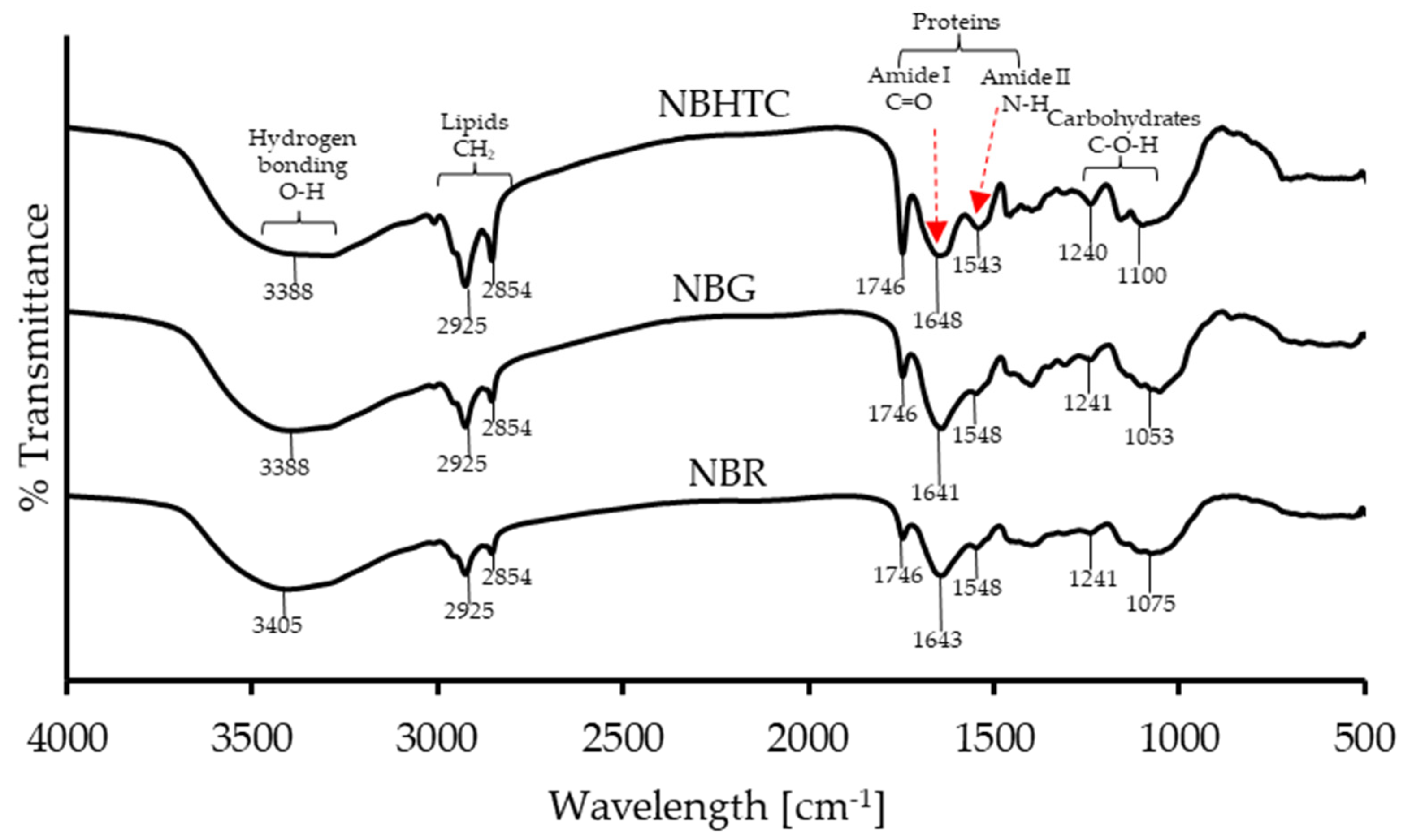
3.4. FTIR Analysis
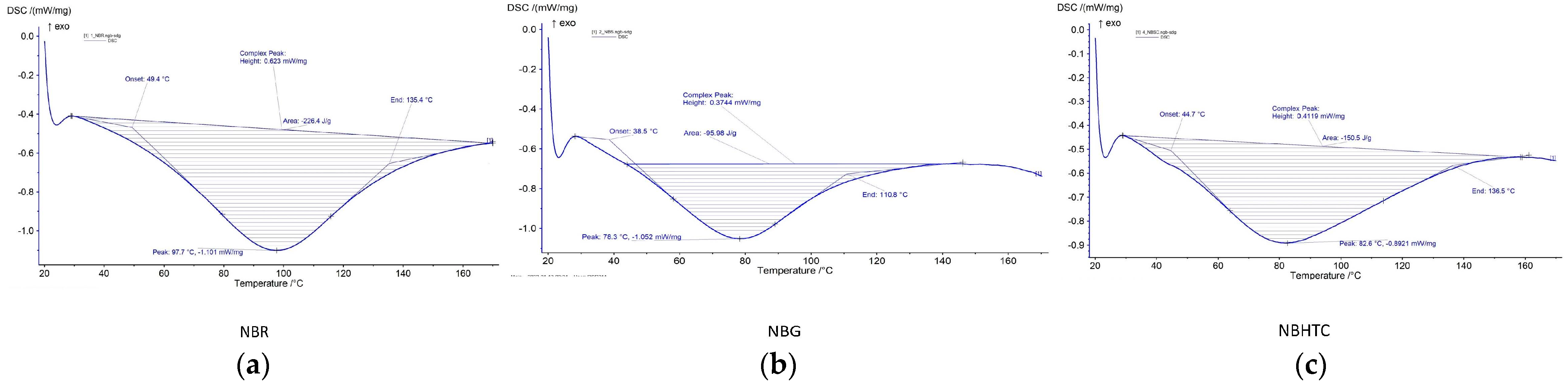
3.5. Thermal Properties
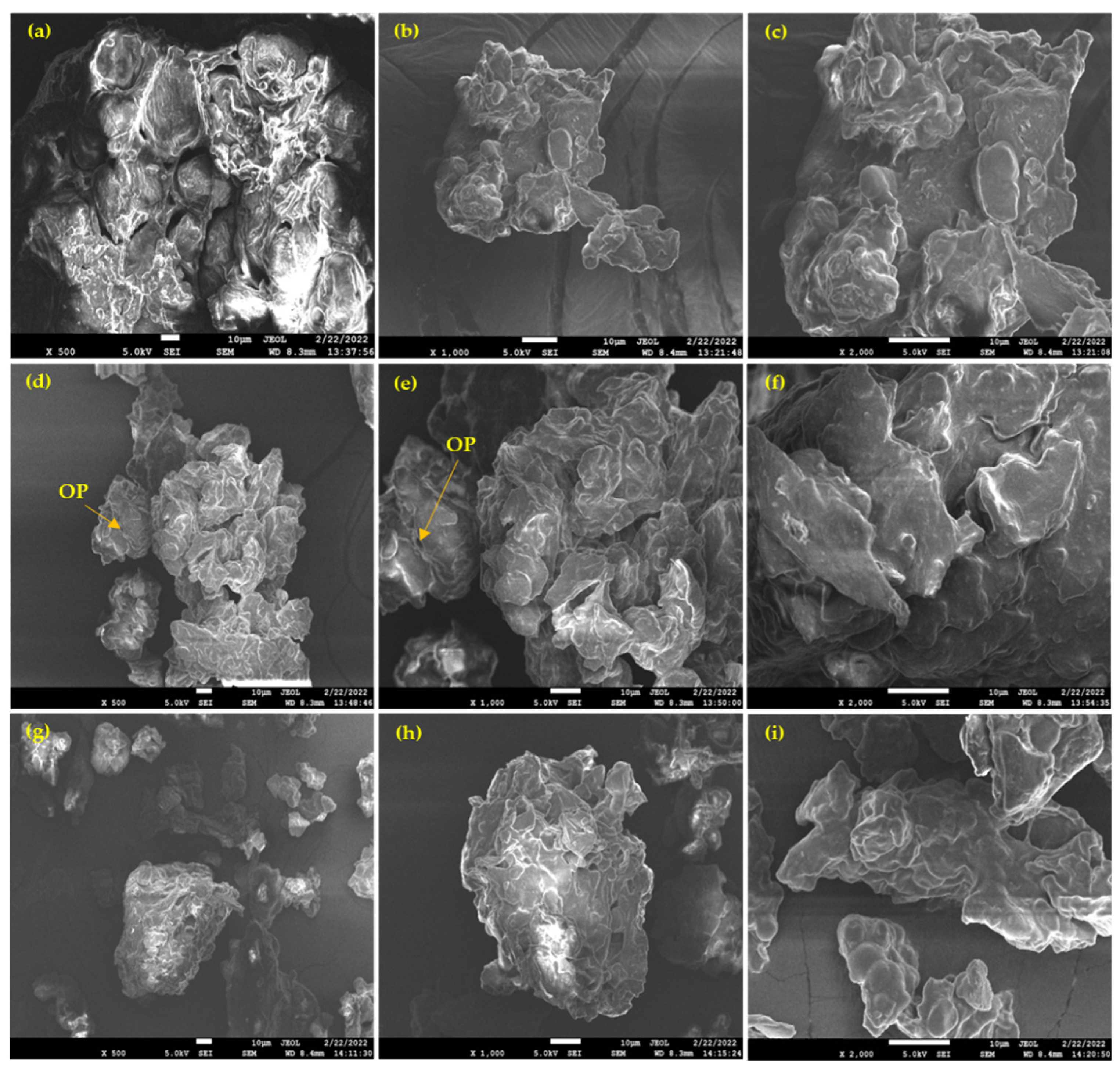
3.6. FE-SEM Analysis
3.7. Total Polyphenol Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathya, A.; Siddhuraju, P. Effect of processing methods on compositional evaluation of underutilized legume, Parkia roxburghii G. Don (yongchak) seeds. J. Food Sci. Technol. 2015, 52, 6157–6169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattab, R.; Arntfield, S.; Nyachoti, C. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Sridaran, A.; Karim, A.A.; Bhat, R. Pithecellobium jiringa legume flour for potential food applications: Studies on their physico-chemical and functional properties. Food Chem. 2012, 130, 528–535. [Google Scholar] [CrossRef]
- Ralte, L.; Khiangte, L.; Thangjam, N.M.; Kumar, A.; Singh, Y.T. GC–MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci. Rep. 2022, 12, 3395. [Google Scholar] [CrossRef]
- Thangjam, R.; Maibam, D.; Sharma, J.G. Detection of genetic diversity in Parkia timoriana (DC.) Merr. using randomly amplified polymorphic DNA analysis. J. Food Agric. Environ. 2003, 1, 46–49. [Google Scholar]
- Angami, T.; Bhagawati, R.; Touthang, L.; Makdoh, B.; Bharati, K.A.; Silambarasan, R.; Ayyanar, M. Traditional uses, phytochemistry and biological activities of Parkia timoriana (DC.) Merr., an underutilized multipurpose tree bean: A review. Genet. Resour. Crop Evol. 2018, 65, 679–692. [Google Scholar] [CrossRef]
- Mohan, V.; Janardhanan, K. Chemical and nutritional evaluation of raw seeds of the tribal pulses Parkia roxburghii G. Don. and Entada phaseoloides (L.) Merr. Int. J. Food Sci. Nutr. 1993, 44, 47–53. [Google Scholar] [CrossRef]
- Ruthiran, P.; Selvaraj, C.I. Phytochemical screening and in vitro antioxidant activity of Parkia timoriana (DC.) Merr. Res. J. Biotechnol. 2017, 12, 46–54. [Google Scholar]
