Quality Preservation of Ready-to-Eat Prickly Pears by Peels Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Peeling and Coating
2.2. Microbiological Analyses and pH Determination of Prickly Pears
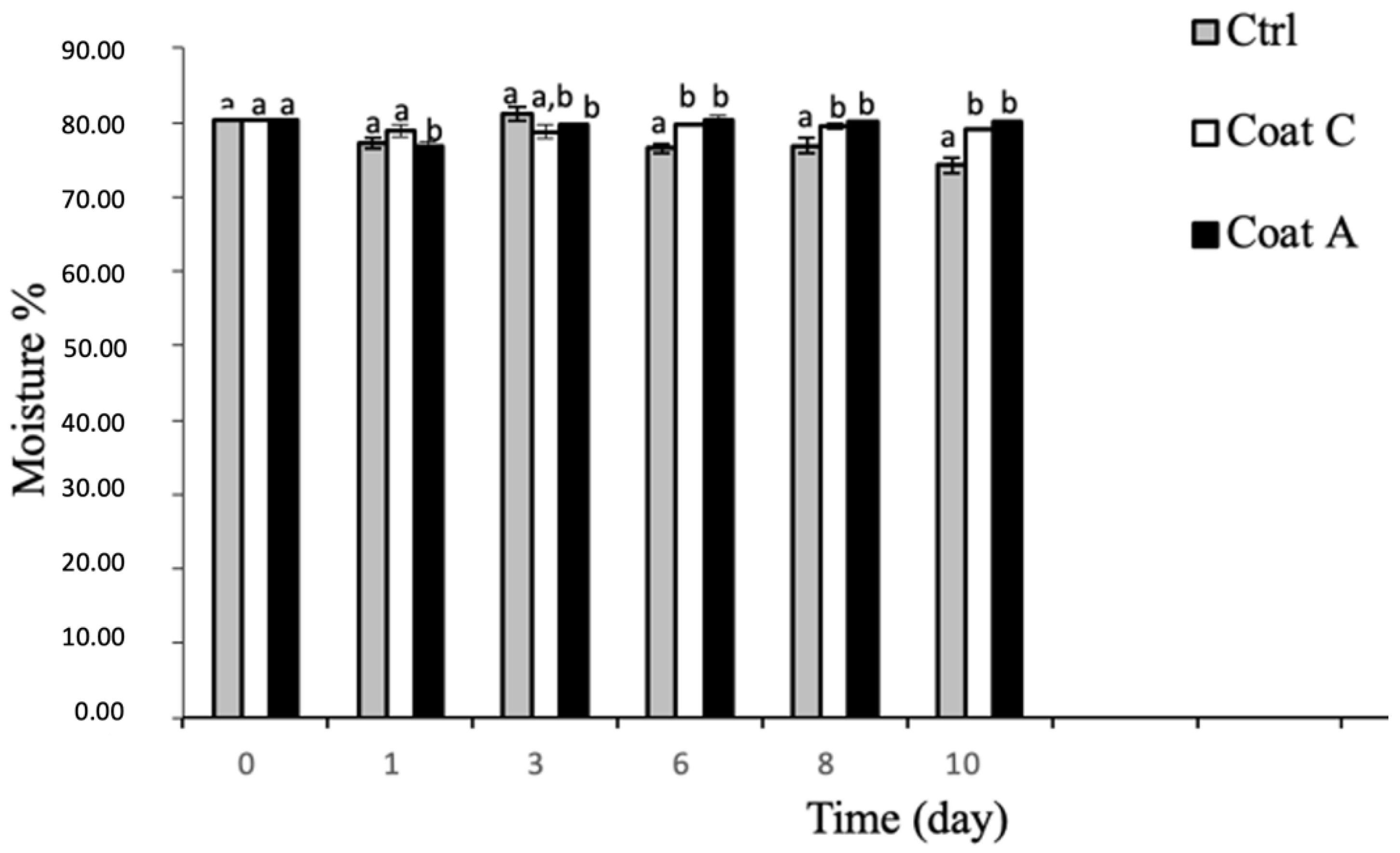
2.3. Weight Loss and Moisture Content of Prickly Pears
2.4. Chemical Analyses of Prickly Pears
2.5. Sensory Analyses of Prickly Pears
2.6. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, L.; Mhaske, P.; Zing, X.; Kasapis, S.; Majzoobi, M.; Farahnaky, A. Cold plasma: Microbial inactivation and effects on quality attributes of fresh and minimally processed fruits and Ready-To-Eat vegetables. Trends Food Sci. Technol. 2021, 116, 146–175. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Buonocore, G.G.; Lavorgna, M.; Del Nobile, M.A. Calcium-alginate coating loaded with silver-montmorillonite nanoparticles to prolong the shelf life of fresh-cut carrots. Food Res. Int. 2012, 48, 164–169. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; García-Pastor, M.E.; Valero, D.; Amorós, A.; Almansa, M.S.; Legua, P.; Hernández, F. Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.). Agriculture 2021, 11, 62. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Ramírez-Moreno, E.; Santos-Buelga, C.; Ferreira, I.C.F.R. Xoconostle fruit (Opuntia matudae Scheinvar cv. Rosa) by-products as potential functional ingredients. Food Chem. 2015, 185, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Palmeri, R.; Parafati, L.; Arena, E.; Grassenio, E.; Restuccia, C.; Fallico, B. Antioxidant and Antimicrobial Properties of Semi-Processed Frozen Prickly Pear Juice as Affected by Cultivar and Harvest Time. Foods 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Madrigal-Santillán, E.; García-Melo, F.; Morales-González, J.A.; Vázquez-Alvarado, P.; Muñoz-Juárez, S.; Zuñiga-Pérez, C.; Sumaya-Martínez, M.T.; Madrigal-Bujaidar, E.; Hernández-Ceruelos, A. Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients 2013, 5, 4145–4158. [Google Scholar] [CrossRef] [Green Version]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and Medicinal Use of Cactus Pear (Opuntia spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- Piga, A. Cactus Pear: A Fruit of nutraceutical and functional importance. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Cruz-Cansino, N.D.S.; Montiel-Columna, N.I.; Bautista-Velueta, P.G.; Pérez-Tinoco, M.R.; Alanís-García, E.; Ramírez-Moreno, E. Optimization of thermo-ultrasound conditions for the processing of a prickly pear juice blend (Opuntia Ficus Indica) using response surface methodology. J. Food Qual. 2016, 39, 780–791. [Google Scholar] [CrossRef]
- Piga, A.; D’Aquino, S.; Agabbio, M.; Emonti, G.; Farris, G.A. Influence of Storage Temperature on Shelf-life of Minimally Processed Cactus Pear Fruits. LWT 2000, 33, 15–20. [Google Scholar] [CrossRef]
