Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing
Abstract
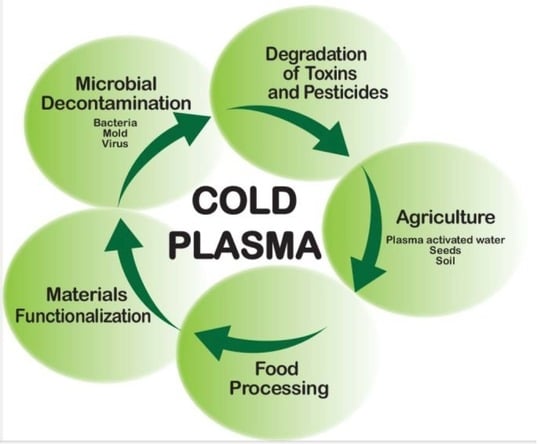
:1. Introduction
2. Product Cleansing and Decontamination
2.1. Microbial Decontamination
2.2. Toxin Removal
2.3. Pesticides Degradation
3. Sustainable Production Improvement
3.1. Plasma Activated Liquids
3.2. Cold Plasma in Agriculture
3.3. Cold Plasma in Food Processing
4. Potential Risks and Regulations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F.; Ionkova, K. What A Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018; ISBN 1464813477. [Google Scholar]
- Olabi, A.G. Circular Economy and Renewable Energy. Energy 2019, 181, 450–454. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [Green Version]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-Package Decontamination of Chicken Breast Using Cold Plasma Technology: Microbial, Quality and Storage Studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation Mechanisms of Non-Thermal Plasma on Microbes: A Review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Peng, H.-J.; Chu, Y.-H. Efficacy of Cold Plasma in Producing Salmonella-Free Duck Eggs: Effects on Physical Characteristics, Lipid Oxidation, and Fatty Acid Profile. J. Food Sci. Technol. 2019, 56, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Popo, E.; Mendonça, A.; Misra, N.N.; Little, A.; Wan, Z.; Moutiq, R.; Coleman, S.; Keener, K. Inactivation of Shiga-Toxin-Producing Escherichia Coli, Salmonella Enterica and Natural Microflora on Tempered Wheat Grains by Atmospheric Cold Plasma. Food Control 2019, 104, 231–239. [Google Scholar] [CrossRef]
- Chaplot, S.; Yadav, B.; Jeon, B. Atmospheric Cold Plasma and Peracetic Acid–Based Hurdle Intervention to Reduce Salmonella on Raw Poultry Meat. J. Food Prot. 2019, 82, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Baek, K.H.; Heo, Y.S.; Yong, H.I.; Jo, C. Synergistic Bactericidal Effect of Clove Oil and Encapsulated Atmospheric Pressure Plasma against Escherichia Coli O157:H7 and Staphylococcus Aureus and Its Mechanism of Action. Food Microbiol. 2021, 93, 103611. [Google Scholar] [CrossRef]
- Sudarsan, A.; Keener, K.M. Inactivation of Salmonella Enterica Serovars and Escherichia Coli O157:H7 Surrogate from Baby Spinach Leaves Using High Voltage Atmospheric Cold Plasma (HVACP). LWT 2022, 155, 112903. [Google Scholar] [CrossRef]
- De Oliveira, S.; Baptista, T.; Cesar, E. Salmonella Spp. and Escherichia Coli O157:H7 Prevalence and Levels on Lettuce: A Systematic Review and Meta-Analysis. J. Food Microbiol. 2019, 84, 103217. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold Plasma Treated Thyme Essential Oil/Silk Fibroin Nanofibers against Salmonella Typhimurium in Poultry Meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A Review of Novel Physical and Chemical Decontamination Technologies for Aflatoxin in Food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Nguyen, T.; Palmer, J.; Phan, N.; Shi, H.; Keener, K.; Flint, S. Control of Aflatoxin M1 in Skim Milk by High Voltage Atmospheric Cold Plasma. Food Chem. 2022, 386, 132814. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal Fungal Infection, Mycotoxins, and Lactic Acid Bacteria Mediated Bioprotection: From Crop Farming to Cereal Products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Ouf, S.A.; Basher, H.; Mohamed, A.H. Inhibitory Effect of Double Atmospheric Pressure Argon Cold Plasma on Spores and Mycotoxin Production of Aspergillus Niger Contaminating Date Palm Fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W. Effect of Dielectric Barrier Discharge Plasma on the Degradation of Malathion and Chlorpyrifos on Lettuce. J. Sci. Food Agric. 2021, 101, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Kalaivendan, T.; Gracy, R.; Gupta, V. Influence of Low-Pressure Nonthermal Dielectric Barrier Discharge Plasma on Chlorpyrifos Reduction in Tomatoes. J. Food Process Eng. 2019, 42, e13242. [Google Scholar] [CrossRef]
