Effects of Drying Methods on Serum Protein Powder Properties
Abstract
:1. Introduction
2. Materials and Methods
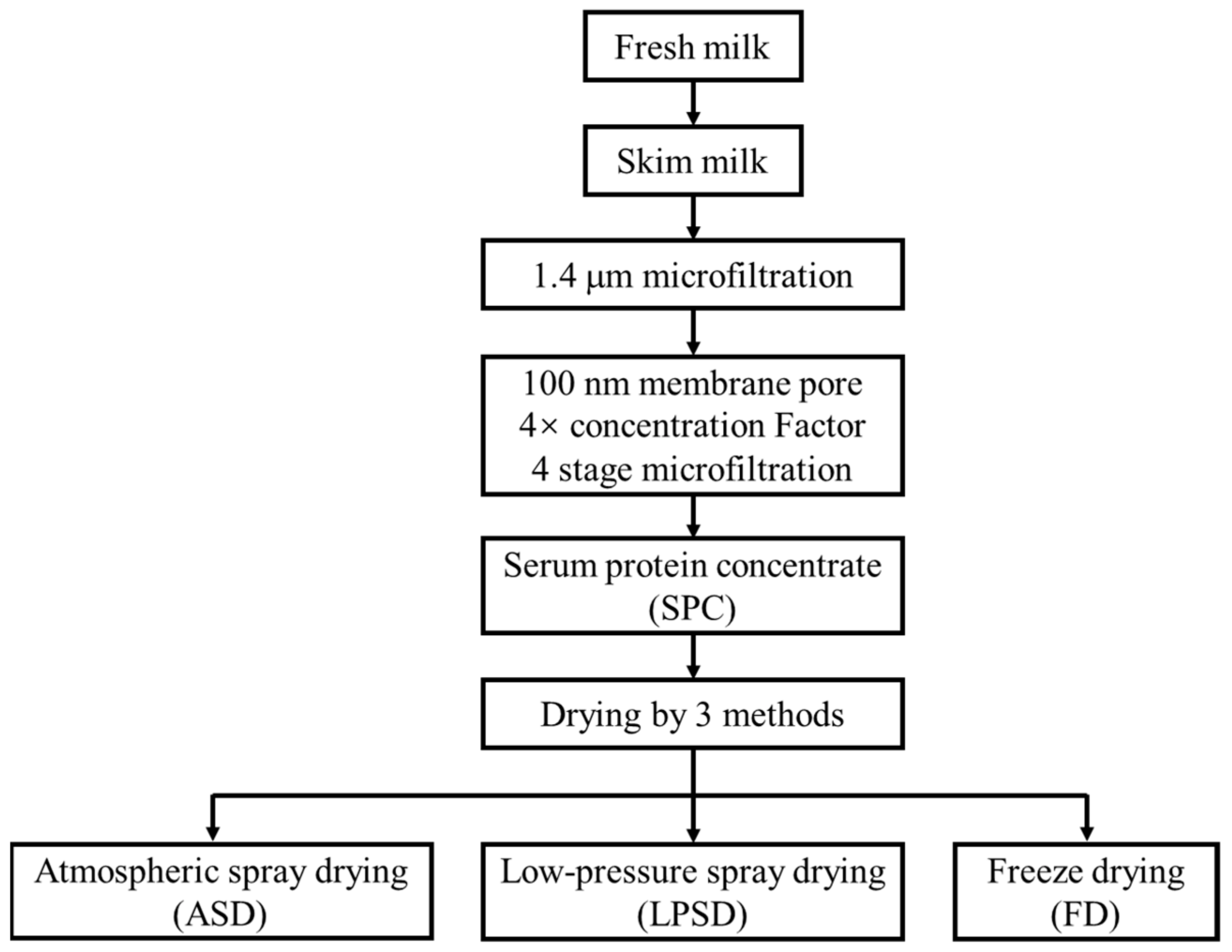
2.1. Manufacture of Serum Protein Powder
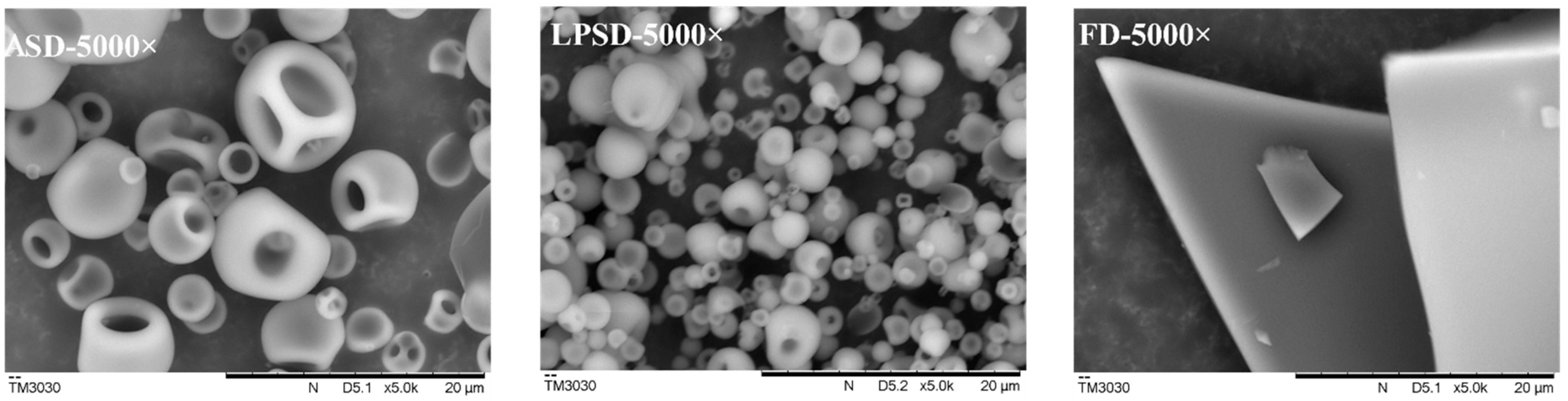
2.2. Microstructure of Serum Protein Powder
2.3. Basic Characteristics of Serum Protein Powder
2.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Protein Analysis by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. LC-MS/MS Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physiochemical Properties of Serum Protein Powder
3.2. Electrophoresis of Serum Protein Powders
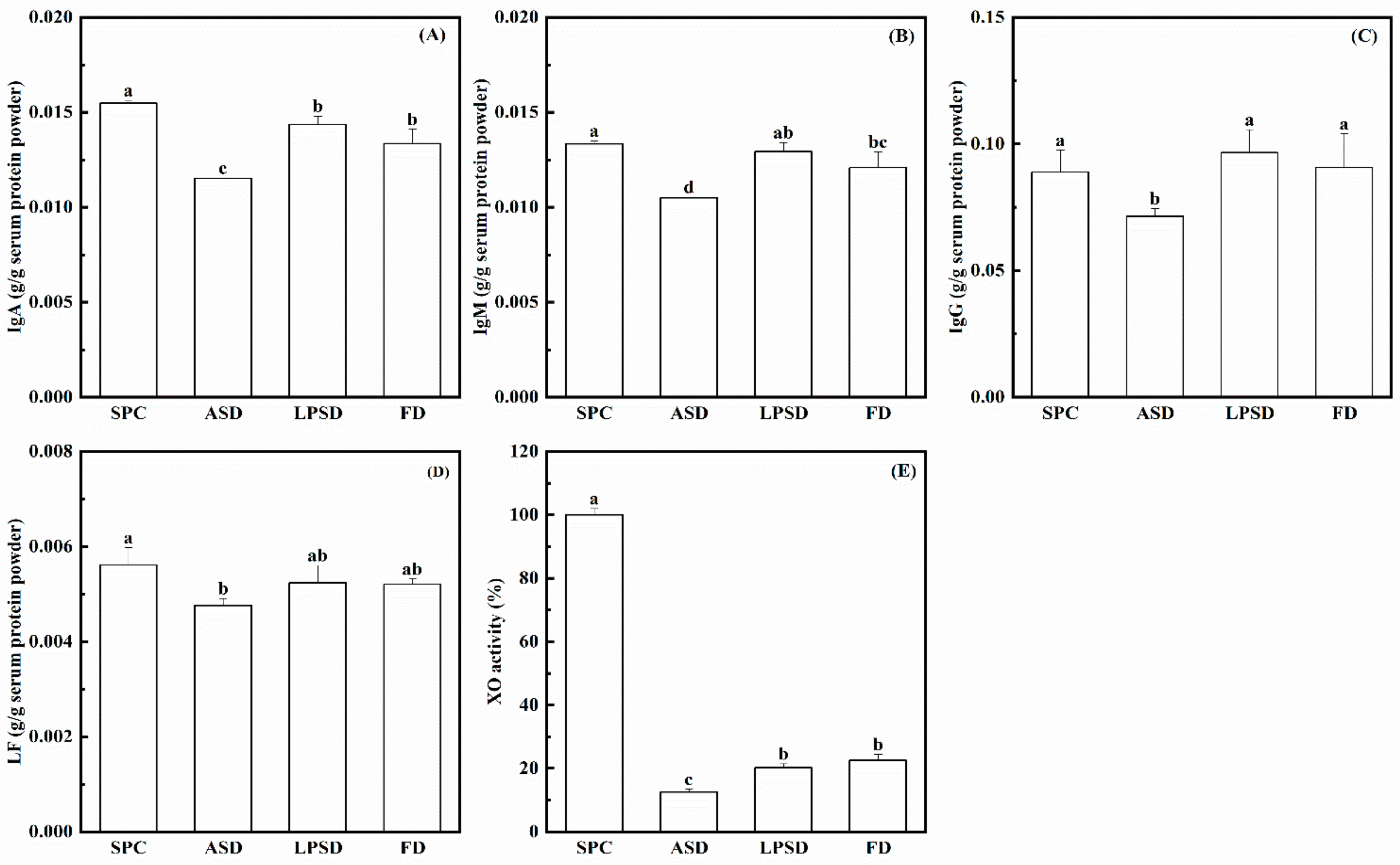
3.3. Concentrations of Proteins in Serum Protein Powder
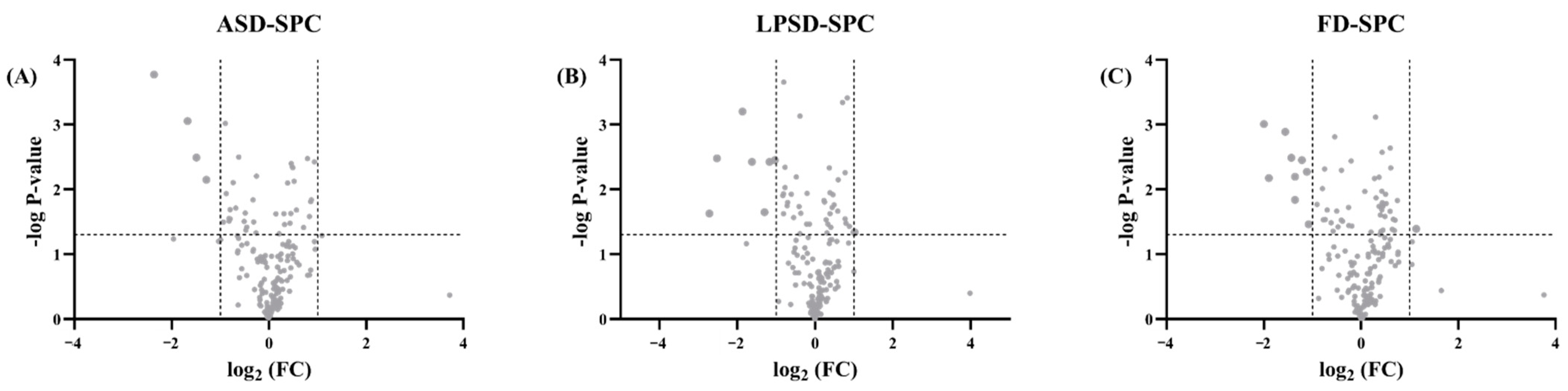
3.4. Proteomics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Whey proteins in infant formula. In Whey Proteins; Elsevier: Amsterdam, The Netherlands, 2019; pp. 439–494. [Google Scholar]
- Borad, S.; Singh, A.; Kapila, S.; Behare, P.; Arora, S.; Sabikhi, L. Influence of unit operations on immunoglobulins and thermal stability of colostrum fractions. Int. Dairy J. 2019, 93, 85–91. [Google Scholar] [CrossRef]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Xu, S.; Zhang, W.; Zhou, P. Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages. Foods 2021, 10, 888. [Google Scholar] [PubMed]