- Hamid, S.; Muzaffar, S.; Wani, I.A.; Masoodi, F.A.; Bhat, M.M. Physical and cooking characteristics of two cowpea cultivars grown in temperate Indian climate. J. Saudi Soc. Agric. Sci. 2016, 15, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hatcher, D.; Toews, R.; Gawalko, E. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT Food Sci. Technol. 2009, 42, 842–848. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Kowalczyk, D.; Złotek, U. Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts. Sci. Hortic. 2012, 140, 87–95. [Google Scholar] [CrossRef]
- Thangjam, U.; Sahoo, U.K. Effects of different pre-treatments and germination media on seed germination and seedling growth of Parkia Timoriana (Dc.) Merr. J. Exp. Biol. 2017, 5, 98–105. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, N.; Sodhi, N.S.; Rana, J.C. Diversity in properties of seed and flour of kidney bean germplasm. Food Chem. 2009, 117, 282–289. [Google Scholar] [CrossRef]
- Medhe, S.; Jain, S.; Anal, A.K. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J. Food Sci. Technol. 2019, 56, 2115–2125. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International, 21st ed.; AOAC: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Xu, L.; Chen, L.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Jin, Z.; Xu, X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coixlachryma-jobi L.). Food Chem. 2017, 229, 312–318. [Google Scholar] [CrossRef]
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT 2021, 140, 110749. [Google Scholar] [CrossRef]
- Alberto, J.; Rivas, G.; Fang, R.; Elizabeth, N.; Francisco, R. Effect of the addition of common bean flour on the cooking quality and antioxidant characteristics of spaghetti. J. Microbiol. Biotechnol. Food Sci. 2021, 2, 730–744. [Google Scholar]
- Gupta, S.; Chhabra, G.S.; Liu, C.; Bakshi, J.S.; Sathe, S.K. Functional properties of select dry bean seeds and flours. J. Food Sci. 2018, 83, 2052–2061. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Soybean germination limits the role of cell wall integrity in controlling protein physicochemical changes during cooking and improves protein digestibility. Food Res. Int. 2021, 143, 110254. [Google Scholar] [CrossRef]
- Miceli, A.; Miceli, C. Effect of thermal treatments on vitality and physical characteristics of bean, chickpea and lentil. J. Stored Prod. Res. 2012, 51, 86–91. [Google Scholar] [CrossRef]