- Van Impe, J.; Smet, C.; Tiwari, B.; Greiner, R.; Ojha, S.; Stulic, V.; Vukusic, T.; Rezek Jambrak, A. State of the Art of Nonthermal and Thermal Processing for Inactivation of Micro-Organisms. J. Appl. Microbiol. 2018, 125, 16–35. [Google Scholar] [CrossRef] [Green Version]
- Bansal, S.; Singh, A.; Mangal, M.; Mangal, A.K. Food Adulteration: Sources, Health Risks, and Detection Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1174–1189. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A Review on Recent Development in Non-Conventional Food Sterilization Technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Goggins, J.A.; Mclachlan, J.B. Salmonella Infection: Interplay between the Bacteria and Host Immune System. Immunol. Lett. 2017, 190, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lata, C.; Keener, K.M.; Misra, N.N. Strategy to Achieve a 5-Log Salmonella Inactivation in Tender Coconut Water Using High Voltage Atmospheric Cold Plasma (HVACP). Food Chem. 2019, 284, 303–311. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Nisar, M.; Nayak, G.; Nagaraja, K.V.; Collins, J.; Bruggeman, P.J.; Goyal, S.M. Bactericidal Efficacy of a Two-Dimensional Array of Integrated, Coaxial, Microhollow, Dielectric Barrier Discharge Plasma Against Salmonella Enterica Serovar Heidelberg. Foodborne Pathog. Dis. 2019, 17, 157–165. [Google Scholar] [CrossRef]
- Erickson, M.C.; Liao, J.; Webb, C.C.; Habteselassie, M.Y.; Cannon, J.L. Inactivation of Escherichia Coli O157:H7 and Salmonella Deposited on Gloves in a Liquid State and Subjected to Drying Conditions. Int. J. Food Microbiol. 2018, 266, 200–206. [Google Scholar] [CrossRef]
- Shah, U.; Ranieri, P.; Zhou, Y.; Schauer, C.L.; Miller, V.; Fridman, G.; Sekhon, J.K. Effects of Cold Plasma Treatments on Spot-Inoculated Escherichia Coli O157:H7 and Quality of Baby Kale (Brassica Oleracea) Leaves. Innov. Food Sci. Emerg. Technol. 2019, 57, 102104. [Google Scholar] [CrossRef]
- Pasquali, F.; Ch, A.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusi, R.; Manfreda, G.; Trevisani, M. Atmospheric Cold Plasma Process for Vegetable Leaf Decontamination: A Feasibility Study on Radicchio (Red Chicory, Cichorium Intybus L.). Food Control 2016, 60, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric Cold Plasma Inactivation of Escherichia Coli, Salmonella Enterica Serovar Typhimurium and Listeria Monocytogenes Inoculated on Fresh Produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric Cold Plasma Inactivation of Escherichia Coli and Listeria Monocytogenes in Tender Coconut Water: Inoculation and Accelerated Shelf- Life Studies. Food Control 2019, 106, 106678. [Google Scholar] [CrossRef]
- Girgin, Z.; Barisci, S.; Dinc, O. Mechanisms of the Escherichia Coli and Enterococcus Faecalis Inactivation by Ozone. LWT-Food Sci. Technol. 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Yeni, F.; Alpas, H.; Soyer, Y. Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef]
