The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Determination of Thawing Loss and Cooking Loss
2.4. Color Measurement
2.5. pH Value
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Raman Spectroscopy Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Thawing and Cooking Loss
3.2. pH Value
3.3. Color
3.4. Thiobarbituric Acid Reactive Substances
3.5. Protein Secondary Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liston, J. Microbiology in fishery science. In Advances in Fish Science and Technology; Fishing News Books: Farnham, UK, 1980; pp. 138–157. [Google Scholar]
- Fan, W.; Chi, Y.; Zhang, S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ramaswamy, H.S.; Simpson, B.K. Effect of high-pressure versus conventional thawing on color, drip loss and texture of Atlantic salmon frozen by different methods. Lebensm.-Wiss. Und-Technol. Food Sci. Technol. 2004, 37, 291–299. [Google Scholar] [CrossRef]
- Zhu, S.M.; Le Bail, A.; Ramaswamy, H.S. Ice crystal formation in pressure shift freezing of atlantic salmon (Salmo salar) as compared to classical freezing methods. J. Food Process. Preserv. 2003, 27, 427–444. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, Z.; Wang, C.; Hu, L.; Ramaswamy, H.S.; Zhu, S. Melting endothermic technique for establishing different phase diagram pathways during high pressure treatment of liquid foods. Innov. Food Sci. Emerg. Technol. 2020, 62, 102361. [Google Scholar] [CrossRef]
- Otero, L.; Sanz, P.; Guignon, B.; Sanz, P.D. Pressure–shift nucleation: A potential tool for freeze concentration of fluid foods. Innov. Food Sci. Emerg. Technol. 2012, 13, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Van Buggenhout, S.; Messagie, I.; Van Loey, A.; Hendrickx, M. Influence of low-temperature blanching combined with high-pressure shift freezing on the texture of frozen carrots. J. Food Sci. 2005, 70, S304–S308. [Google Scholar] [CrossRef]
