Investigating the Impact of Dragon Fruit Peel Waste on Starch Digestibility, Pasting, and Thermal Properties of Flours Used in Asia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dragon Fruit Peel Powder (DFP) Preparation
2.3. Proximate Analysis of DFP
2.4. Determination of Total Phenolic Content (TPC), Total Betacyanin Content (TBC), and Antioxidant Activity
2.5. Chemical Properties of DFP Pectin
2.6. Physicochemical Properties of DFP
2.6.1. Oil Holding Capacity (OHC)
2.6.2. Water Holding Capacity (WHC)
2.6.3. Swelling Capacity (SC)
2.7. In Vitro Starch Digestion and Starch Fractions
2.8. Determination of Total Phenolic Content (TPC), Total Betacyanin Content (TBC), and Antioxidant Activity during In Vitro Digestion
2.9. Thermal, Pasting Properties and Fourier Transform Infrared (FT-IR) Spectroscopy
2.9.1. Thermal Properties
2.9.2. Pasting Properties
2.9.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions, Bioactive Compounds, and Antioxidant Activity of DFP
3.2. Pectin and Physicochemical Properties of DFP
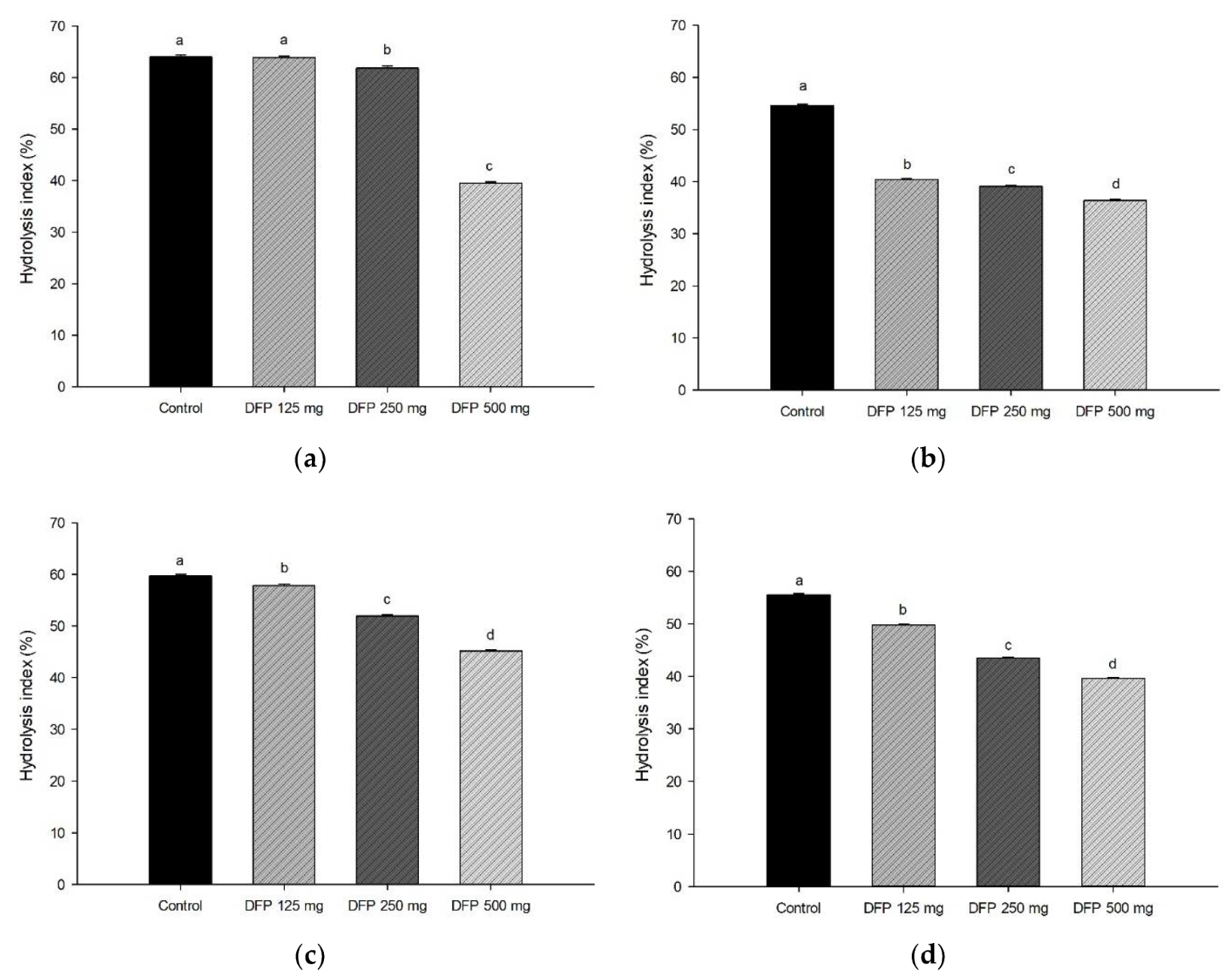
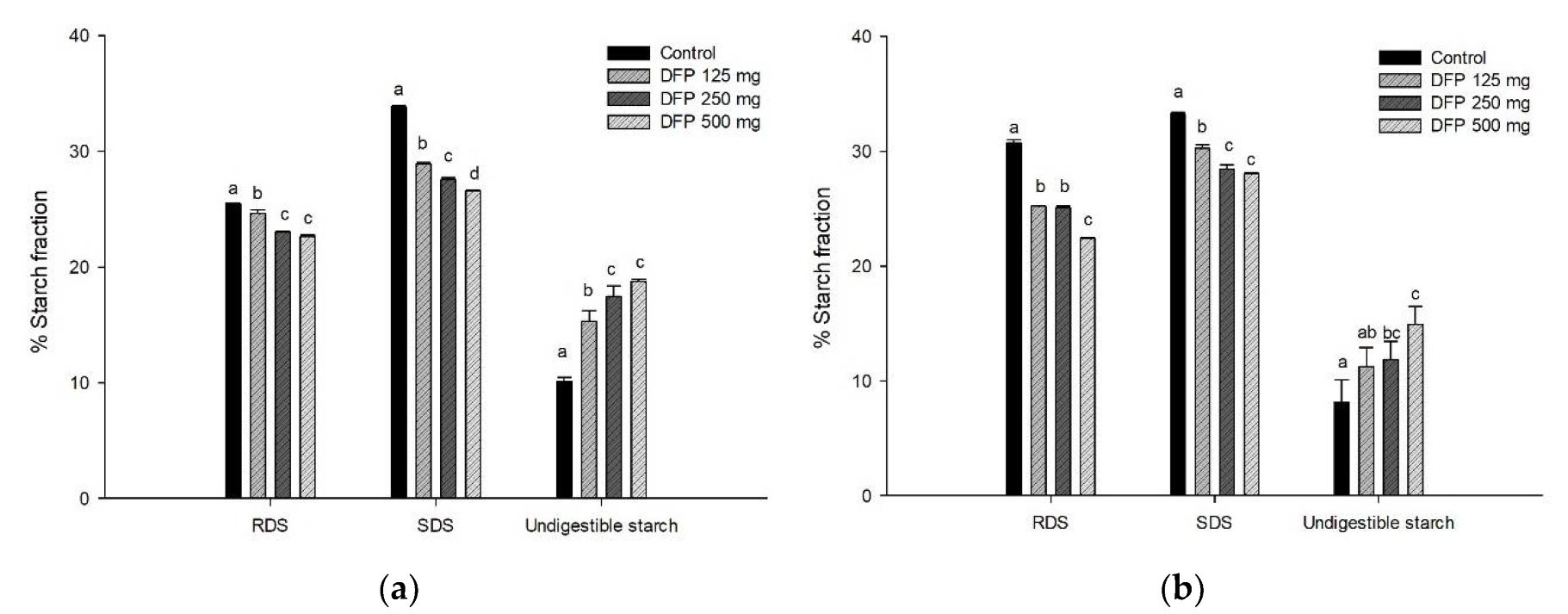
3.3. Effect of DFP on In Vitro Starch Digestion, Hydrolysis Index, and Starch Fraction
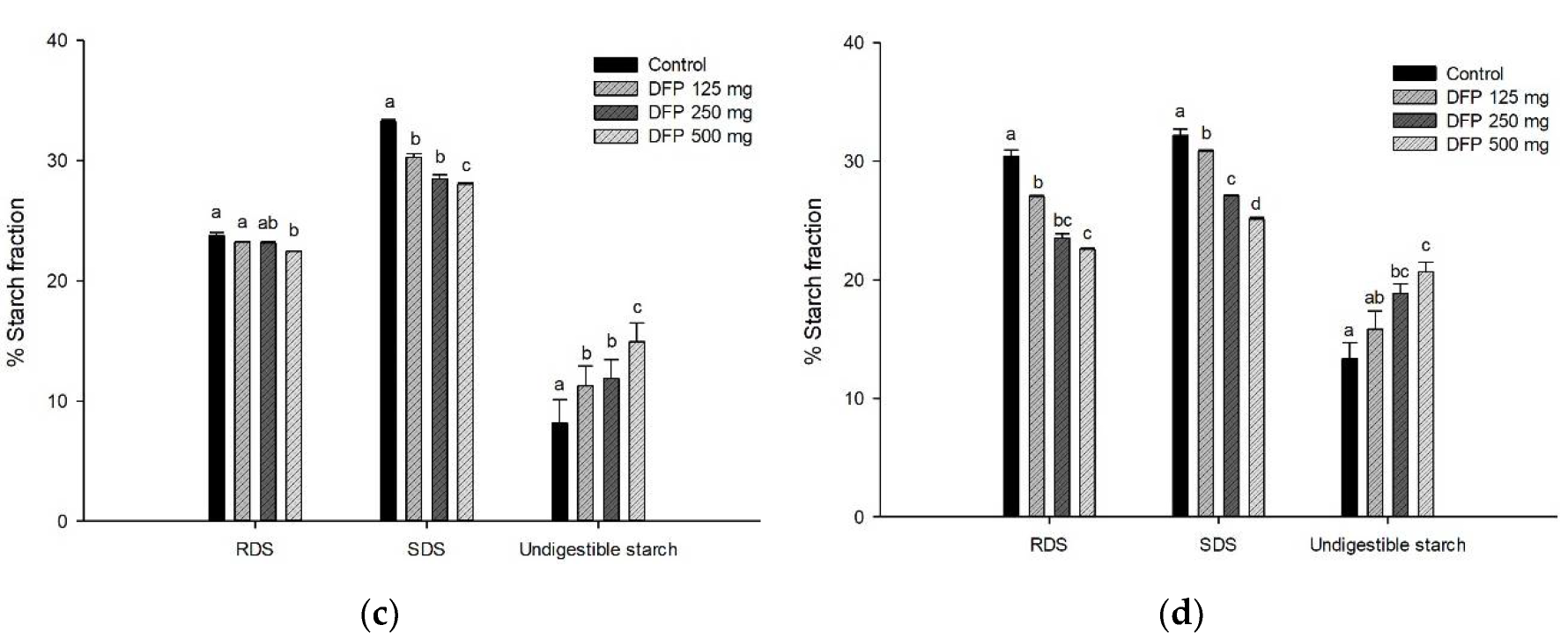
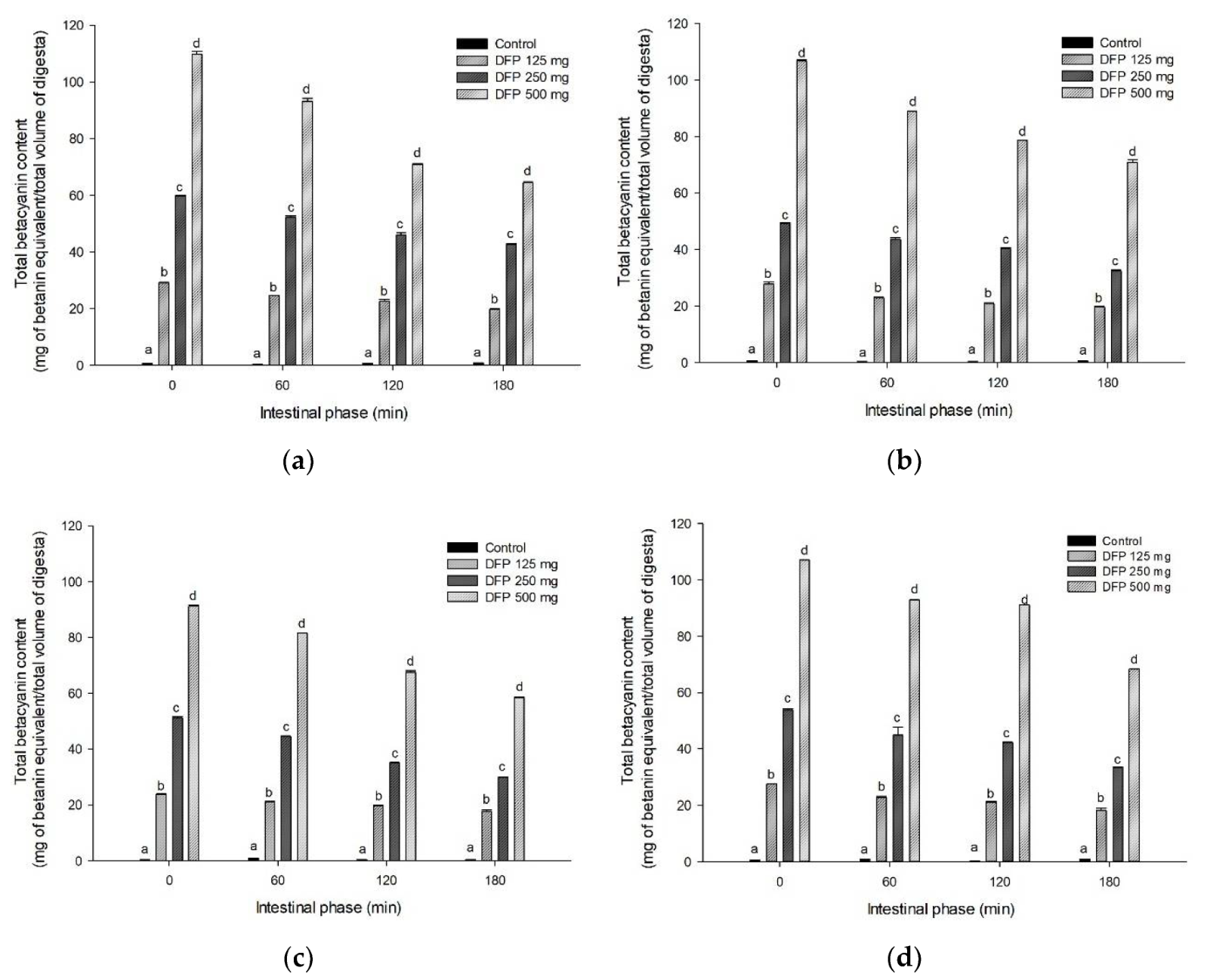
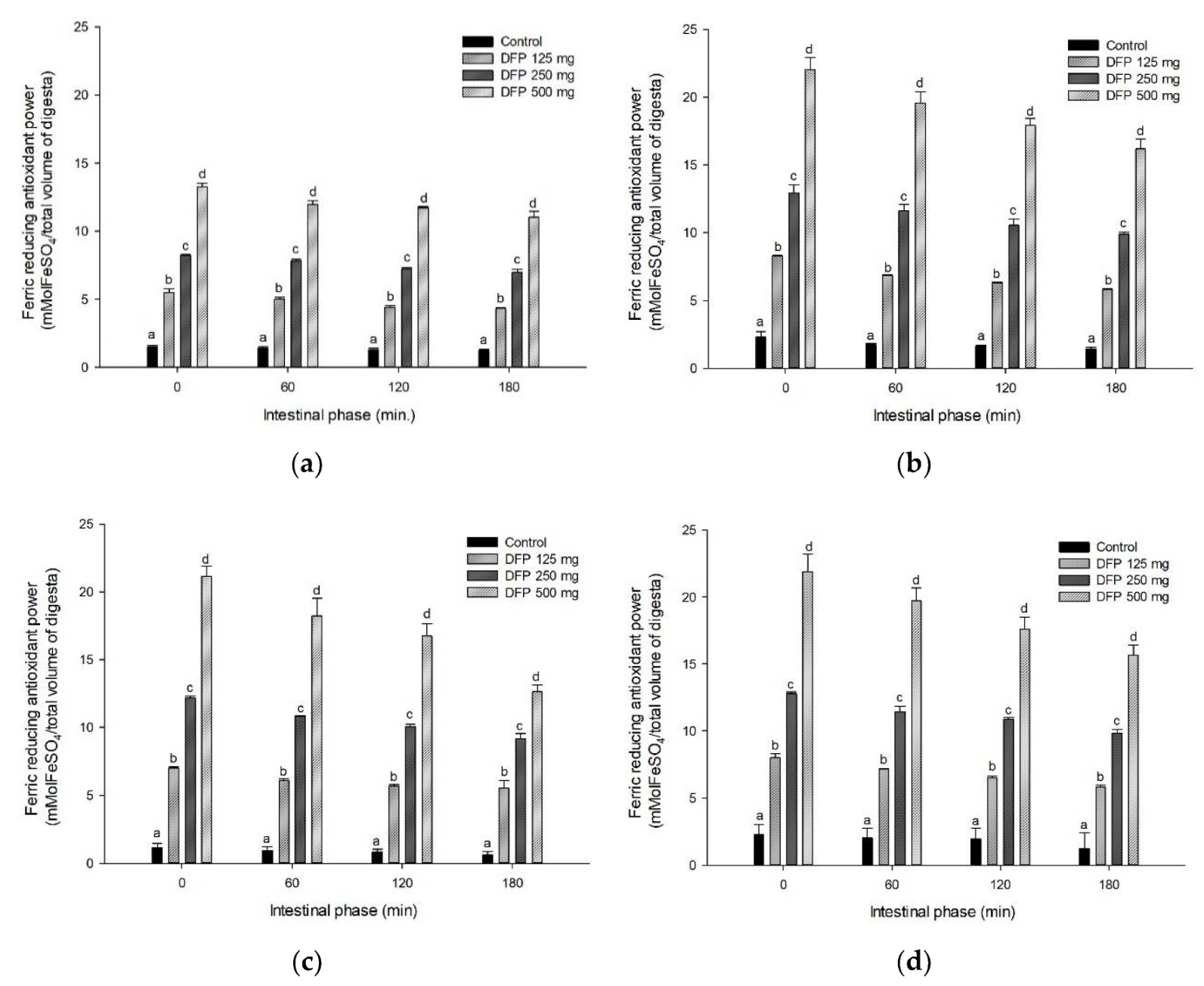
3.4. Effect of DFP on TPC, TBC, and Antioxidant Activity during In Vitro Digestion
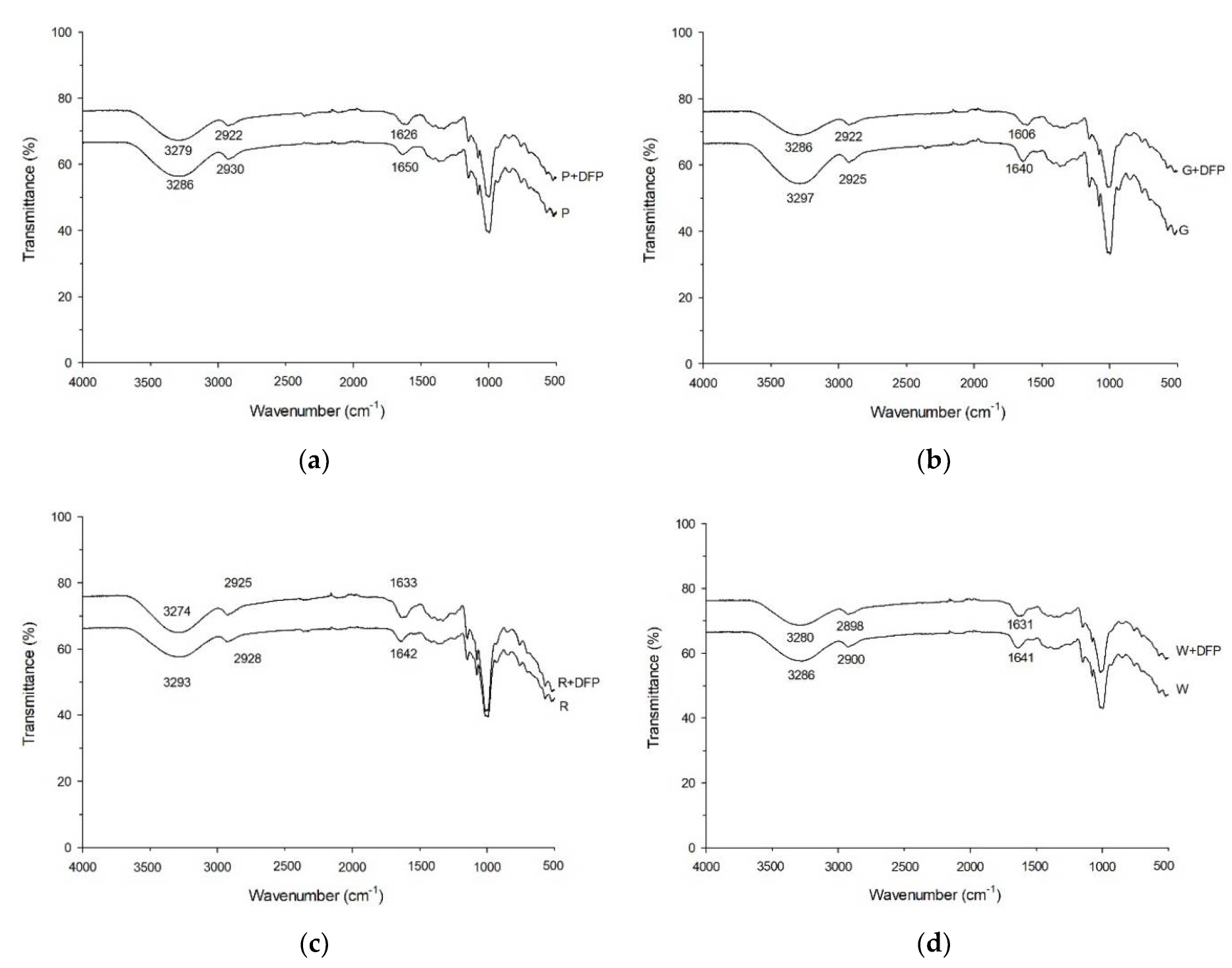