- Gómez, D.; Iguácel, L.P.; Rota, M.C.; Carramiñana, J.J.; Ariño, A.; Yangüela, J. Occurrence of Listeria Monocytogenes in Ready-to-Eat Meat Products and Meat Processing Plants in Spain. Foods 2015, 4, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Taylor, B.J.; Quinn, A.R.; Kataoka, A. Listeria Monocytogenes in Low-Moisture Foods and Ingredients. Food Control 2019, 103, 153–160. [Google Scholar] [CrossRef]
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The Effects of Atmospheric Cold Plasma on Inactivation of Listeria Monocytogenes and Staphylococcus Aureus and Some Quality Characteristics of PastıRma—A Dry-Cured Beef Product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. [Google Scholar] [CrossRef]
- Srey, S.; Park, S.Y.; Jahid, I.K.; Ha, S. Do Reduction Effect of the Selected Chemical and Physical Treatments to Reduce L. Monocytogenes Biofilms Formed on Lettuce and Cabbage. Food Res. Int. 2014, 62, 484–491. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Drishya, C.; Yoha, K.S.; Perumal, A.B.; Moses, J.A.; Anandharamakrishnan, C.; Balasubramaniam, V.M. Impact of Nonthermal Food Processing Techniques on Mycotoxins and Their Producing Fungi. Int. J. Food Sci. Technol. 2022, 57, 2140–2148. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Abuagela, M.O.; Boz, Z.; Marshall, S.M.; Goodrich, R.; Charles, S.; Maurice, A.S.; Macintosh, A.J.; Welt, B.A. Effects of Atmospheric Pressure Plasma Jet Treatment on Aflatoxin Level, Physiochemical Quality, and Sensory Attributes of Peanuts. J. Food Process. Preserv. 2019, 44, e14305. [Google Scholar] [CrossRef]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A Holistic Study to Understand the Detoxification of Mycotoxins in Maize and Impact on Its Molecular Integrity Using Cold Atmospheric Plasma Treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of Cold Plasma on Fungal Growth and Aflatoxins Production on Groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Shi, H.; Ileleji, K.; Stroshine, R.L.; Keener, K.; Jensen, J.L. Reduction of Aflatoxin in Corn by High Voltage Atmospheric Cold Plasma. Food Bioprocess Technol. 2017, 10, 1042–1052. [Google Scholar] [CrossRef]
- Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, I.; Audenaert, K.; Gelder, L. De Biodegradation of Mycotoxins: Tales from Known and Unexplored Worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol Decontamination in Raw and Germinating Barley Treated by Plasma-Activated Water and Intense Pulsed Light. Food Bioprocess Technol. 2019, 12, 246–254. [Google Scholar] [CrossRef]
- Pivovarov, A.; Mykolenko, S.; Shcherbakov, S.; Agrarian, D.S. Plasma-Chemically Activated Water Influence on Staling and Safety of Sprouted Bread. Technol. Food Saf. 2018, 12, 100–107. [Google Scholar] [CrossRef]
- Abbasian, E.G.; Nosrati, A.C.; Ghoranneviss, M. Study of the Effect of Plasma Jet on Fusarium Isolates with Ability to Produce DON Toxins. Clin. Res. Methods 2017, 15, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Phan, K.; Phan, H.T.; Boonyawan, D.; Intipunya, P.; Brennan, C.S.; Regenstein, J.M.; Phimolsiripol, Y. Non-Thermal Plasma for Elimination of Pesticide Residues in Mango. Innov. Food Sci. Emerg. Technol. 2018, 48, 164–171. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-Package Nonthermal Plasma Degradation of Pesticides on Fresh Produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Sarangapani, C.; Misra, N.N.; Milosavljevic, V.; Bourke, P.; O’Regan, F.; Cullen, P.J. Pesticide Degradation in Water Using Atmospheric Air Cold Plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Wardenier, N.; Gorbanev, Y.; Van Moer, I.; Nikiforov, A.; Van Hulle, S.W.H.; Surmont, P.; Lynen, F.; Leys, C.; Bogaerts, A.; Vanraes, P. Removal of Alachlor in Water by Non-Thermal Plasma: Reactive Species and Pathways in Batch and Continuous Process. Water Res. 2019, 161, 549–559. [Google Scholar] [CrossRef]
