Squalene-Rich Amaranth Oil Pickering Emulsions Stabilized by Native α-Lactalbumin Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Native α-LA Nanoparticles
2.3. Measurements of ζ Potential, Particle Size, and Size Distribution of the α-LA Nanoparticles
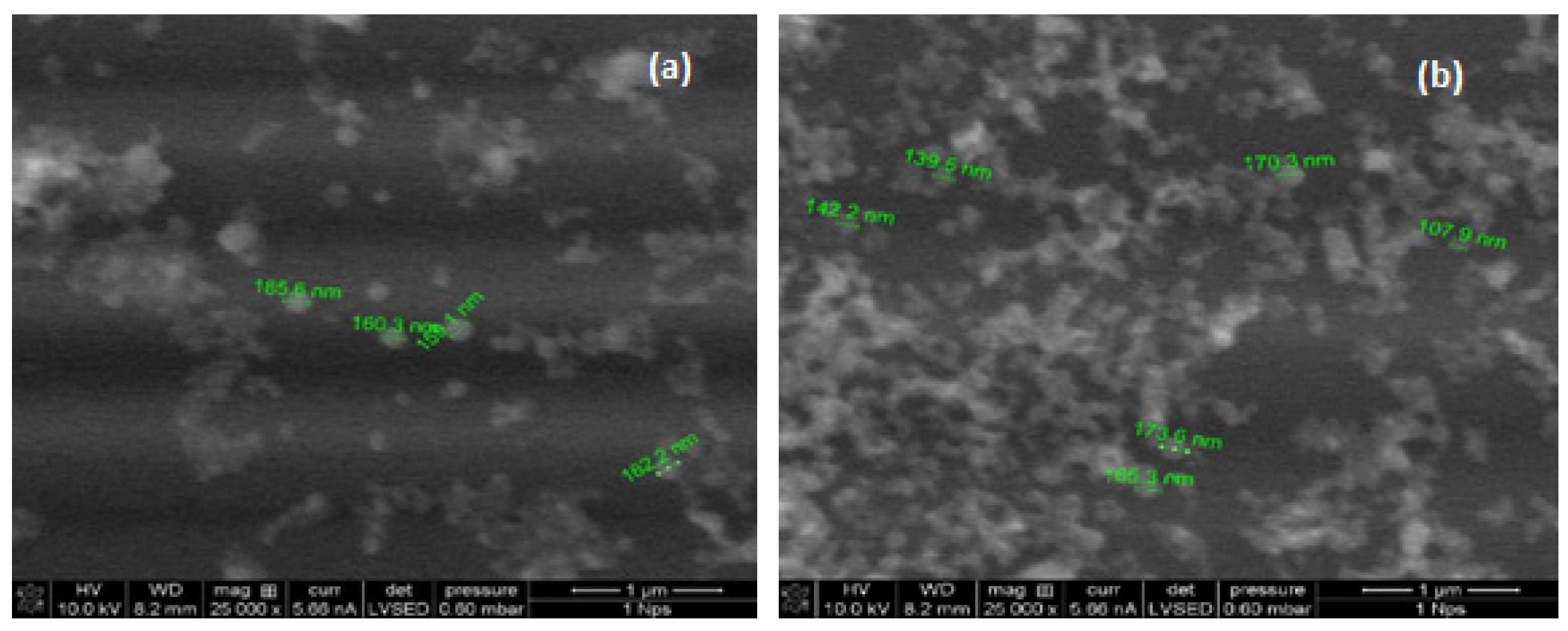
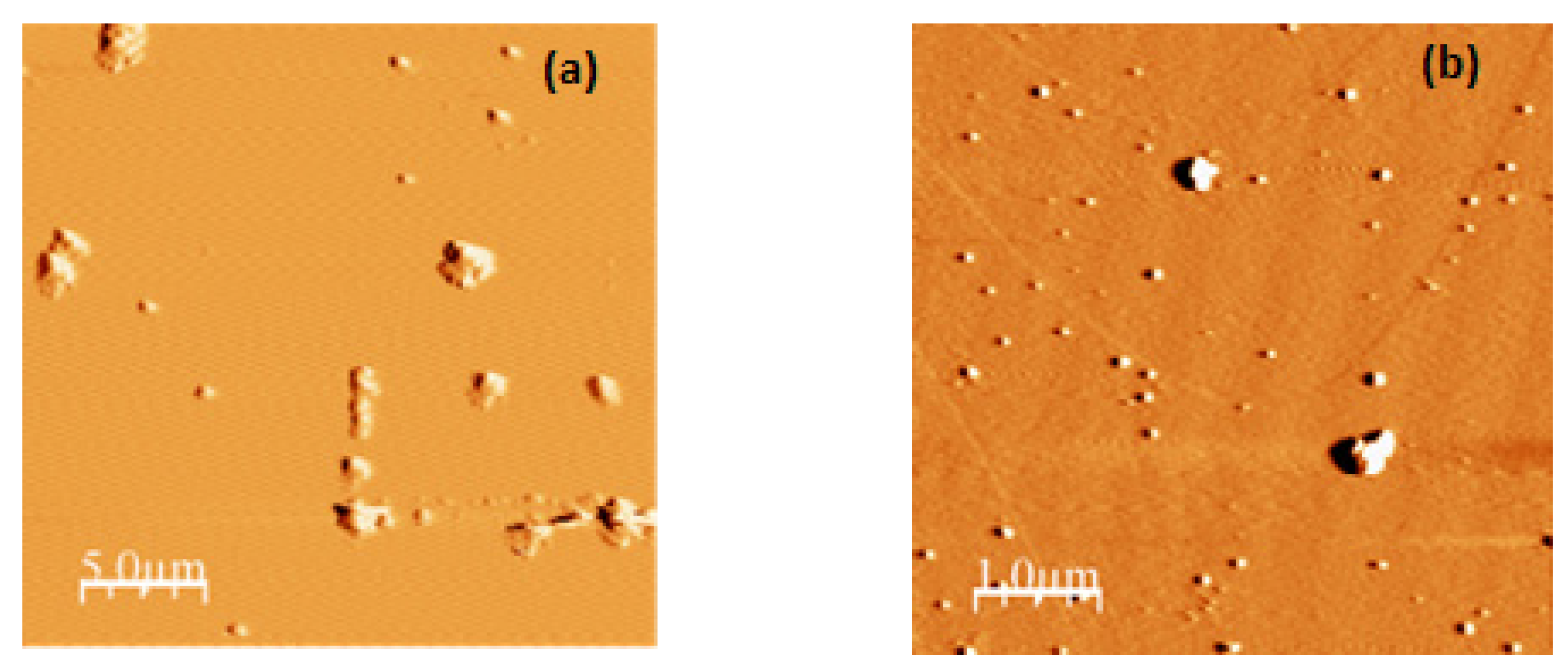
2.4. Microscopy Analysis of the α-LA Nanoparticles
2.5. Preparation of the Pickering Emulsions
2.6. ζ Potential, Particle Size, and Size Distribution of the Pickering Emulsions Droplets
2.7. Emulsion Stability
2.8. Emulsification Efficiency
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of α-LA Nanoparticles
3.2. Droplet Size, Polydispersity Index, and ζ Potential of the Pickering Emulsions
3.3. Confocal Laser Scanning Microscopy (CLSM)
3.4. Stability of Pickering Emulsions Determined by Light Backscattering
3.5. Emulsification Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binks, B.P.; Dezhong, Y. Pickering emulsions stabilized by hydrophilic nanoparticles: In situ surface modification by oil. Soft Matter 2016, 12, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Ridel, L.; Bolzinger, M.A.; Gilon-Delepine, N.; Dugas, P.Y.; Chevalier, Y. Pickering emulsions stabilized by charged nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef] [PubMed]
- De Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B. Fabrication and characterization of Pickering emulsions stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloids Surf. B 2015, 127, 96–104. [Google Scholar] [CrossRef]
- Leal-Castañeda, E.J.; Farcía-Tejeda, Y.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Téllez-Medina, D.I.; Calderón-Domínguez, G.; García, H.S.; Gutiérrez-López, G.F. Pickering emulsions stabilized with native and lauroylated amaranth starch. Food Hydrocoll. 2018, 80, 177–185. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of antioxidant and stable conjugated linoleic acid Pickering emulsion with protein nanofibers by microwave-assisted self-assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Yang, L.; Yuan, L.; Xu, Z.; Ge, L.; Mu, C.; Li, D. Stability enhanced Pickering emulsions based on gelatin and dialdehyde starch nanoparticles as simple strategy for structuring liquid oils. Food Bioprocess Technol. 2021, 14, 1600–1610. