Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Nutrients Composition of OE
2.2. Approval for Animal Experiments
2.3. Animal Diet and Care
2.4. Animal Experiment
2.5. Analysis of Serum Biochemical Parameters
2.6. Analysis of Gene Expression
2.7. Analysis of Fecal SCFA Compositions
2.8. Analysis of 16S rRNA Amplicon Sequence
2.9. Statistical Analysis of Data
3. Results
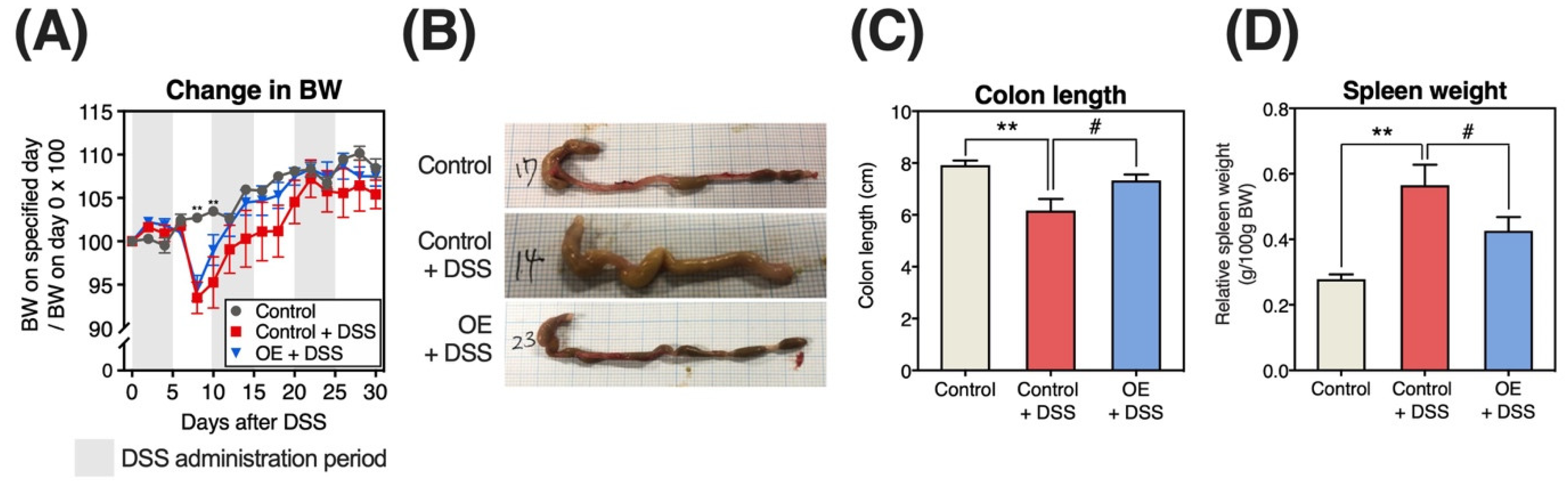
3.1. Growth Parameters, Change of BW, Colon Length, and Organs Weight
3.2. Change in DAI Scores
3.3. Serum Biochemical Parameters
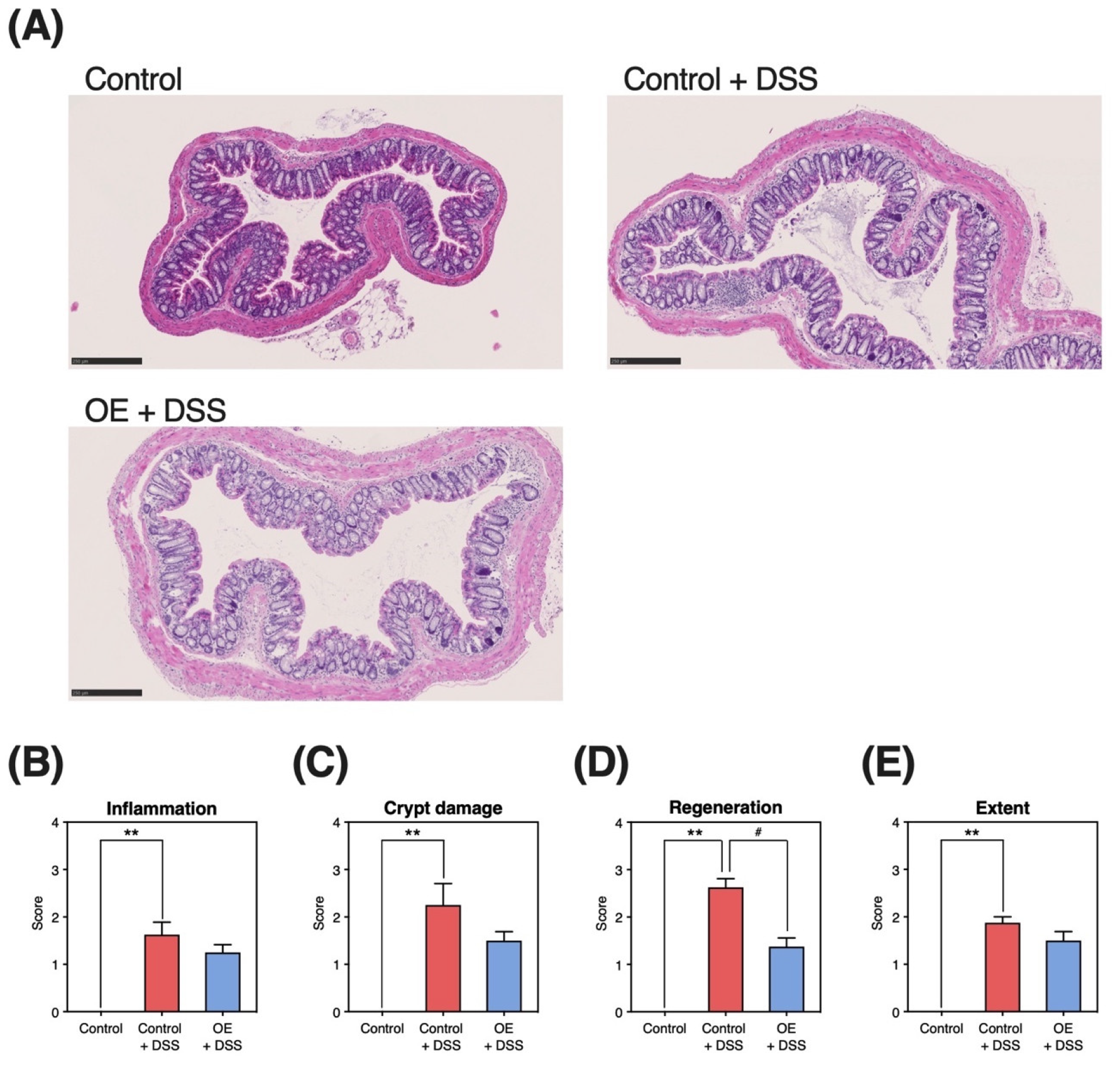
3.4. Histopathological Damage in the Colonic Tissue
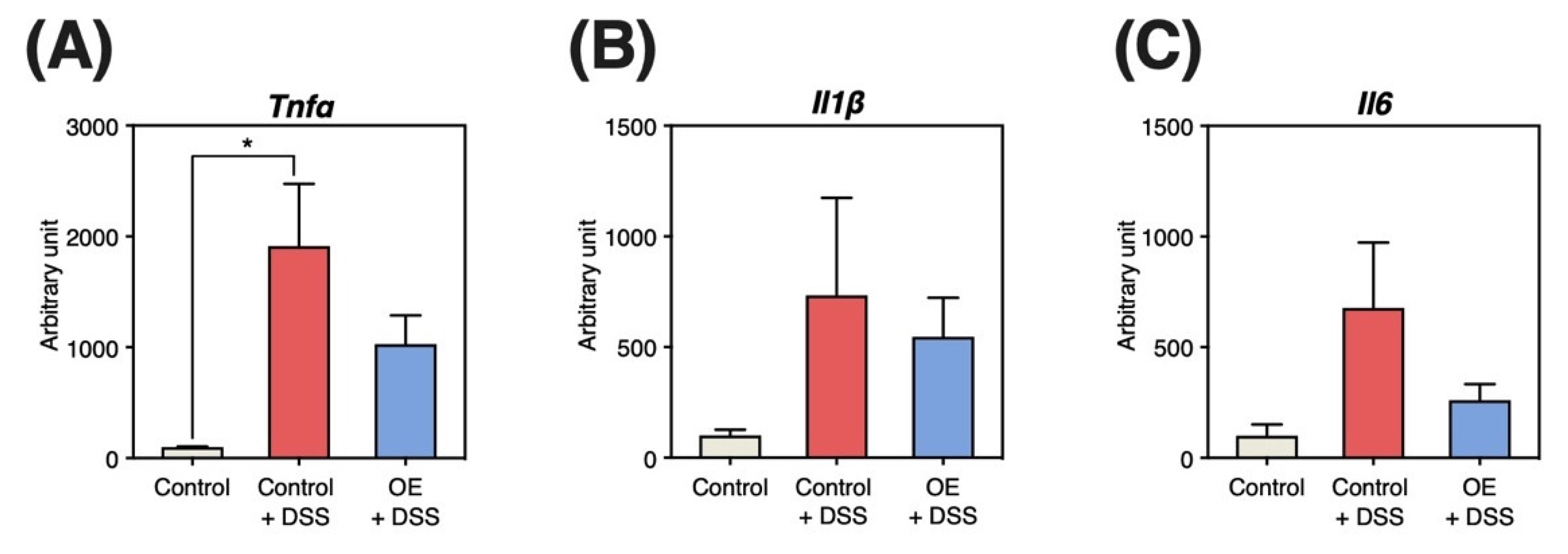
3.5. Gene Expression Levels in the Colonic Mucosa
3.6. Fecal SCFA Contents and Compositions
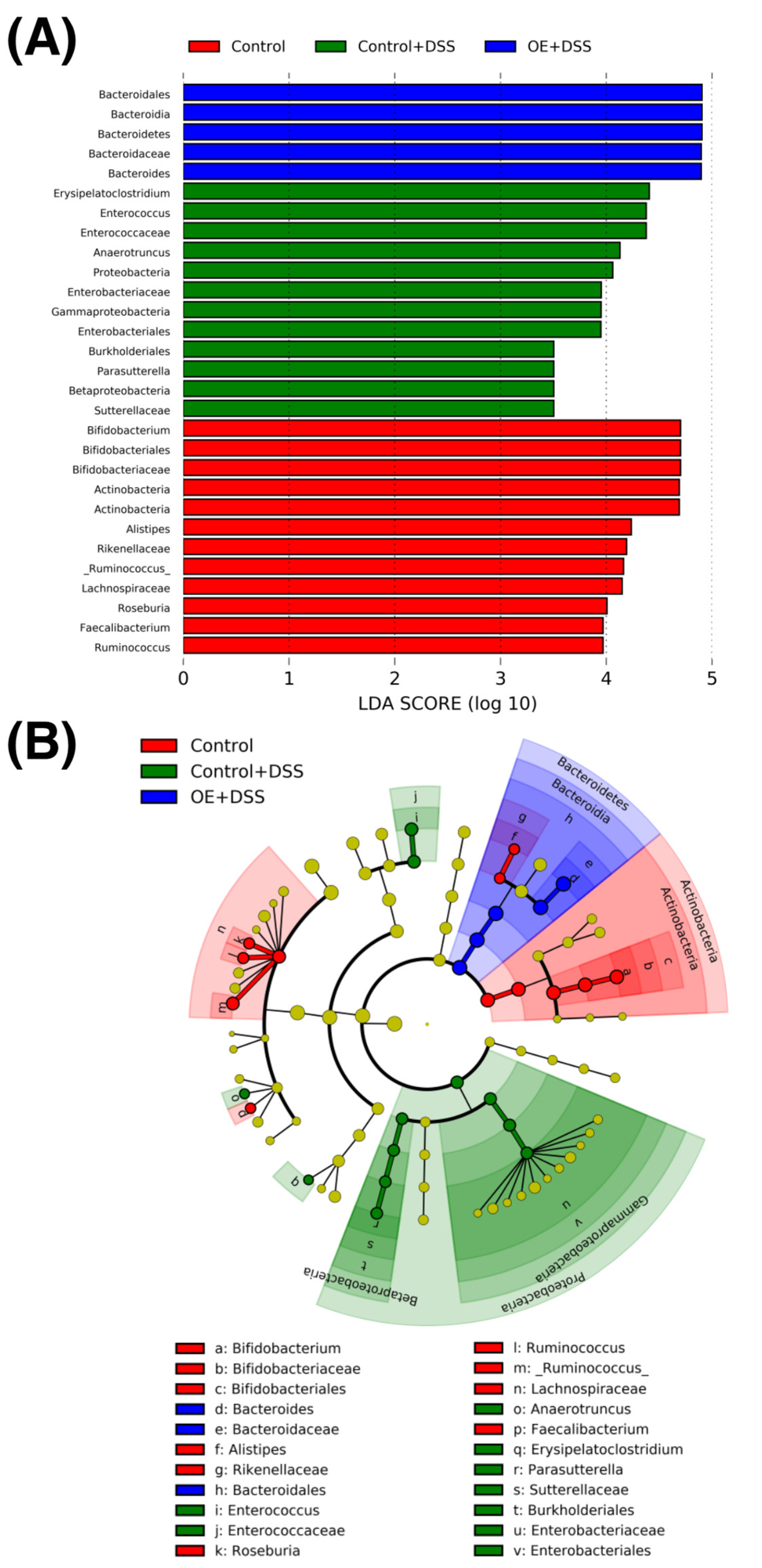
3.7. Diversity and Composition of Fecal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Liu, Y.; Tian, X.; Tian, Z. Antimicrobial capacity and antioxidant activity of enzymatic hydrolysates of protein from Rushan Bay Oyster (Crassostrea gigas). J. Food Process. Preserv. 2015, 39, 404–412. [Google Scholar] [CrossRef]