- Su, G.; Ramaswamy, H.S.; Zhu, S.; Yu, Y.; Hu, F.; Xu, M. Thermal characterization and ice crystal analysis in pressure shift freezing of different muscle (shrimp and porcine liver) versus conventional freezing method. Innov. Food Sci. Emerg. Technol. 2014, 26, 40–50. [Google Scholar] [CrossRef]
- Zhu, S.M.; Ramaswamy, H.S.; Le Bail, A. Ice-crystal formation in gelatin gel during pressure shift versus conventional freezing. J. Food Eng. 2005, 66, 69–76. [Google Scholar] [CrossRef]
- Martino, M.N.; Otero, L.; Sanz, P.D.; Zaritzky, N.E. Size and location of ice crystals in pork frozen by high-pressure-assisted freezing as compared to classical methods. Meat Sci. 1998, 50, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Otero, L.; Martino, M.; Zaritzky, N.; Solas, M.; Sanz, P.D. Preservation of microstructure in peach and mango during high-pressure–shift freezing. J. Food Sci. 2000, 65, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.-P.; Choi, M.-J. Comparison of the quality characteristics of abalone processed by high-pressure sub-zero temperature and pressure–shift freezing. Innov. Food Sci. Emerg. Technol. 2016, 33, 19–25. [Google Scholar] [CrossRef]
- Park, S.H.; Hong, G.P.; Min, S.G.; Choi, M.J. Combined High Pressure and Subzero Temperature Phase Transition on the Inactivation of Escherichia coli ATCC 10536. Int. J. Food Eng. 2008, 4, 8. [Google Scholar] [CrossRef]
- Picart, L.; Dumay, E.; Guiraud, J.P.; Cheftel, J.C. Combined high pressure-sub-zero temperature processing of smoked salmon mince: Phase transition phenomena and inactivation of Listeria innocua. J. Food Eng. 2005, 68, 43–56. [Google Scholar] [CrossRef]