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From sharks to yeasts: Squalene in the development of vaccine adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Squalene and its potential clinical uses. Altern. Med. Rev. 1999, 4, 29–36. [Google Scholar] [PubMed]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a step toward sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Corke, H. Oil and squalene in Amaranthus grain and leaf. J. Agric. Food Chem. 2003, 51, 7913–7920. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.M.; Lu, R.R. Alpha-Lactalbumin: Its production technologies and bioactive peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–211. [Google Scholar] [CrossRef]
- Permyakov, E.A. α-Lactalbumin, amazing calcium-binding protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef]
- Nareswara, A.R.; Alamsyah, A.Z.; Afifah, D.N.; Panunggal, B.; Sulchan, M.; Khumaeni, A.; Anjani, G. Encapsulation efficiency of vitamin D3 in α-lactalbumin during storage. Food Res. 2020, 4 (Suppl. S3), 141–146. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Shi, R.; Ma, C.; Gantumur, M.A.; Qayum, A.; Bilawal, A.; Liang, G.; Oh, K.C.; Jiang, Z. Comparison of carrying mechanisms between three fat-soluble vitamins and alpha-lactalbumin: Effects on structure and physicochemical properties of alpha-lactalbumin. Food Hydrocoll. 2021, 116, 106662. [Google Scholar] [CrossRef]
- Mehravar, R.; Jahanshani, M.; Saghatoleslami, N. Production of biological nanoparticles from α-lactalbumin for drug delivery and food science application. Afr. J. Biotechnol. 2009, 8, 6822–6827. [Google Scholar]
- Graveland-Bikker, J.F.; de Kruif, C.G. Unique milk protein based nanotubes: Food and nanotechnology meet. Trends Food Sci. Technol. 2006, 17, 196–203. [Google Scholar] [CrossRef]
- Balandrán-Quintana, R.R.; Valdéz-Covarrubias, M.A.; Mendoza-Wilson, A.M.; Sotelo-Mundo, R.R. α-Lactalbumin hydrolysate spontaneously produces disk-shaped nanoparticles. Int. Dairy J. 2013, 32, 133–135. [Google Scholar] [CrossRef]
- Liu, B.; Liu, B.; Wang, R.; Li, Y. α-Lactalbumin self-assembled nanoparticles with various morphologies, stiffnesses, and sizes as Pickering stabilizers for oil-in-water emulsions and delivery of curcumin. J. Agric. Food Chem. 2021, 69, 2485–2492. [Google Scholar] [CrossRef]