- Falade, K.O.; Akinrinde, I.M. Physical, chemical and adsorption isotherm characteristics of fermented soybean cultivars, and cracked and dehulled African locust bean using selected Bacillus Spp. J. Food Sci. Technol. 2021, 58, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some Indian kidney bean (Phaseolus vulgaris L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Bueno, D.B.; da Silva Júnior, S.I.; Chiarotto, A.B.S.; Cardoso, T.M.; Neto, J.A.; dos Reis, G.C.L.; Glória, M.B.A.; Tavano, O.L. The germination of soybeans increases the water-soluble components and could generate innovations in soy-based foods. LWT 2020, 117, 108599. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of germination on the microstructural and physicochemical properties of different legume types. Plants 2021, 10, 592. [Google Scholar] [CrossRef]
- Inyang, C.; Zakari, U. Effect of germination and fermentation of pearl millet on proximate, chemical and sensory properties of instant “Fura”-a Nigerian cereal food. Pak. J. Nutr. 2008, 7, 9–12. [Google Scholar] [CrossRef]
- Piecyk, M.; Wołosiak, R.; Drużynska, B.; Worobiej, E. Chemical composition and starch digestibility in flours from Polish processed legume seeds. Food Chem. 2012, 135, 1057–1064. [Google Scholar] [CrossRef]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Regulation of storage lipid metabolism in developing and germinating lupin (Lupinus spp.) seeds. Acta Physiol. Plant. 2015, 37, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hatcher, D.; Tyler, R.; Toews, R.; Gawalko, E. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Díaz, M.F.; Aguilera, Y.; Benítez, V.; Mollá, E.; Esteban, R.M. Influence of germination on the soluble carbohydrates and dietary fibre fractions in non-conventional legumes. Food Chem. 2008, 107, 1045–1052. [Google Scholar] [CrossRef]
- Mahadevamma, S.; Tharanathan, R. Processing of legumes: Resistant starch and dietary fiber contents. J. Food Qual. 2004, 27, 289–303. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Ariza, N.; Esteban, R.; Mollá, E.; Waldron, K.; López-Andréu, F.J. Effect of germination on the carbohydrate composition of the dietary fiber of peas (Pisum sativum L.). J. Agric. Food Chem. 2003, 51, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, M.; Ghadle, M. Effect of sprouting on nutrients, antinutrients and in vitro digestibility of the MACS-13 soybean variety. Plant Foods Hum. Nutr. 2003, 58, 1–11. [Google Scholar] [CrossRef]