- Prestamo, G.; Pedrazuela, A.; Guignon, B.; Sanz, P.D. Synergy between high-pressure, temperature and ascorbic acid on the inactivation of Bacillus cereus. Eur. Food Res. Technol. 2007, 225, 693–698. [Google Scholar] [CrossRef]
- Fernandez, P.P.; Sanz, P.D.; Molina-Garcia, A.D.; Otero, L.; Guignon, B.; Vaudagna, S.R. Conventional freezing plus high pressure-low temperature treatment: Physical properties, microbial quality and storage stability of beef meat. Meat Sci. 2007, 77, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Sanz, P.D.; Guignon, B.; Aparido, C. Experimental determination of the amount of ice instantaneously formed in high-pressure shift freezing. J. Food Eng. 2009, 95, 670–676. [Google Scholar] [CrossRef]
- Li, T.; Xiao, T.; Zheng, Z.; Li, Y.; Zhu, S.; Ramaswamy, H.S.; Hu, L.; Yu, Y. Facilitating high pressure phase-transition research and kinetics studies at subzero temperatures using self-cooling laboratory units. Food Res. Int. 2022, 151, 110857. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Lu, W.; Shen, H.; Luo, Y. Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control 2011, 22, 1197–1202. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Li, X.; Wei, Y.; Li, J. Effect of ultrasonic-assisted immersion freezing and quick-freezing on quality of sea bass during frozen storage. LWT-Food Sci. Technol. 2021, 154, 112737. [Google Scholar] [CrossRef]
- DenHertogMeischke, M.J.A.; VanLaack, R.; Smulders, F.J.M. The water-holding capacity of fresh meat. Vet. Q. 1997, 19, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M. The influence of superchilling storage methods on the location/distribution of ice crystals during storage of Atlantic salmon (Salmo salar). Food Control 2015, 52, 19–26. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Tironi, V.; Lebail, A.; De Ilamballerie, M. Effects of pressure–shift freezing and pressure-assisted thawing on sea bass (Dicentrarchus labrax) quality. J. Food Sci. 2007, 72, C381–C387. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Saha, A.; Xiong, R.; Owens, C.M.; Meullenet, J.F. Changes in broiler breast fillet tenderness, water-holding capacity, and color attributes during long-term frozen storage. J. Food Sci. 2008, 73, E162–E168. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Chung, Y.B.; Park, S.H.; Kim, J.S.; Chun, H.H. Effect of tempering methods on quality changes of pork loin frozen by cryogenic immersion. Meat Sci. 2017, 124, 69–76. [Google Scholar] [CrossRef]
- Choi, M.-J.; Min, S.-G.; Hong, G.-P. Effects of pressure–shift freezing conditions on the quality characteristics and histological changes of pork. LWT-Food Sci. Technol. 2016, 67, 194–199. [Google Scholar] [CrossRef]
- Han-Jun, M.; Ledward, D.A. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 2004, 68, 347–355. [Google Scholar]
- Khan, M.A.; Parrish, C.C.; Shahidi, F. Quality indicators of cultured Newfoundland blue mussels (Mytilus edulis) during storage on ice: Microbial growth, pH, lipid oxidation, chemical composition characteristics, and microbial fatty acid contents. J. Agric. Food Chem. 2005, 53, 7067–7073. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W.; Li, X. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2013, 138, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhao, S.; Han, X.; Guan, W.; Liu, B.; Chen, A.; Sun, Y.; Wang, J. Effect of static magnetic field extended supercooling preservation on beef quality. Food Chem. 2022, 370, 131264. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT-Food Sci. Technol. 2014, 59, 122–129. [Google Scholar] [CrossRef]
- Grunert, K.G.; Bredahl, L.; Brunso, K. Consumer perception of meat quality and implications for product development in the meat sector—A review. Meat Sci. 2004, 66, 259–272. [Google Scholar] [CrossRef]