- Fuciños, C.; Míguez, M.; Fuciños, P.; Pastrana, L.M.; Rúa, M.L.; Vicente, A.A. Creating functional nanostructures: Encapsulation of caffeine into α-lactalbumin nanotubes. Innov. Food Sci. Emerg. Technol. 2017, 40, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Maya, I.J.; Hernández-Sánchez, H.; Jiménez-Cruz, E.; Camarillo-Cadena, M.; Hernández-Arana, A. α-Lactalbumin nanoparticles prepared by desolvation and cross-linking: Structure and stability of the assembled protein. Biophys. Chem. 2014, 193–194, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Etorki, A.M.; Gao, M.; Sadeghi, R.; Maldonado-Mejía, L.F.; Kokini, J.L. Effects of desolvating agent types, ratios, and temperature on size and nanostructure of nanoparticles from α-lactalbumin and ovalbumin. J. Food Sci. 2016, 81, E2511–E2520. [Google Scholar] [CrossRef]
- Dhayal, S.K.; Gruppen, H.; de Vries, R.; Wierenga, P.A. Controlled formation of protein nanoparticles by enzymatic cross-linking of α-lactalbumin with horseradish peroxidase. Food Hydrocoll. 2014, 36, 53–59. [Google Scholar] [CrossRef]
- Dhayal, S.K.; Delahaije, R.J.; de Vries, R.; Gruppen, H.; Wierenga, P.A. Enzymatic cross-linking of α-lactalbumin to produce nanoparticles with increased foam stability. Soft Matter 2015, 11, 7888–7898. [Google Scholar] [CrossRef]
- Nudelman, R.; Gavriely, S.; Bychenko, D.; Barzilay, M.; Gulakhmedova, T.; Gazit, E.; Richter, S. Bio-assisted synthesis of bimetallic nanoparticles featuring antibacterial and photothermal properties for the removal of biofilms. J. Nanobiotechnol. 2021, 19, 452. [Google Scholar] [CrossRef]
- Grobelny, J.; Del Rio, F.W.; Pradeep, N.; Kim, D.I.; Hackley, V.A.; Cook, R.F. Size measurement of nanoparticles using Atomic Force Microscopy. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.A., Ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2011; pp. 71–82. [Google Scholar]
- Liu, Z.; Yang, X.; Zhang, Q. Turbiscan: History, development, applications to colloids and dispersions. Adv. Mater. Res. 2014, 936, 1592–1596. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Lopera, S.M.; Guzmán, C.; Cataño, C.; Gallardo, C. Development and characterization of folic acid microparticles formed by spray drying with gum arabic and maltodextrin. Vitae 2009, 16, 55–65. [Google Scholar]
- Chaganti, L.K.; Venkatakrishnan, N.; Bose, K. An efficient method for FITC labelling of proteins using tandem affinity purification. Biosci. Rep. 2018, 38, BSR20181764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, P.M.; Felicio, M.R.; Santos, N.C.; Gonçalves, S.; Domingues. Application of Light Scattering Techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, R.F. Impact