3.5. Effect of DFP and Various Types of Flour on Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, Y.-J.; Lee, H.-S.; Lee, J.-W. Association of carbohydrate and fat intake with metabolic syndrome. Clin. Nutr. 2018, 37, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Prentice, R.L. Associations of fats and carbohydrates with cardiovascular disease and mortality-PURE and simple? Lancet 2018, 391, 1677. [Google Scholar] [CrossRef]
- Schmiele, M.; Sampaio, U.M.; Pedrosa Silva Clerici, M.T. Chapter 1-Basic principles: Composition and properties of starch. In Starches for Food Application; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–22. ISBN 978-0-12-809440-2. [Google Scholar]
- Agama-Acevedo, E.; Flores-Silva, P.C.; Bello-Perez, L.A. Chapter 3-Cereal starch production for food applications. In Starches for Food Application; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 71–102. ISBN 978-0-12-809440-2. [Google Scholar]
- Behall, K.M.; Hallfrisch, J. Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur. J. Clin. Nutr. 2002, 56, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-amylose wheat lowers the postprandial glycemic response to bread in healthy adults: A randomized controlled crossover trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Henry, C.J. Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 670–702. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef]
- Bae, I.Y.; Jun, Y.; Lee, S.; Lee, H.G. Characterization of apple dietary fibers influencing the in vitro starch digestibility of wheat flour gel. LWT-Food Sci. Technol. 2016, 65, 158–163. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea flower extract on the in vitro enzymatic digestibility of starch and its application in bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.-E.S. Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance—A review. J. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Paw, N.J. Effect of dragon fruit on glycemic control in prediabetes and type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0184577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakar, J.; Shuc, E.; Muhammad, S.; Hashim, D.M.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2011, 18, 279–286. [Google Scholar]
- Phrueksanan, W.; Yibchok-anun, S.; Adisakwattana, S. Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res. Vet. Sci. 2014, 97, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Wesarachanon, K.; Anantsuphasak, K.; Daenphetploy, T.; Thien-Ngern, S.; Thilavech, T.; Pasukamonset, P.; Ngamukote, S.; Adisakwattana, S. Protein glycation inhibitory activity and antioxidant capacity of clove extract. J. Food Sci. Techno. 2015, 52, 3843–3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, B.M.N.; Pirak, T. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Chia, S.L.; Chong, G.H. Effect of drum drying on physico-chemical characteristics of dragon fruit peel (Hylocereus polyrhizus). Int. J. Food Eng. 2015, 11, 285–293. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Yousif, A.; Nhepera, D.; Johnson, S. Influence of sorghum flour addition on flat bread in vitro starch digestibility, antioxidant capacity and consumer acceptability. Food Chem. 2012, 134, 880–887. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, M.; Yang, S.; Li, Z.; Liu, M.; Yang, X.; Lin, N.; Liu, J. Physicochemical properties and in vitro digestibility of proso millet starch after addition of Proanthocyanidins. Int. J. Biol. Macromol. 2021, 168, 784–791. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Mahawar, M.K.; Bibwe, B.; Kannaujia, P. Postharvest profile, processing and waste utilization of dragon fruit (Hylocereus spp.): A review. Food Rev. Int. 2020, 38, 733–759. [Google Scholar] [CrossRef]