- Mitrović, T.; Lazović, S.; Nastasijević, B.; Pašti, I.A.; Vasić, V.; Lazareviić-Pašti, T. Non-Thermal Plasma Needle as an e Ff Ective Tool in Dimethoate Removal from Water. J. Environ. Manag. 2019, 246, 63–70. [Google Scholar] [CrossRef]
- Giardina, A.; Tampieri, F.; Biondo, O.; Marotta, E.; Paradisi, C. Air Non-Thermal Plasma Treatment of the Herbicides Mesotrione and Metolachlor in Water. Chem. Eng. J. 2019, 372, 171–180. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Dai, X. Cold Atmospheric Plasma: An Effective Approach for Fast Benazoxystrobin Degradation via Generating Reactive Oxygen Species. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Dang, J.; Wang, S.; Liu, Z.; Fang, J. Reduction of Phoxim Pesticide Residues from Grapes by Atmospheric Pressure Non-Thermal Air Plasma Activated Water. J. Hazard. Mater. 2019, 377, 98–105. [Google Scholar] [CrossRef]
- Gianella, M.; Reuter, S.; Press, S.A.; Schmidt-Bleker, A.; van Helden, J.H.; Ritchie, G.A.D. HO2 Reaction Kinetics in an Atmospheric Pressure Plasma Jet Determined by Cavity Ring-down Spectroscopy. Plasma Sources Sci. Technol. 2018, 27, 095013. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of Plasma-Activated Water (PAW) as a Novel Disinfectant: Effectiveness on Escherichia Coli and Listeria Innocua, Physicochemical Properties, and Storage Stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Ng, S.W.; Slikboer, E.; Dickenson, A.; Walsh, J.L.; Lu, P.; Boehm, D.; Bourke, P. Characterization of an Atmospheric Pressure Air Plasma Device under Different Modes of Operation and Their Impact on the Liquid Chemistry. J. Appl. Phys. 2021, 129, 123303. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, X.; Zhao, Z.; Wang, W.; Yang, D.; Yuan, H. Reactive Oxygen and Nitrogen Species in Ar + N2 + O2 Atmospheric-Pressure Nanosecond Pulsed Plasmas in Contact with Liquid. Phys. Plasmas 2019, 26, 023521. [Google Scholar] [CrossRef]
- Ma, M.; Duan, J.; Lu, X.; He, G. Genotoxic and Mutagenic Properties of Atmospheric Pressure Plasma Jet on Human Liver Cell Line L02. Phys. Plasmas 2019, 26, 023523. [Google Scholar] [CrossRef]
- Daniel Cortázar, O.; Megía-Macías, A.; Moreno, S.; Brun, A.; Gómez-Casado, E. Vulnerability of SARS-CoV-2 and PR8 H1N1 Virus to Cold Atmospheric Plasma Activated Media. Sci. Rep. 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Mahnot, N.K.; Mahanta, C.L. Tender Coconut Water Processing: Hurdle Approach, Quality, and Accelerated Shelf-Life Measurements. J. Food Meas. Charact. 2021, 16, 102–113. [Google Scholar] [CrossRef]
- Griseti, E.; Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Rols, M.-P.; Yousfi, M.; Merbahi, N.; Golzio, M. Pulsed Electric Field Treatment Enhances the Cytotoxicity of Plasma-Activated Liquids in a Three-Dimensional Human Colorectal Cancer Cell Model. Sci. Rep. 2019, 9, 7583. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving Reactive Species Specificity within Plasma-Activated Water through Selective Generation Using Air Spark and Glow Discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, K.; Nakamura, T. Comparative Study of Discharge Schemes for Production Rates and Ratios of Reactive Oxygen and Nitrogen Species in Plasma Activated Water. J. Phys. D Appl. Phys. 2019, 52, 385202. [Google Scholar] [CrossRef]