- Zhu, S.M.; Le Bail, A.; Chapleau, N.; Ramaswamy, H.S.; de Lamballerie-Anton, M. Pressure shift freezing of pork muscle: Effect on color, drip loss, texture, and protein stability. Biotechnol. Prog. 2004, 20, 939–945. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. Lebensm.-Wiss. Und-Technol. Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef]
- Hernandez Saluena, B.; Saenz Gamasa, C.; Dineiro Rubial, J.M.; Alberdi Odriozola, C. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Ines lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef]
- Rubio, B.; Martinez, B.; Dolores Garcia-Cachan, M.; Rovira, J.; Jaime, I. Effect of the packaging method and the storage time on lipid oxidation and colour stability on dry fermented sausage salchichon manufactured with raw material with a high level of mono and polyunsaturated fatty acids. Meat Sci. 2008, 80, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Thanasak, S.-l.; Soottawat, B.; Nalan, G.; Sitthipong, N. Changes in lipids and fishy odour development in skin from Nile tilapia (Oreochromis niloticus) stored in ice. Food Chem. 2013, 141, 2466–2472. [Google Scholar]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze-thaw cycles. Food Chem. 2001, 72, 207–217. [Google Scholar] [CrossRef]
- Bello, R.A.; Luft, J.H.; Pigott, G.M. Ultrastructural—Study of skeletal fish muscle after freezing at different rates. J. Food Sci. 1982, 47, 1389–1394. [Google Scholar] [CrossRef]
- Sequeira-Munoz, A.; Chevalier, D.; Simpson, B.K.; Le Bail, A.; Ramaswamy, H.S. Effect of pressure–shift freezing versus air-blast freezing of carp (Cyprinus carpio) fillets: A storage study. J. Food Biochem. 2005, 29, 504–516. [Google Scholar] [CrossRef]