- Shiga, T.M.; Cordenunsi, B.R.; Lajolo, F.M. Effect of cooking on non-starch polysaccharides of hard-to-cook beans. Carbohydr. Polym. 2009, 76, 100–109. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Khalil, A.W.; Zeb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shah, H. Comparison of sprout quality characteristics of desi and kabuli type chickpea cultivars (Cicer arietinum L.). LWT Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Odeny, D.A. Microsatellite Development and Application in Pigeonpea (Cajanus cajan (L.) Millsp.). Ph.D. Thesis, University of Bonn, Bonn, Germany, 2007. [Google Scholar]
- Chinma, C.E.; Adewuyi, O.; Abu, J.O. Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Res. Int. 2009, 42, 1004–1009. [Google Scholar] [CrossRef]
- Ohizua, E.R.; Adeola, A.A.; Idowu, M.A.; Sobukola, O.P.; Afolabi, T.A.; Ishola, R.O.; Ayansina, S.O.; Oyekale, T.O.; Falomo, A. Nutrient composition, functional, and pasting properties of unripe cooking banana, pigeon pea, and sweetpotato flour blends. Food Sci. Nutr. 2017, 5, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Singh, A.; Singh, B. Effect on germination time and temperature on techno-functional properties and protein solubility of pigeon pea (Cajanus cajan) flour. Qual. Assur. Saf. Crops Foods 2019, 11, 305–312. [Google Scholar] [CrossRef]
- Aguilera, Y.; Esteban, R.M.; Benitez, V.; Molla, E.; Martin-Cabrejas, M.A. Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 10682–10688. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT-Food Sci. Technol. 2016, 65, 137–144. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Saleh, F.; Al-Oweini, R.; Maroun, R.G.; Louka, N. Food fraud detection in commercial pomegranate molasses syrups by UV–VIS spectroscopy, ATR-FTIR spectroscopy and HPLC methods. Food Control. 2017, 78, 132–137. [Google Scholar] [CrossRef]