- Virk, B.S.; Sogi, D.S. Extraction and characterization of pectin from apple (Malus Pumila. Cv Amri) peel waste. Int. J. Food Prop. 2004, 7, 693–703. [Google Scholar] [CrossRef]
- International Pectin Producers’ Association (IPPA). Pectin Commercial Production and Pectin in Organic Food Products. Available online: https://ippa.info/ippa-resources/pectin-in-organic-food-products (accessed on 15 October 2021).
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical characterization of five types of citrus dietary fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Qi, X.; Al-Ghazzewi, F.H.; Tester, R.F. Dietary Fiber, Gastric emptying, and carbohydrate digestion: a mini-review. Starch-Stärke 2018, 70, 1700346. [Google Scholar] [CrossRef]
- Oboh, H.; Obayiuwana, O.A.; Aihie, E.O.; Iyayi, J.I.; Udoh, E.J. Beetroot (Beta vulgaris) juice inhibits key carbohydrate metabolising enzymes associated with type II diabetes. Niger. J. Basic Appl. Sci. 2021, 28, 1–6. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch Structure influences its digestibility: a review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Choo, K.Y.; Ong, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus). Food Sci. Biotechnol. 2019, 28, 1163–1169. [Google Scholar] [CrossRef]
- Abedimanesh, N.; Asghari, S.; Mohammadnejad, K.; Daneshvar, Z.; Rahmani, S.; Shokoohi, S.; Farzaneh, A.H.; Hosseini, S.H.; Anarkooli, I.J.; Noubarani, M.; et al. The anti-diabetic effects of betanin in streptozotocin-induced diabetic rats through modulating AMPK/SIRT1/NF-κB signaling pathway. Nutr. Metab. 2021, 18, 92. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef]
- Sevilmis, B.; Sensoy, I. Effects of psyllium fiber on in vitro digestion and structure of different types of starches. J. Sci. Food Agric. 2022, 102, 3213–3226. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.; Tang, X.; Zhou, J.; Li, H.; Zhang, H.; Liu, J. Physicochemical properties and microstructure of corn flour-cellulose fiber extrudates. Food Sci. Nutr. 2021, 9, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Ö.; Yurt, B.; Baştürk, A.; Toker, O.; Yilmaz, M.; Karaman, S.; Dağlıoğlu, O. Pasting properties, texture profile and stress–relaxation behavior of wheat starch/dietary fiber systems. Food Res. Int. 2013, 53, 278–290. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Siripatrawan, U.; Henry, C.J.K. Pasting behaviour, textural properties and freeze–thaw stability of wheat flour–crude malva nut (Scaphium scaphigerum) gum system. J. Food Eng. 2011, 105, 557–562. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, L.; Wang, X.; He, J.; Wang, Q. The influence of Konjac glucomannan on the functional and structural properties of wheat starch. Food Sci. Nutr. 2020, 8, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—a joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Kohyama, K.; Suzuki, Y.; Okamoto, K.; Noel, T.R.; Ring, S.G. Physicochemical characteristics of waxy rice starch influencing the in vitro digestibility of a starch gel. Food Chem. 2009, 116, 137–142. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Q.; Shi, Z.; Ye, J. Interactions between wheat starch and cellulose derivatives in short-term retrogradation: Rheology and FTIR study. Food Res. Int. 2017, 100, 858–863. [Google Scholar] [CrossRef]