- Herrero, A.M.; Cambero, M.I.; Ordonez, J.A.; De la Hoz, L.; Carmona, P. Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics. Food Chem. 2008, 109, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Zhang, W.; Cao, A.; Cao, M.; Li, J. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum). Ultrason. Sonochem. 2019, 55, 96–104. [Google Scholar] [CrossRef]
- Vanwart, H.E. Biochemical applications of raman and resonance raman spectroscopies—Carey, pr. J. Am. Chem. Soc. 1984, 106, 6463. [Google Scholar]
- Xiong, G.; Han, M.; Kang, Z.; Zhao, Y.; Xu, X.; Zhu, Y. Evaluation of protein structural changes and water mobility in chicken liver paste batters prepared with plant oil substituting pork back-fat combined with pre-emulsification. Food Chem. 2016, 196, 388–395. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Chang, T.; Wang, C.; Yang, H.; Xiong, S.; Liu, Y.; Liu, R. Effects of the Acid- and Alkali-Aided Processes on Bighead Carp (Aristichthys nobilis) Muscle Proteins. Int. J. Food Prop. 2016, 19, 1863–1873. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Li, H.; Wang, Z.; Emara, A.M.; Hu, Y.; He, Z. Effects of in vitro oxidation on myofibrillar protein charge, aggregation, and structural characteristics. Food Chem. 2020, 332, 127396. [Google Scholar] [CrossRef] [PubMed]
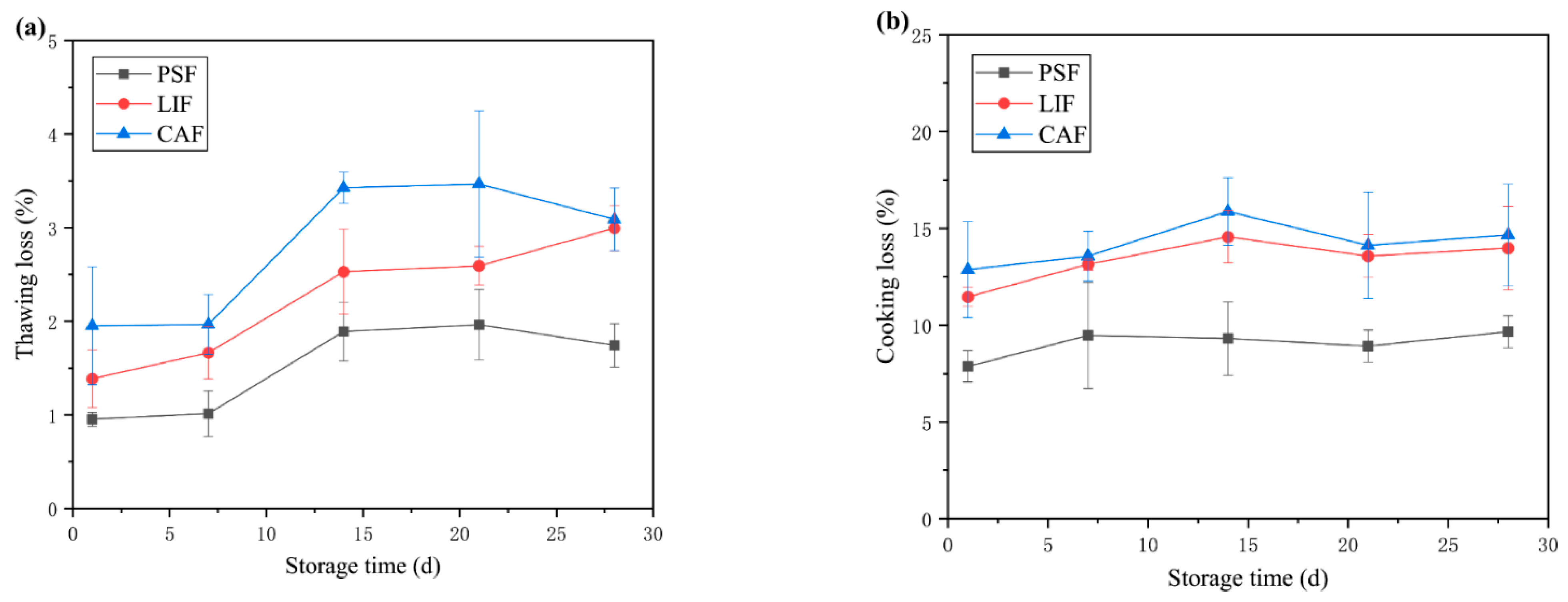
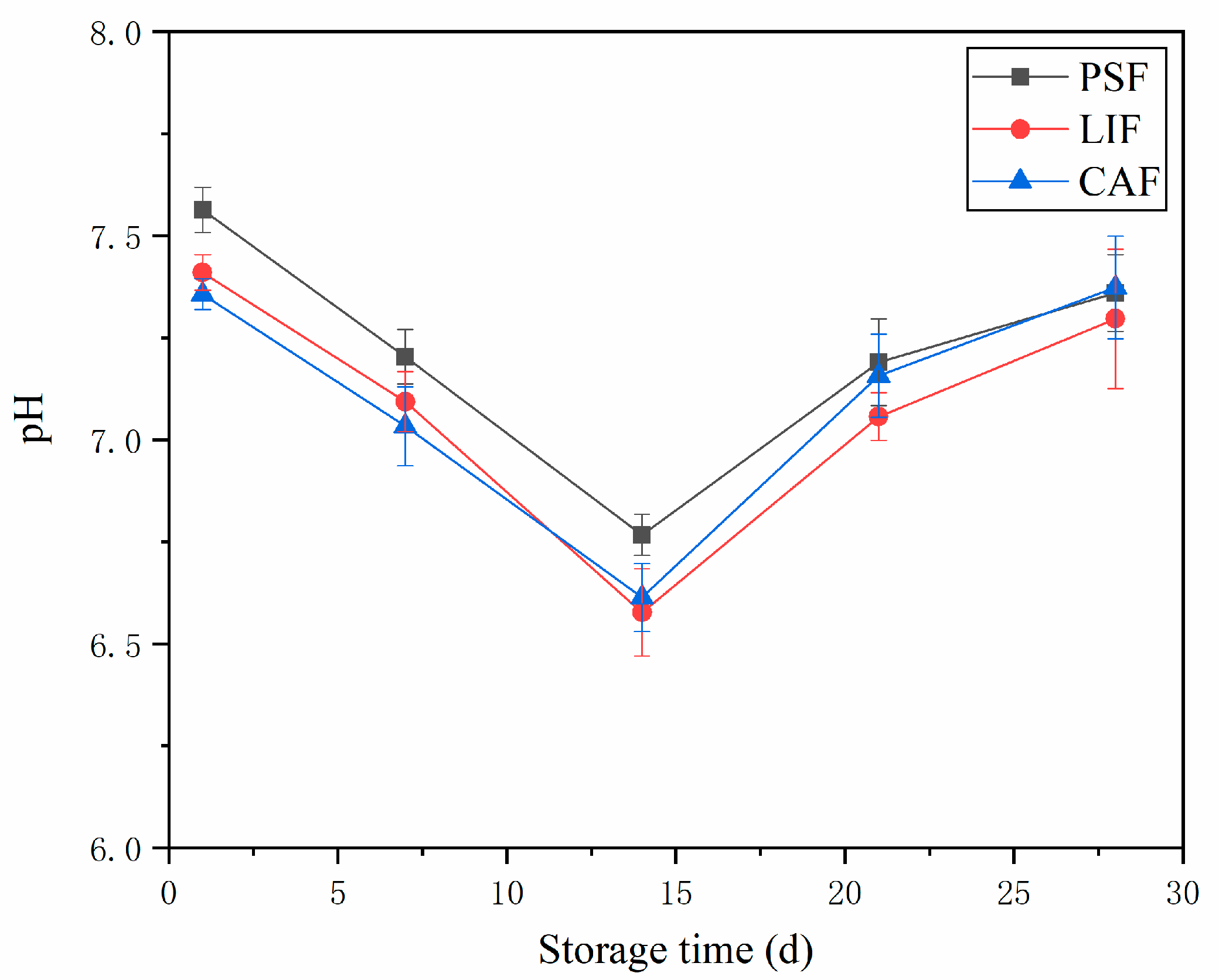
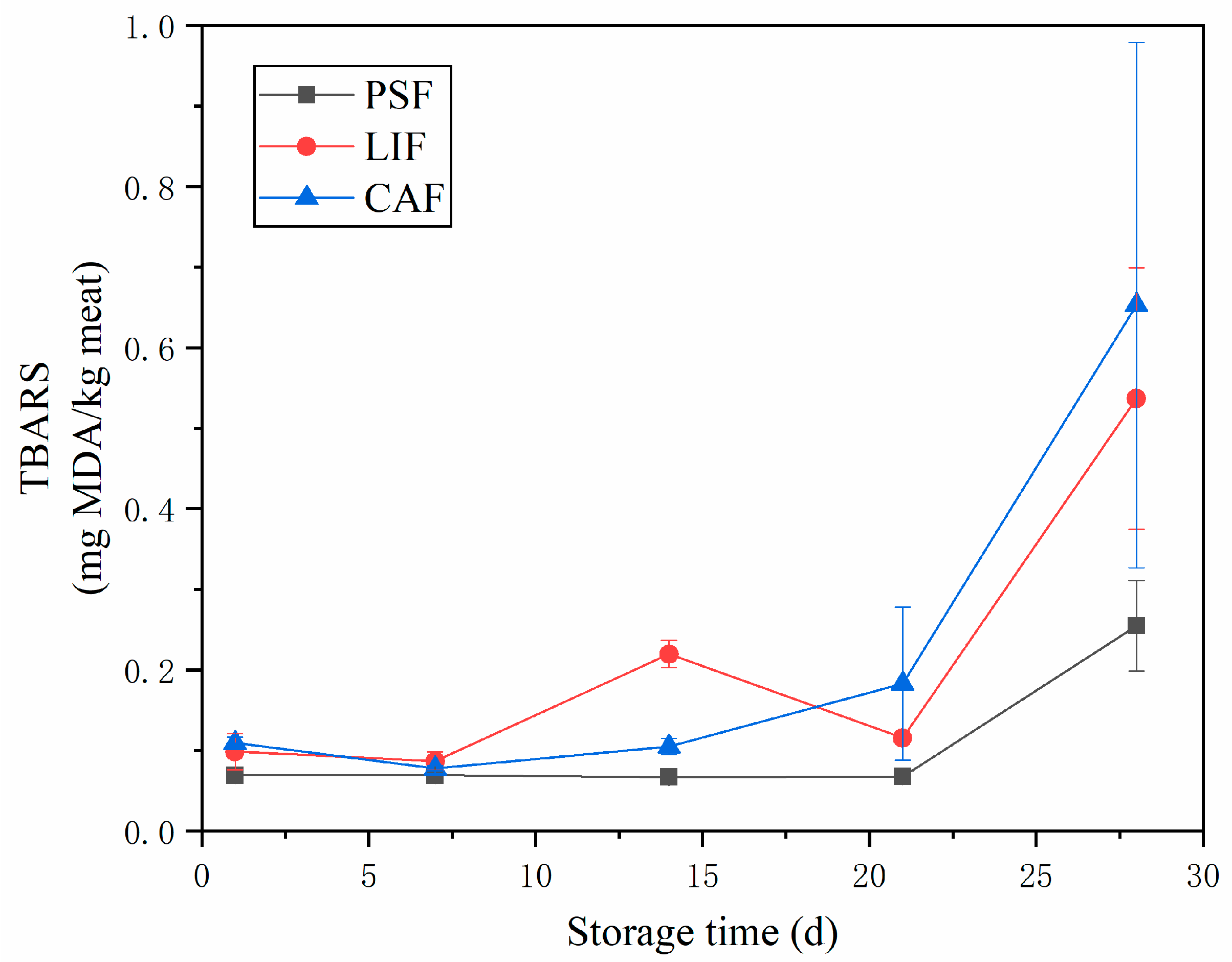
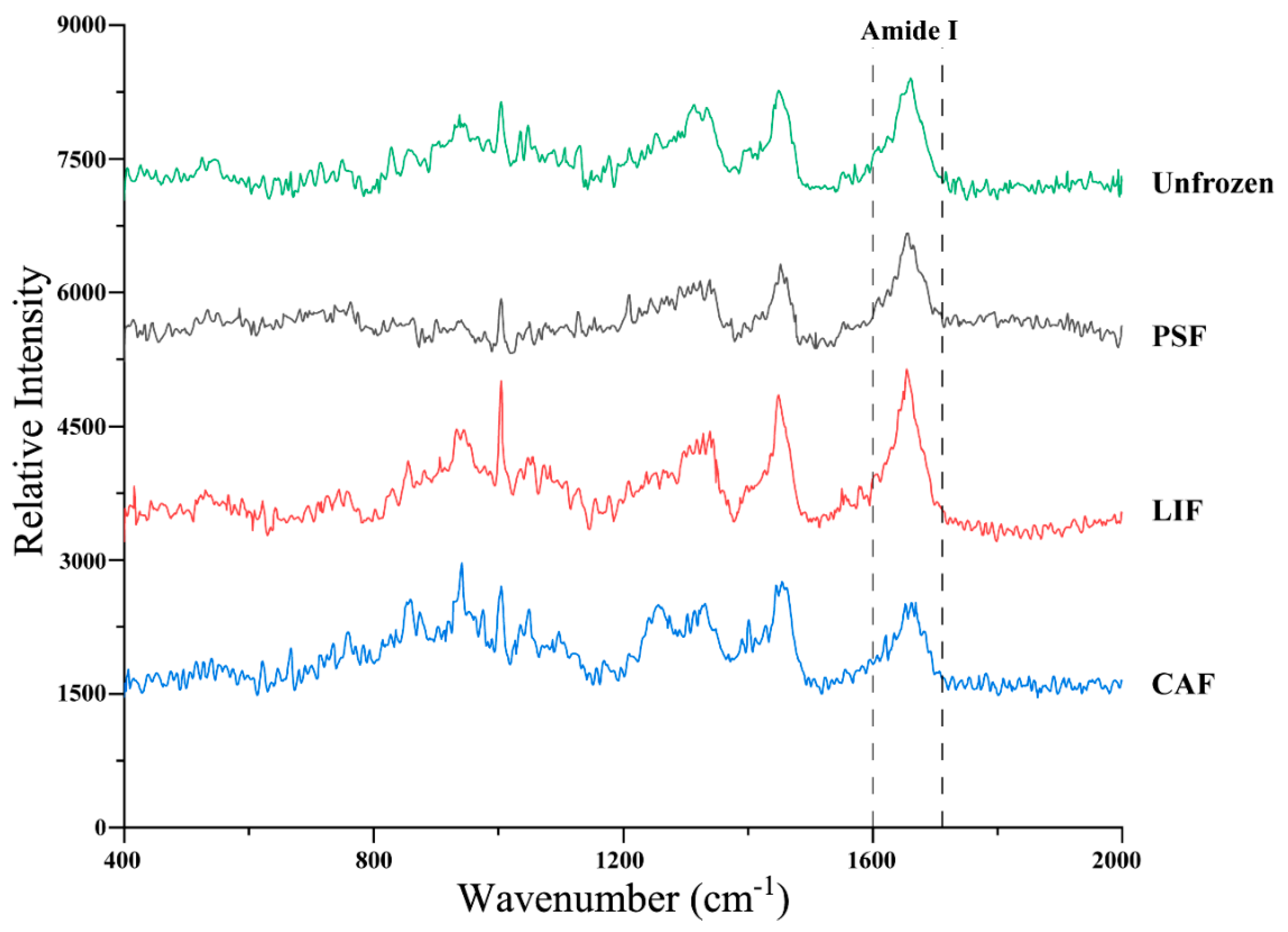
| Treatments | Time (Days) | L* | a* | b* |
|---|---|---|---|---|
| PSF | 1 | 38.8 ± 2.19 a | −3.61±0.33 a | −1.35 ± 0.69 a |
| 7 | 38.5 ± 0.91 a | −3.38 ± 0.12 a | −0.74 ± 0.56 a | |
| 14 | 40.2 ± 2.81 a,b | −3.80 ± 0.46 a | −0.07 ± 0.74 a,b | |
| 21 | 42.1 ± 2.66 a,b | −3.47 ± 0.52 a | 0.39 ± 0.25 a,b | |
| 28 | 43.8 ± 3.33 b | −3.71 ± 0.14 a | 1.15 ± 1.66 b | |
| LIF | 1 | 36.3 ± 2.45 a | −2.95 ± 0.49 a | −1.66 ± 0.27 a |