- Scalone, D.; Stuto, A.; Licciardello, F.; Muratore, G.; Todaro, A.; Spagna, G. Strategies for the extension of the shelf life of ready to eat prickly pear fruits. It. J. Food Sci. 2012, 24, 174–177. [Google Scholar]
- Díaz-Delgado, G.L.; Rodríguez-Rodríguez, E.M.; Dorta, E.; Lobo, M.G. Effects of Peeling, Film Packaging, and Cold Storage on the Quality of Minimally Processed Prickly Pears (Opuntia ficus-indica L. Mill.). Agriculture 2022, 12, 281. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J.A. Postharvest quality of peeled prickly pear fruit treated with acetic acid and chitosan. Postharv. Biol. Technol. 2014, 92, 139–145. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Scrocco, C.; Brescia, I. New strategies for minimally processed cactus pear packaging. Inn. Food Sci. Emerg. Technol. 2009, 10, 356–362. [Google Scholar] [CrossRef]
- Wan, C.; Kahramanoğlu, İ.; Okatan, V. Application of plant natural products for the management of postharvest diseases in fruits. Folia Hortic. 2021, 33, 203–215. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carboh. Pol. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Wong, C.H.; Mak, I.E.K.; Li, D. Bilayer edible coating with stabilized Lactobacillus plantarum 299v improved the shelf life and safety quality of fresh-cut apple slices. Food Packag. Shelf Life 2021, 30, 100746. [Google Scholar] [CrossRef]
- Manzoor, S.; Gull, A.; Wani, S.M.; Ganaie, T.A.; Masoodi, F.A.; Bashir, K.; Malik, A.R.; Dar, B.N. Improving the shelf life of fresh cut kiwi using nanoemulsion coatings with antioxidant and antimicrobial agents. Food Biosci. 2021, 41, 101015. [Google Scholar] [CrossRef]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Setanni, L.; Inglese, P.; D’Anna, F.; Miceli, A. Effect of Opuntia ficus-indica mucilage edible coating on quality, nutraceutical, and sensorial parameters of minimally processed cactus pear fruits. J. Food Qual. 2021, 11, 1963. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT Food Sci. Technol. 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinozad, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Cardaror-Martinez, A.; Jimenez-Martinez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Food Sci. Technol. 2011, 31, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Morsel, J. Recovered lipids from prickly pear [Opuntia ficus-indica (L.) Mill] peel: A good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem. 2003, 83, 447–456. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. LWT 2020, 124, 109155. [Google Scholar] [CrossRef]
- Hegazy, E.M.; Ibrahim, N.M.; Saleh, N.S.M. Determination of antioxidant and antifungal activities in cookies fortified with solar dried prickly pear peels powder. Pak. J. Biol. Sci. 2020, 23, 590–601. [Google Scholar] [CrossRef]