- Li, Y.; Obadi, M.; Shi, J.; Xu, B.; Shi, Y.C. Rheological and thermal properties of oat flours and starch affected by oat lipids. J. Cereal Sci. 2021, 102, 103337. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physicochemical and functional properties of flours from three Black gram (Phaseolus mungo L.) cultivars. Int. J. Food Sci. Technol. 2013, 48, 771–777. [Google Scholar] [CrossRef]
- Rui, X.; Boye, J.I.; Ribereau, S.; Simpson, B.K.; Prasher, S.O. Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res. Int. 2011, 44, 2497–2504. [Google Scholar] [CrossRef]
- Błaszczak, W.; Doblado, R.; Frias, J.; Vidal-Valverde, C.; Sadowska, J.; Fornal, J. Microstructural and biochemical changes in raw and germinated cowpea seeds upon high-pressure treatment. Food Res. Int. 2007, 40, 415–423. [Google Scholar] [CrossRef]
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination andcooking on nutrients, anti-nutrients, fatty acid composition andantioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Salawu, S.O.; Bester, M.J.; Duodu, K.G. Phenolic composition and bioactive properties of cell wall preparations and whole grains of selected cereals and legumes. J. Food Biochem. 2014, 38, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Chipurura, B.; Baudi, J.S.; Munodawafa, T.; Benhura, C. Effect of soaking, dehulling and boiling on protein, polyphenolicand antinutrient content of cowpeas (Vigna unguiculata L. Walp). Nutrafoods 2018, 17, 205–211. [Google Scholar]
- Chutipanyaporn, P.; Kruawan, K.; Chupeerach, C.; Santivarangkna, C.; Suttisansanee, U. The effect of cooking process on antioxidant activities and total phenolic compounds of five colored beans. Food Appl. Biosci. J. 2014, 2, 183–191. [Google Scholar]
| Parameter | NBR | NBG | NBHTC |
|---|---|---|---|
| Length (mm) | 17.39 ± 0.14 a | 23.75 ± 0.17 c | 19.86 ± 0.16 b |
| Width (mm) | 9.14 ± 0.10 a | 11.08 ± 0.12 b | 10.67 ± 0.11 b |
| Thickness (mm) | 5.53 ± 0.07 b | 6.69 ± 0.07 c | 5.09 ± 0.04 a |
| Diameter (mm) | 9.53 ± 0.08 a | 12.03 ± 0.08 c | 10.22 ± 0.07 b |
| Sphericity (%) | 54.99 ± 0.36 b | 50.77 ± 0.27 a | 51.61 ± 0.22 a |
| Aspect ratio | 0.53 ± 0.01 b | 0.47 ± 0.00 a | 0.54 ±0.01 b |
| Volume (mm3) | 252.19 ± 5.53 a | 491.72 ± 9.07 c | 306.50 ± 6.11 b |