| Pectin Chemical Property | Physicochemical Property | ||||||
|---|---|---|---|---|---|---|---|
| EW (g/mol) | MeO (%) | DE (%) | AUA (%) | OHC (g of Oil/g of Sample) | WHC (g of Water/g of Sample) | SC (mL of Water/g of Sample) | |
| CMC | N.A. | N.A. | N.A. | N.A. | 1.63 ± 0.06 a | 5.64 ± 0.16 a | 2.63 ± 0.06 a |
| DFP | 397.63 ± 1.51 | 5.22 ± 0.11 | 46.90 ± 3.03 | 79.45 ± 0.53 | 1.93 ± 0.03 a | 9.44 ± 0.19 b | 4.60 ± 0.03 b |
| Experiments | AUCGlucose | AUCTPC | AUCTBC | AUCFRAP |
|---|---|---|---|---|
| P | 8900.6 ± 82.5 a | 208.1 ± 9.0 a | N.D. | 241.4 ± 13.8 a |
| P + DFP125 | 8815.9 ± 46.6 a | 334 ± 10.1 b | 4250.3 ± 28.8 a | 858.8 ± 14.6 b |
| P + DFP250 | 8520.9 ± 62.6 b | 394.9 ± 4.2 c | 8878.2 ± 28.7 b | 1350.0 ± 19.1 c |
| P + DFP500 | 5474.2 ± 34.0 c | 643.6 ± 16.5 d | 14,545.4 ± 32.6 c | 2146.2 ± 20.8 d |
| G | 7551.1 ± 111.5 a | 236.3 ± 3.2 a | N.D. | 307.4 ± 15.3 a |
| G + DFP125 | 5597.8 ± 88.7 b | 349.6 ± 2.5 b | 4045.1 ± 6.6 a | 1199.4 ± 50.7 b |
| G + DFP250 | 5405.5 ± 78.6 b | 466.1 ± 1.6 c | 7464.8 ± 18.9 b | 1990.9 ± 50.4 c |
| G + DFP500 | 5020.2 ± 78.0 c | 699.6 ± 4.1 d | 15,363.8 ± 16.3 c | 3447.7 ± 29.5 d |
| R | 8261.6 ± 27.3 a | 209.6 ± 10.0 a | N.D. | 155.7 ± 27.5 a |
| R + DFP125 | 7989.2 ± 47.1 b | 303.7 ± 2.3 b | 3705.3 ± 20.8 a | 1069.5 ± 11.1 b |
| R + DFP250 | 7179.2 ± 46.8 c | 414.8 ± 3.2 c | 6984 ± 10.0 b | 1887.2 ± 20.7 c |
| R + DFP500 | 6244.2 ± 15.0 d | 606.4 ± 4.6 d | 13,490 ± 26.7 c | 3270.9 ± 48.1 d |