- Okatan, V. Antioxidant properties and phenolic profile of the most widely appreciated cultivated berry species: A comparative study. Folia Hort. 2020, 32, 79–85. [Google Scholar] [CrossRef]
- Zarifikhosroshahia, M.; Tugba Murathanb, Z.; Kafkasa, E.; Okatan, V. Variation in volatile and fatty acid contents among Viburnum opulus L. Fruits growing different locations. Sci. Hortic. 2020, 264, 109160. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia Ficus Indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dilucia, F.; Lacivita, V.; Nobile, M.A.D.; Conte, A. Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach. Foods 2021, 10, 1972. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Restuccia, C.; Fallico, B. Application of prickly pear fruit extract to improve domestic shelf life, quality and microbial safety of sliced beef. Food Chem. Toxicol. 2018, 118, 355–360. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Mahloko, L.M.; Silungwe, H.; Mashau, M.E.; Kgatla, T.E. Bioactive compounds, antioxidant activity and physical characteristics of wheat-prickly pear and banana biscuits. Heliyon 2019, 5, e02479. [Google Scholar] [CrossRef] [Green Version]
- Folin-Ciocalteu Index. Off. J. Eur. Communities 1992, 178–179.
- Huang, Y.S.; Ho, S.C. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010, 119, 868–873. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Danza, A.; Conte, A.; Mastromatteo, M.; Nobile, M.A.D. A New Example of Nanotechnology Applied to Minimally Processed Fruit: The Case of Fresh-Cut Melon. J. Food Process. Technol. 2015, 6, 439. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A. Quality parameters of fresh-cut fruit and vegetable products. In Fresh-Cut Fruits and Vegetables; Lamikanra, O., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 11–20. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Pinna, I.; Agabbio, M. Changes in ascorbic acid, polyphenol content and antioxidant activity in minimally processed cactus pear fruits. LWT 2003, 36, 257–262. [Google Scholar] [CrossRef]
- Palma, A.; Continella, A.; Malfa, S.; D’Aquino, S. Changes in Physiological and Some Nutritional, Nutraceuticals, Chemical-Physical, Microbiological and Sensory Quality of Minimally Processed Cactus Pears Cvs “Bianca”, “Gialla” and “Rossa” Stored under Passive Modified Atmosphere. J. Sci. Food Agric. 2017, 98, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Panza, O.; Conte, A.; Del Nobile, M.A. Pomegranate By-Products as Natural Preservative to Prolong the Shelf Life of Breaded Cod Stick. Molecules 2021, 26, 2385. [Google Scholar] [CrossRef] [PubMed]
- Artés, F.; Gómez, P.A.; Artés-Hernández, F. Physical, physiological and microbial deterioration of minimally fresh processed fruits and vegetables. Food Sci. Technol. Int. 2007, 13, 177–188. [Google Scholar] [CrossRef]