- Kim, S.-H.; Chang, Y.-H.; Kwak, H.-S. Physicochemical properties of reconstituted milk made from freeze-dried milk powder or spray-dried milk powder. Food Sci. Anim. Resour. 2010, 30, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhao, G.; Yan, Y.; Chen, C.; Sun, C.; Zou, X.; Jin, Q.; Wang, X. Effects of freeze drying and spray drying on the microstructure and composition of milk fat globules. RSC Adv. 2016, 6, 2520–2529. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Singh, A.K.; Kumar, D.; Sharma, H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT 2020, 134, 110117. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Yang, T.; Liu, X.; Hemar, Y.; Regenstein, J.M.; Zhou, P. Effects of skim milk pre-acidification and retentate pH-restoration on spray-drying performance, physico-chemical and functional properties of milk protein concentrates. Food Chem. 2019, 272, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Wu, W.D.; Saunders, J.; Chen, X.D. Characteristics of milk powders produced by spray freeze drying. Dry. Technol. 2008, 26, 404–412. [Google Scholar] [CrossRef]
- Claeys, W.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control. 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Han, B.; Zhang, L.; Zhou, P. Changes in bioactive milk serum proteins during milk powder processing. Food Chem. 2020, 314, 126177. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, K.; van Valenberg, H.; De Vries, S.; Boeren, S.; Van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef] [PubMed]