| W | 7684.8 ± 42.8 a | 320.6 ± 2.7 a | N.D. | 370.0 ± 41.8 a |
| W + DFP125 | 6884.4 ± 28.1 b | 392.3 ± 2.5 b | 4011.6 ± 15.0 a | 1218.4 ± 14.3 b |
| W + DFP250 | 6001.2 ± 19.0 c | 481.4 ± 6.0 c | 8024.5 ± 13.5 b | 2007.7 ± 18.1 c |
| W + DFP500 | 5478.8 ± 87.2 d | 632.7 ± 5.7 d | 15,818.4 ± 7.4 c | 3217.8 ± 45.7 d |
| AUCGlucose | AUCTPC | AUCTBC | AUCFRAP | |
|---|---|---|---|---|
| AUCGlucose | 1 | −0.786 * | −0.688 * | −0.755 * |
| AUCTPC | 1 | 0.965 * | 0.948 * | |
| AUCTBC | 1 | 0.948 * | ||
| AUCFRAP | 1 |
| Experiments | Thermal Properties | |||
|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
| P | 64.85 ± 0.35 | 71.75 ± 0.55 | 79.95 ± 0.45 | 11.81 ± 0.10 |
| P + DFP250 | 68.90 ± 0.10 * | 76.75 ± 0.15 * | 83.65 ± 0.65 * | 4.96 ± 0.13 * |
| G | 63.50 ± 1.30 | 69.10 ± 0.40 | 74.15 ± 0.45 | 5.47 ± 1.52 |
| G + DFP250 | 66.80 ± 0.40 * | 74.50 ± 0.10 * | 79.30 ± 0.50 * | 4.60 ± 1.58 |
| R | 67.15 ± 2.95 | 74.75 ± 2.35 | 82.05 ± 0.25 | 8.02 ± 0.17 |
| R + DFP250 | 66.70 ± 0.00 | 72.50 ± 0.50 | 86.10 ± 0.50 * | 3.61 ± 0.96 * |
| W | 57.35 ± 0.25 | 63.70 ± 0.30 | 69.60 ± 0.30 | 5.93 ± 0.18 |
| W + DFP250 | 61.55 ± 0.05 * | 68.00 ± 1.00 * | 72.50 ± 1.40 * | 3.15 ± 0.27 * |
| Experiments | Thermal Properties | FTIR Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| PV (RVU) | Trough (RVU) | BD (RVU) | FV (RVU) | Setback (RVU) | PT (°C) | 1047/1022 cm−1 | 1022/1000 cm−1 | |
| P | 267.08 ± 1.25 | 87.20 ± 0.13 | 179.88 ± 1.13 | 128.71 ± 0.04 | 41.50 ± 0.08 | 71.83 ± 0.08 | 1.107 | 1.024 |
| P + DFP250 | 573.17 ± 1.42 * | 287.42 ± 1.50 * | 285.75 ± 0.08 * | 395.79 ± 4.04 * | 108.38 ± 5.55 * | 70.50 ± 0.40 * | 1.103 | 1.042 |