| Surface area (mm2) | 243.36 ± 4.81 a | 391.14 ± 3.65 c | 281.88 ± 3.89 b |
| Weight (g/100 seed) | 54.31 ± 0.28 a | 94.19 ± 0.65 c | 69.18 ± 2.03 b |
| Volume (mL/100 seed) | 43.67 ± 0.88 a | 89.00 ± 3.06 c | 65.33 ± 3.93 b |
| Husk content (%) | 37.90 ± 0.48 | -- | -- |
| Parameter | NBR | NBG | NBHTC |
|---|---|---|---|
| Energy (Kcal) | 385.51± 0.58 a | 422.66 ± 0.20 b | 502.37 ± 0.65 c |
| Moisture | 8.87 ± 0.21 c | 6.61 ± 0.03 b | 0.98 ± 0.02 a |
| Total proteins | 17.27 ± 0.01 a | 37.34 ± 0.24 b | 36.62 ± 0.02 c |
| Total fats | 8.72 ±0.06 a | 15.57 ± 0.02 b | 24.02 ± 0.08 c |
| Total carbohydrates * | 68.86 ± 0.29 c | 40.78 ± 0.25 b | 36.18 ± 0.07 a |
| Total dietary fibers | 56.52 ± 0.44 c | 26.86 ± 0.22 a | 32.49 ± 0.24 b |
| Soluble dietary fibers | 12.70 ± 0.56 c | 1.47 ± 0.02 a | 7.81 ± 0.26 b |
| Insoluble dietary fibers | 43.82 ± 0.98 b | 25.39 ± 0.22 a | 24.67 ± 0.31 a |
| Ash | 5.15 ± 0.03 b | 6.32 ± 0.03 c | 3.18 ± 0.02 a |
| Nitta Bean | Hunter Color Values | ||||
|---|---|---|---|---|---|
| L | a* | b* | Croma | Hue Angle | |
| NBRF | 41.84 ± 0.10 a | 1.59 ± 0.01 c | 10.98 ± 0.01 a | 11.09 ± 0.01 a | 101.11 ± 0.03 b |
| NBGF | 48.47 ± 0.01 c | −3.47 ± 0.01 a | 17.65 ± 0.02 c | 17.99 ± 0.03 c | 101.11 ± 0.03 b |
| NBHTCF | 43.84 ± 0.06 b | 0.20 ± 0.01 b | 15.78 ± 0.01 b | 15.78 ± 0.01 b | 89.29 ± 0.04 a |
| NBR | 21.62 ± 0.04 A | 2.37 ± 0.05 B | 3.64 ± 0.00 A | 4.35 ± 0.03 A | 56.90 ± 0.58 A |
| NBG | 56.107 ± 0.07 C | −4.67 ± 0.07 A | 30.60 ± 0.07 C | 30.96 ± 0.07 C | 98.68 ± 0.13 C |
| NBHTC | 35.96 ± 0.17 B | 4.53 ± 0.04 C | 21.08 ± 0.12 B | 21.56 ± 0.12 B | 77.86 ± 0.06 B |
| Sample | Concentration of Flours (g/100 mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 4% | 6% | 8% | 10% | 12% | 14% | 16% | 18% | 20% | |
| NBRF | − | − | − | − | − | − | ± | ± | + | + |
| NBGF | − | − | − | − | ± | + | + | + | + | + |
| NBHTCF | − | − | − | − | − | − | − | − | ± | ± |
| Parameter | NBR | NBG | NBHTC |
|---|---|---|---|
| EC (%) | 51.84 ± 3.74 b | 58.33 ± 1.67 b | 3.22 ± 0.06 a |
| ES (%) | 31.97 ± 7.79 b | 63.89 ± 2.00 c | 0.96 ± 0.03 a |
| SC (mL/seed) | 0.03 ±0.00 a | 0.12 ± 0.04 a | 0.11 ± 0.03 a |
| SI | 0.01±0.00 a | 0.02 ±0.01 a | 0.01± 0.00 a |