| Samples | MAL (Day) | Microbiological Shelf Life (Day) | ||
|---|---|---|---|---|
| MALTMB | MALTPB | MALYeast | ||
| Ctrl Coat C Coat A | 3.96 ± 0.37 a 6.41 ± 0.89 b 6.79 ± 0.52 b | 6.45 ± 0.66 >10 >10 | 7.3 ± 0.47 >10 >10 | 3.96 ± 0.37 a 6.41 ± 0.89 b 6.79 ± 0.52 b |
| Samples | Time (Day) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | 10 | |
| Lactic Acid Bacteria | ||||||
| Ctrl | 1.60 ± 0.43 a | 4.08 ± 0.09 a | 5.50 ± 0.28 a | 5.13 ± 0.32 a | 5.30 ± 0.00 a | 7.70 ± 0.01 b |
| Coat C | 1.39 ± 0.55 a | 2.80 ± 0.28 b | 4.70 ± 0.50 b | 4.31 ± 0.19 b | 4.48 ± 0.52 b | 8.20 ± 0.28 a |
| Coat A | 1.00 ± 0.00 a | 3.56 ± 0.40 a | 3.17 ± 0.24 c | 4.31 ± 0.19 b | 4.69 ± 0.18 a,b | 8.00 ± 0.00 a,b |
| Molds | ||||||
| Ctrl | 2.0 ± 0.10 a | 4.48 ± 0.10 a | 4 ± 0.10 a | 4.24 ± 0.34 a | 5.50 ± 0.71 a | 6.45 ± 0.64 a |
| Coat C | 2.0 ± 0.10 a | 3.50 ± 0.71 a,b | 3.24 ± 0.34 b | 3.0 ± 0.10 b | 3.74 ± 0.37 b | 3.50 ± 0.71 b |
| Coat A | 2.0 ± 0.10 a | 2.5 ± 0.71 b | 3.0 ± 0.0 b | 3.15 ± 0.21b | 3.50 ± 0.71 b | 3.65 ± 0.49 b |
| Samples | Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 8 | 10 | |
| Ctrl Coat C Coat A | 5.76 ± 0.01 a 5.73 ± 0.00 a 5.70 ± 0.00 a | 5.58 ± 0.02 b 5.65 ± 0.01 a 5.72 ± 0.06 a | 5.42 ± 0.08 b 5.57 ± 0.01 a 5.61 ± 0.03 a | 4.92 ± 0.09 c 5.51 ± 0.02 a 5.34 ± 0.10 b | 4.89 ± 0.19 a 5.02 ± 0.21 a 4.82 ± 0.02 a | 5.37 ± 0.01 a 4.95 ± 0.01 b 5.19 ± 0.01 a |
| Sample | TPC | TFC | ABTS | |||
|---|---|---|---|---|---|---|
| Mg of Gallic Acid/g of Dry Weight | Mg of Quercetin/g of Dry Weight | Mg Trolox Equivalents/g of Dry Weight | ||||
| t 0 | t 10 | t 0 | t 10 | t 0 | t 10 | |
| Ctrl | 1.86 ± 0.85 a | 1.31 ± 0.11 a | 0.92 ± 0.04 a,b | 0.69 ± 0.11 a | 4.55 ± 0.73 a | 3.53 ± 0.50 a |
| Coat C | 1.85 ± 0.30 a | 0.76 ± 0.11 b | 1.01 ± 0.02 b | 0.52 ± 0.06 b | 4.50 ± 0.64 a | 2.50 ± 0.31 b |
| Coat A | 1.57 ± 0.02 a | 0.86 ± 0.33 b | 0.89 ± 0.07 a | 0.47 ± 0.02 b | 3.80 ± 0.28 a | 2.24 ± 0.27 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panza, O.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Quality Preservation of Ready-to-Eat Prickly Pears by Peels Recycling. Foods 2022, 11, 2016. https://doi.org/10.3390/foods11142016
Panza O, Lacivita V, Conte A, Del Nobile MA. Quality Preservation of Ready-to-Eat Prickly Pears by Peels Recycling. Foods. 2022; 11(14):2016. https://doi.org/10.3390/foods11142016
Chicago/Turabian StylePanza, Olimpia, Valentina Lacivita, Amalia Conte, and Matteo Alessandro Del Nobile. 2022. "Quality Preservation of Ready-to-Eat Prickly Pears by Peels Recycling" Foods 11, no. 14: 2016. https://doi.org/10.3390/foods11142016
APA StylePanza, O., Lacivita, V., Conte, A., & Del Nobile, M. A. (2022). Quality Preservation of Ready-to-Eat Prickly Pears by Peels Recycling. Foods, 11(14), 2016. https://doi.org/10.3390/foods11142016