| G | 387.13 ± 2.83 | 254.75 ± 1.58 | 132.38 ± 0.79 | 318.88 ± 1.05 | 64.13 ± 0.55 | 71.78 ± 0.02 | 1.151 | 0.890 |
| G + DFP250 | 521.77 ± 0.01 * | 403.96 ± 2.21 * | 117.79 ± 2.21 * | 584.25 ± 2.17 * | 180.30 ± 4.38 * | 66.48 ± 0.48 * | 1.084 | 1.174 |
| R | 156.13 ± 0.21 | 149.21 ± 0.13 | 6.92 ± 0.09 | 259.25 ± 0.58 | 110.04 ± 0.46 | 90.05 ± 0.35 | 1.167 | 0.905 |
| R + DFP250 | 456.09 ± 2.84 * | 433.08 ± 3.50 * | 23.00 ± 0.67 * | 604.25 ± 1.67 * | 171.17 ± 1.83 * | 70.53 ± 0.38 * | 1.122 | 1.159 |
| W | 108.25 ± 0.92 | 66.63 ± 0.70 | 41.63 ± 0.20 | 151.88 ± 0.21 | 85.25 ± 0.92 | 88.83 ± 0.02 | 1.140 | 1.005 |
| W + DFP250 | 456.50 ± 1.00 * | 373.42 ± 2.34 * | 83.09 ± 1.34 * | 553.96 ± 2.04 * | 180.54 ± 0.29 * | 58.85 ± 0.45 * | 1.078 | 1.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumroenvidhayakul, S.; Thilavech, T.; Abeywardena, M.; Adisakwattana, S. Investigating the Impact of Dragon Fruit Peel Waste on Starch Digestibility, Pasting, and Thermal Properties of Flours Used in Asia. Foods 2022, 11, 2031. https://doi.org/10.3390/foods11142031
Chumroenvidhayakul S, Thilavech T, Abeywardena M, Adisakwattana S. Investigating the Impact of Dragon Fruit Peel Waste on Starch Digestibility, Pasting, and Thermal Properties of Flours Used in Asia. Foods. 2022; 11(14):2031. https://doi.org/10.3390/foods11142031
Chicago/Turabian StyleChumroenvidhayakul, Siriwan, Thavaree Thilavech, Mahinda Abeywardena, and Sirichai Adisakwattana. 2022. "Investigating the Impact of Dragon Fruit Peel Waste on Starch Digestibility, Pasting, and Thermal Properties of Flours Used in Asia" Foods 11, no. 14: 2031. https://doi.org/10.3390/foods11142031