| HC (g/seed) | 0.04 ± 0.00 a | 0.01 ± 0.01 a | 0.38 ±0.00 b |
| HI | 0.53 ±0.00 a | 0.96 ±0.00 c | 0.68 ± 0.01 b |
| WHC (mL/g) | 0.81 ± 0.03 a | 0.82 ± 0.00 a | 0.83 ± 0.0 a |
| OHC (g/g) | 0.86 ± 0.03 a | 0.81 ± 0.03 a | 0.80 ± 0.05 a |
| Treatments | Functional Group Characteristic Absorption Peak | |||
|---|---|---|---|---|
| -OH | Lipid | Protein | Carbohydrate | |
| NBR | 3404.87 | 3010.11, 2924.88, 2853.93, 1745.73 | 1643.38, 1548.1, 1398.06, 1312.64 | 1240.51, 1074.64 |
| NBG | 3388.28 (+) | 3010.28 (+), 2925.05 (+), 2853.94 (+), 1745.77(+) | 1641.27 (+), 1548.28 (+), 1455 (+), 1398.98 (+), 1309.38 (+) | 1240.95 (+), 1102.61 (+), 1053.1 (−), 895.44 (−) |
| NBHTC | 3388.28 (+) | 3009.35 (+), 2924.93 (+), 2853.95 (+), 1746.29 (+) | 1647.85 (+), 1542.91 (+), 1456.28 (+), 1398.09 (+), 1310.65 (+) | 1239.78 (+), 1158.68, 1099.7 (+) |
| Sample | ORAC (µM TE/g) | DPPH (mM TE/g) | Polyphenol (mg GE/g) |
|---|---|---|---|
| NBR | 391.51 ± 4.5 b | 58.49 ± 3.8 b | 3.89 ± 0.05 b |
| NBG | 505.33 ± 4.7 c | 61.21 ± 3.9 c | 6.88 ± 0.05 c |
| NBHTC | 324.79 ± 2.9 a | 41.50 ± 2.1 a | 1.92 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhe, S.V.; Kamble, M.T.; Kettawan, A.K.; Monboonpitak, N.; Kettawan, A. Effect of Hydrothermal Cooking and Germination Treatment on Functional and Physicochemical Properties of Parkia timoriana Bean Flours: An Underexplored Legume Species of Parkia Genera. Foods 2022, 11, 1822. https://doi.org/10.3390/foods11131822
Medhe SV, Kamble MT, Kettawan AK, Monboonpitak N, Kettawan A. Effect of Hydrothermal Cooking and Germination Treatment on Functional and Physicochemical Properties of Parkia timoriana Bean Flours: An Underexplored Legume Species of Parkia Genera. Foods. 2022; 11(13):1822. https://doi.org/10.3390/foods11131822
Chicago/Turabian StyleMedhe, Seema Vijay, Manoj Tukaram Kamble, Aurawan Kringkasemsee Kettawan, Nuntawat Monboonpitak, and Aikkarach Kettawan. 2022. "Effect of Hydrothermal Cooking and Germination Treatment on Functional and Physicochemical Properties of Parkia timoriana Bean Flours: An Underexplored Legume Species of Parkia Genera" Foods 11, no. 13: 1822. https://doi.org/10.3390/foods11131822