| 7 | 38.8 ± 1.75 a,b | −3.32 ± 0.09 a | −1.48 ± 0.53 a | |
| 14 | 38.9 ± 0.80 a,b | −3.15 ± 0.27 a | −0.50 ± 0.99 a,b | |
| 21 | 40.8 ± 1.00 b | −3.23 ± 0.02 a | 0.54 ± 0.69 b | |
| 28 | 44.2 ± 0.61 c | −3.39 ± 0.23 a | 1.13 ± 1.63 b | |
| CAF | 1 | 37.0 ± 0.58 a | −3.13 ± 0.50 a | −0.89 ± 0.14 a |
| 7 | 39.59 ± 0.26 b | −3.46 ± 0.27 a | −0.49 ± 0.53 a | |
| 14 | 39.4 ± 0.48 b | −3.44 ± 0.18 a | −0.97 ± 0.20 a | |
| 21 | 41.2 ± 1.16 c | −3.21 ± 0.37 a | −0.49 ± 0.79 a | |
| 28 | 43.8 ± 1.20 d | −3.52 ± 0.19 a | 0.43 ± 1.39 a |
| Secondary Structure Content (%) | α-Helix | β-Sheet | β-Turn | Random Coil |
|---|---|---|---|---|
| Unfrozen | 59.3 ± 7.22 a | 23.7 ± 5.20 a | 6.14 ± 2.61 a | 10.8 ± 2.68 a |
| PSF | 48.5 ± 3.43 b | 26.5 ± 6.78 a | 11.2 ± 3.47 a | 13.8 ± 3.36 a |
| LIF | 39.1 ± 2.35 c | 29.9 ± 3.71 a | 11.4 ± 3.06 a | 19.7 ± 3.19 b |
| CAF | 33.4 ± 4.21 c | 24.2 ± 3.76 a | 20.3 ± 3.41 b | 22.1 ± 3.16 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Kuang, S.; Xiao, T.; Hu, L.; Nie, P.; Ramaswamy, H.S.; Yu, Y. The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage. Foods 2022, 11, 1842. https://doi.org/10.3390/foods11131842
Li T, Kuang S, Xiao T, Hu L, Nie P, Ramaswamy HS, Yu Y. The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage. Foods. 2022; 11(13):1842. https://doi.org/10.3390/foods11131842
Chicago/Turabian StyleLi, Ting, Shiyao Kuang, Ting Xiao, Lihui Hu, Pengcheng Nie, Hosahalli S. Ramaswamy, and Yong Yu. 2022. "The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage" Foods 11, no. 13: 1842. https://doi.org/10.3390/foods11131842
APA StyleLi, T., Kuang, S., Xiao, T., Hu, L., Nie, P., Ramaswamy, H. S., & Yu, Y. (2022). The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage. Foods, 11(13), 1842. https://doi.org/10.3390/foods11131842