- Garrido, B.C.; Souza, G.H.; Lourenço, D.C.; Fasciotti, M. Proteomics in quality control: Whey protein-based supplements. J. Proteom. 2016, 147, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ram, D.; Romano, B.; Schechter, I. Immunochemical studies on the cercarial-specific calcium binding protein of Schistosoma mansoni. Parasitology 1994, 108, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; McMahon, R.J.; Woo, J.G.; Davidson, B.S.; Morrow, A.L.; Zhang, Q. Temporal changes in milk proteomes reveal developing milk functions. J. Proteome Res. 2012, 11, 3897–3907. [Google Scholar] [CrossRef] [PubMed]
- Contrepas, A.; Walker, J.; Koulakoff, A.; Franek, K.J.; Qadri, F.; Giaume, C.; Corvol, P.; Schwartz, C.E.; Nguyen, G. A role of the (pro) renin receptor in neuronal cell differentiation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R250–R257. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Shirai, K.; Ohta, T.; Kobayashi, J.; Yokoyama, S.; Oikawa, S.; Bujo, H.; Ishibashi, S.; Arai, H.; Yamashita, S.; et al. Diagnosis and management of type I and type V hyperlipoproteinemia. J. Atheroscler. Thromb. 2011, 1111290444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchkovich, N.J.; Maguire, T.G.; Alwine, J.C. Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection. J. Virol. 2010, 84, 7005–7017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, G.; Wieliczko, R.; Pryor, S.; Broadhurst, M.; Wheeler, T.; Haigh, B. The abundance of milk cathelicidin proteins during bovine mastitis. Vet. Immunol. Immunopathol. 