- Tarabová, B.; Lukeš, P.; Janda, M.; Hensel, K.; Šikurová, L.; Machala, Z. Specificity of Detection Methods of Nitrites and Ozone in Aqueous Solutions Activated by Air Plasma. Plasma Process. Polym. 2018, 15, e1800030. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current Status and Future Prospects of Agricultural Applications Using Atmospheric-Pressure Plasma Technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-Thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-Activation of Tap Water Using DBD for Agronomy Applications: Identification and Quantification of Long Lifetime Chemical Species and Production/Consumption Mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum Sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Hosseini, S.I.; Mohsenimehr, S.; Hadian, J.; Ghorbanpour, M.; Shokri, B. Physico-Chemical Induced Modification of Seed Germination and Early Development in Artichoke (Cynara Scolymus L.) Using Low Energy Plasma Technology. Phys. Plasmas 2018, 25, 013525. [Google Scholar] [CrossRef]
- Porto, C.L.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma Activated Water and Airborne Ultrasound Treatments for Enhanced Germination and Growth of Soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; de Dieu Muhire, J.; et al. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Kim, T.H.; Choe, W.; Jo, C. Effect of Atmospheric Pressure Dielectric Barrier Discharge Plasma on the Biological Activity of Naringin. Food Chem. 2014, 160, 241–245. [Google Scholar] [CrossRef]
- Almarashi, J.Q.M.; El-Zohary, S.E.; Ellabban, M.A.; Abomohra, A.E.-F. Enhancement of Lipid Production and Energy Recovery from the Green Microalga Chlorella Vulgaris by Inoculum Pretreatment with Low-Dose Cold Atmospheric Pressure Plasma (CAPP). Energy Convers. Manag. 2019, 204, 112314. [Google Scholar] [CrossRef]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The Use of Atmospheric Pressure Plasma as a Curing Process for Canned Ground Ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Lim, Y.; Choe, W.; Yong, H.I.; Jo, C. Direct Infusion of Nitrite into Meat Batter by Atmospheric Pressure Plasma Treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 113–118. [Google Scholar] [CrossRef]
- Yong, H.I.; Han, M.; Kim, H.J.; Suh, J.Y.; Jo, C. Mechanism Underlying Green Discolouration of Myoglobin Induced by Atmospheric Pressure Plasma. Sci. Rep. 2018, 8, 9790. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of Cold Atmospheric Gas Phase Plasma on Anthocyanins and Color in Pomegranate Juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold Atmospheric Plasma Manipulation of Proteins in Food Systems. Crit. Rev. Food Sci. Nutr. 2017, 58, 2583–2597. [Google Scholar] [CrossRef] [Green Version]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold Plasma Technologies: Their Effect on Starch Properties and Industrial Scale-up for Starch Modification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Yepez, X.V.; Keener, K.M. High-Voltage Atmospheric Cold Plasma (HVACP) Hydrogenation of Soybean Oil without Trans-Fatty Acids. Innov. Food Sci. Emerg. Technol. 2016, 38, 169–174. [Google Scholar] [CrossRef]
- Yepez, X.V.; Baykara, H.; Xu, L.; Keener, K.M. Cold Plasma Treatment of Soybean Oil with Hydrogen Gas. JAOCS J. Am. Oil Chem. Soc. 2021, 98, 103–113. [Google Scholar] [CrossRef]
- Food and Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1563 (accessed on 10 June 2022).