of particle size and Polydispersity Index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Cruz, E.; Cuevas-Gómez, A.P.; Unsworth, L.; Cornejo-Mazón, M.; Arroyo-Maya, I.J.; Hernández-Sánchez, H. Poly-L-lysine-coated α-lactalbumin nanoparticles: Preparation, effect of pH, and stability under in vitro simulated gastrointestinal conditions. J. Chem. Technol. Biotechnol. 2022, 97, 1597–1603. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Rodiles-López, J.O.; Cornejo-Mazón, M.; Gutiérrez-López, G.F.; Hernández-Arana, A.; Toledo-Núñez, C.; Barbosa-Cánovas, G.V.; Flores-Flores, J.O.; Hernández-Sánchez, H. Effect of different treatments on the ability of α-lactalbumin to form nanoparticles. J. Dairy Sci. 2012, 95, 6204–6214. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Shi, C.; He, Y.; Ding, M.; Wang, Y.; Zhong, J. Nanoimaging of food proteins by atomic force microscopy. Part II: Application for food proteins from different sources. Trends Food Sci. Technol. 2019, 87, 14–25. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Graveland-Bikker, J.F.; Schaap, I.A.T.; Schimdt, C.F.; de Kruif, C.G. Structural and mechanical study of a self-assembling protein nanotube. Nano Lett. 2006, 6, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of failure to meet assumptions underlying fixed effects analyses of variance and covariance. Rev. Educ. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Belgheisi, S.; Motamedzadegan, A.; Milani, J.M.; Rashidi, L.; Rafe, A. Impact of ultrasound processing parameters on physical characteristics of lycopene emulsion. J. Food Sci. Technol. 2021, 58, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ngwabebhoh, F.A.; Erdagi, S.I.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Xia, W.; Jiang, Q. Enhancement of storage stability of surimi particles stabilized novel Pickering emulsions: Effect of different sequential ultrasonic processes. Ultrason. Sonochem. 2021, 79, 105802. [Google Scholar] [CrossRef]
- Gauthier, G.; Capron, I. Pickering nanoemulsions: An overview of manufacturing processes, formulations, and applications. JCIS Open 2021, 4, 100036. [Google Scholar] [CrossRef]
- Rayner, M.; Marku, D.; Eriksson, M.; Dejmek, P.; Wahlgren, M. Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications. Colloids Surf. A 2014, 458, 48–62. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Z.L.; Zhu, J.Y.; Yin, S.W.; Tang, C.H.; Wu, L.Y.; Yang, X.Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef]