2011, 143, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Kerbiriou, M.; Taylor, C.J.; Cozza, G.; Lascu, I.; Postel, E.H.; Cassidy, D.; Trouvé, P.; Mehta, A.; Robson, L.; et al. Role of interaction and nucleoside diphosphate kinase B in regulation of the cystic fibrosis transmembrane conductance regulator function by cAMP-dependent protein kinase A. PLoS ONE 2016, 11, e0149097. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | ASD | LPSD | FD |
|---|---|---|---|
| Total solids | 95.8% ± 0.1% b | 95.0% ± 1.9% b | 98.2% ± 1.9% a |
| Solubility (%) | 98.3 ± 0.6 a | 98.6 ± 1.8 a | 98.1 ± 0.7 a |
| Water activity | 0.169 ± 0.004 b | 0.277 ± 0.007 a | 0.036 ± 0.038 c |
| Fat | ND | ND | ND |
| Lactose | <2% | <2% | <2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Y.; Zhou, P. Effects of Drying Methods on Serum Protein Powder Properties. Foods 2022, 11, 1996. https://doi.org/10.3390/foods11141996
Zhang J, Li Y, Zhou P. Effects of Drying Methods on Serum Protein Powder Properties. Foods. 2022; 11(14):1996. https://doi.org/10.3390/foods11141996
Chicago/Turabian StyleZhang, Jie, Yadi Li, and Peng Zhou. 2022. "Effects of Drying Methods on Serum Protein Powder Properties" Foods 11, no. 14: 1996. https://doi.org/10.3390/foods11141996
APA StyleZhang, J., Li, Y., & Zhou, P. (2022). Effects of Drying Methods on Serum Protein Powder Properties. Foods, 11(14), 1996. https://doi.org/10.3390/foods11141996