| Target | Product | Description/Results | Equipment | Processing Conditions | Quality | References |
|---|---|---|---|---|---|---|
| Salmonella spp. | Eggs | Spot inoculation, initial load 8.6 log CFU/cm2, reduction 4 log CFU/cm2@40 s | Arc plasma (APPL-10k Taiwan) | Air, RH * 65%, 12 V/9 A/24,000 W, T < 50C, 0–40 s | Texture, color, pH, acid value, T-bars, fatty acid profile. Not affected | [8] |
| Wheat | Mist inoculation, reduction of 4.4 log CFU/g@20 min | DBD plasma, variac/step-up transformer | 44 kV, 56.6 W, 60 Hz, air, 0–20 min | NA * | [9] | |
| Meat | Reduction of 4.7 log CFU/cm2, with cold plasma and 200 ppm of peracetic acid | Pulsed DBD plasma (PG 100-3D, Advanced Plasma Solutions, Malvern, PA, USA) | 0–30 kV, 0–2 mA, 3.5 kHz, 0–6 min | Color, moisture content. Affected | [10] | |
| E. coli | Beef jerky | Combined clove oil and ACP treatment reduced 0.9 log CFU/g@15 min, and 7.5 log CFU/mL on media. | Encapsulated DBD plasma | 2.2 kHz, 8.4 kV, 0–15 min | NA | [11] |
| Spinach | Reduction of 3.77 log CFU/sample, after storage for 14 days/5 °C | DBD (Phenix Technologies Inc., Accident, MD, USA) | 90 kV, 60 Hz, 85% RH, nitrogen gas, 0–5 min | Texture, moisture, color. Not affected | [12] | |
| Coconut water | Reduction of 5 log@2 min, combined ACP and ascorbic acid | DBD Plasma | 90 kV, 60 Hz, 65% O2–30% CO2–5%N2, 0–2 min | Reduced pH, color. Total soluble solids and acidity, not affected. | [13] | |
| L. monocytogenes | Radicchio | Reduction 2.2 log CFU/cm2@30 min, after 3 days of storage at 4 °C | DBD Plasma | 15 kV, 12.5 KHz, 60% RH, air speed 1.5m/s, 0–30 min | Antioxidant activity not affected. Color and sensory analysis. | [14] |
| Aflatoxin | Peanuts | Reduction 38%, increase temperature to 78C@2 min | Plasma jet surface treatment | 4.4 kV, 70–90 kHz, 650 W, air flow 107 L/min, 0–2 min | Peroxide value, free fatty acid content, acidity value, oxidative stability index. | [15] |
| Milk | Reduction 78.9% Aflatoxin M1@20 min | DBD plasma BK-130 (Phenix Technologies, Accident, MD, USA) | 80 kV, 60 Hz, 200 w. Gas: 65% O2–30% CO2–5%N2, 18–22 °C, 0–5 min | Color not affected. pH affected. | [16] | |
| Fumonisin | Maize | Reduction 64%@10 min | Plasma jet | 6 kV, 20 kHz, mixture of oxygen and 0.75% helium gas, 0–10 min | NA | [17] |
| Ochratoxin | Date palm | Reduction of 24.83 ug/100 mm2 with@7.5 min | Plasma jet | 25 kV, 25 kHz, 0–9 min | NA | [18] |
| Malathion Chlorpyrifos | Lettuce | Degradation 64.6% of malathion, 62.7% chlorpyrifos@180 s | DBD (Phenix Technologies, Accident, MD, USA) | 80 kV, 50 Hz, air, 0–180 s | Color and chlorophyll content not affected. Ascorbic acid content. | [19] |
| Benazoxystrobin | Water | Degradation 90%@60 V/9 min | DBD, power supply (CTP-2000 K), axial flow reactor | 8.8 kHz, 30–60 V, oxygen gas, 0–9 min | NA | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepez, X.; Illera, A.E.; Baykara, H.; Keener, K. Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods 2022, 11, 1833. https://doi.org/10.3390/foods11131833
Yepez X, Illera AE, Baykara H, Keener K. Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods. 2022; 11(13):1833. https://doi.org/10.3390/foods11131833
Chicago/Turabian StyleYepez, Ximena, Alba E. Illera, Haci Baykara, and Kevin Keener. 2022. "Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing" Foods 11, no. 13: 1833. https://doi.org/10.3390/foods11131833
APA StyleYepez, X., Illera, A. E., Baykara, H., & Keener, K. (2022). Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods, 11(13), 1833. https://doi.org/10.3390/foods11131833