| Nanoparticle | Size (nm) | Polydispersity Index (PDI) | ζ Potential (mV) |
|---|---|---|---|
| NP1 (pH 9) | 143 ± 8 a | 0.09 ± 0.01 a | −29.7 ± 1.42 a |
| NP2 (pH 11) | 152 ± 1 b | 0.06 ± 0.04 b | −25.4 ± 2.35 b |
| Concentration of α-LA Nanoparticles (%) | Droplet Size (μm) | PDI | ζ Potential | Global TSI | Emulsification Efficiency (%) |
|---|---|---|---|---|---|
| NP1-3 | 5.8 ± 0.4 | 0.73 ± 0.16 | −59.8 ± 8.3 | 8.63 ± 0.7 | 40 |
| NP1-5 | 5.0 ± 0.4 | 0.65 ± 0.17 | −39.7 ± 1.95 a | 6.46 ± 1.3 | 48 |
| NP1-10 | 4.15 ± 0.75 | 0.42 ± 0.03 a | −44.7 ± 3.2 a,b | 1.86 ± 0.07 b | 92 |
| NP1-15 | 3.6 ± 0.66 | 0.49 ± 0.10 a | −39.7 ± 15.8 a,b | 1.98 ± 0.2 b | 84 |
| NP1-20 | 1.1 ± 0.5 | 0.74 ± 0.04 b | −40.6 ± 5.4 | 2.06 ± 0.7 | 87 |
| NP2-3 | 5.9 ± 0.2 | 0.88 ± 0.12 | −42.5 ± 3.4 a,b | 4.7 ± 2.7 | 46 |
| NP2-5 | 5.1 ± 0.37 | 0.61 ± 0.11 | −41.7 ± 5.4 a,b | 2.0 ± 0.7 | 51 |
| NP2-10 | 3.52 ± 0.34 | 0.60 ± 0.16 | −41.9 ± 20 | 1.6 ± 0.04 a | 94 |
| NP2-15 | 2.56 ± 0.36 | 0.66 ± 0.16 | −33.4 ± 9.9 | 1.8 ± 0.8 | 86 |
| NP2-20 | 2.2 ± 1.4 | 0.88 ± 0.15 | −48.7 ± 3.7 b | 1.7 ± 0.1 a,b | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Gómez, A.P.; González-Magallanes, B.; Arroyo-Maya, I.J.; Gutiérrez-López, G.F.; Cornejo-Mazón, M.; Hernández-Sánchez, H. Squalene-Rich Amaranth Oil Pickering Emulsions Stabilized by Native α-Lactalbumin Nanoparticles. Foods 2022, 11, 1998. https://doi.org/10.3390/foods11141998
Cuevas-Gómez AP, González-Magallanes B, Arroyo-Maya IJ, Gutiérrez-López GF, Cornejo-Mazón M, Hernández-Sánchez H. Squalene-Rich Amaranth Oil Pickering Emulsions Stabilized by Native α-Lactalbumin Nanoparticles. Foods. 2022; 11(14):1998. https://doi.org/10.3390/foods11141998
Chicago/Turabian StyleCuevas-Gómez, Andrea P., Berenice González-Magallanes, Izlia J. Arroyo-Maya, Gustavo F. Gutiérrez-López, Maribel Cornejo-Mazón, and Humberto Hernández-Sánchez. 2022. "Squalene-Rich Amaranth Oil Pickering Emulsions Stabilized by Native α-Lactalbumin Nanoparticles" Foods 11, no. 14: 1998. https://doi.org/10.3390/foods11141998
APA StyleCuevas-Gómez, A. P., González-Magallanes, B., Arroyo-Maya, I. J., Gutiérrez-López, G. F., Cornejo-Mazón, M., & Hernández-Sánchez, H. (2022). Squalene-Rich Amaranth Oil Pickering Emulsions Stabilized by Native α-Lactalbumin Nanoparticles. Foods, 11(14), 1998. https://doi.org/10.3390/foods11141998