- Lee, H.-J.; Saravana, P.S.; Cho, Y.-N.; Haq, M.; Chun, B.-S. Extraction of bioactive compounds from oyster (Crassostrea gigas) by pressurized hot water extraction. J. Supercrit. Fluids 2018, 141, 120–127. [Google Scholar] [CrossRef]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and characterization of a phenolic antioxidant from the Pacific oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.C.; Velmurugan, S.; Donio, M.B.S.; Michaelbabu, M.; Citarasu, T. Studies on the antimicrobial potential and structural characterization of fatty acids extracted from Sydney rock oyster Saccostrea glomerata. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Yoshida, M. Examination of the anti-tumor action of oyster extract for P388 leukemia. J. Jpn. Health Med. Assoc. 2004, 13, 3–10. [Google Scholar]
- Matsuda, Y.; Izuta, S.; Fujita, T.; Murata, M.; Doi, E.; Ishizu, H.; Ohta, T.; Nakatsuka, M.; Tsubouti, R.; Shibata, Y. Influence of oyster extract on the platelet aggregation, the nucleotides and minerals of blood in diabetes mellitus patients. Trace Nutr. Res. 1992, 9, 67–73. [Google Scholar]
- Tanaka, K.; Nishizono, S.; Kugino, K.; Tamari, M.; Kurumiya, M.; Abe, N.; Ikeda, I. Effects of dietary oyster extract on lipid metabolism, blood pressure, and blood glucose in SD rats, hypertensive rats, and diabetic rats. Biosci. Biotechnol. Biochem. 2006, 70, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Hosomi, R.; Matsuda, Y.; Ishimaru, A.; Takemura, S.; Fukunaga, K.; Yoshida, M. Effect of dietary oyster extract on the colonic aberrant crypt foci in mice. Trace Nutr. Res. 2007, 24, 71–75. [Google Scholar]
- Matsudo, A.; Nishimura, N.; Hosomi, R.; Harumatsu, S.; Fukuda, S.; Matsuda, Y.; Yoshida, M.; Fukunaga, K. Dietary oyster-extract decreases cholesterol content by suppressing hepatic HMG-CoA reductase mRNA expression level and enhancing fecal steroids excretions in rats fed high-fat diet. Trace Nutr. Res. 2016, 33, 43–48. [Google Scholar]
- Hosomi, R.; Matsuda, Y.; Watanabe, Y.; Fukunaga, K.; Yoshida, M. Effect of dietary oyster extract on the p-aminophenol-induced nephrotoxicity in rats. Trace Nutr. Res. 2008, 25, 91–95. [Google Scholar]
- Amato, K.R.; Arrieta, M.-C.; Azad, M.B.; Bailey, M.T.; Broussard, J.L.; Bruggeling, C.E.; Claud, E.C.; Costello, E.K.; Davenport, E.R.; Dutilh, B.E.; et al. The human gut microbiome and health inequities. Proc. Natl. Acad. Sci. USA 2021, 118, e2017947118. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, Y.; Hosomi, R.; Matsuda, Y.; Arai, H.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Effects of dietary oyster extract on the gut microbiome in rats. Trace Nutr. Res. 2018, 35, 41–46. [Google Scholar]
- Nakamura, J.; Ikeda, Y.; Hosomi, R.; Ishida, T.; Matsui, H.; Matsuda, Y.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; et al. Effects of dietary oyster-extract on the composition of the microbiota and short-chain fatty acids in the feces of mice. Trace Nutr. Res. 2019, 36, 21–28. [Google Scholar]
- Ishida, T.; Matsui, H.; Matsuda, Y.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Fukunaga, K.; Yoshida, M. Oyster (Crassostrea gigas) extract attenuates dextran sulfate sodium-induced acute experimental colitis by improving gut microbiota and short-chain fatty acids compositions in mice. Foods 2022, 11, 373. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Liu, Y.D.; Zhu, J.Q.; Zhou, Y.L.; Chen, H.T.; Xu, J.; Zhao, H.L.; Guo, X.; Shi, W.; et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Optimization of a Chronic Colitis Model in Mice Induced by Dextran Sulfate Sodium (DSS) and Effects of Drugs Thereon. Available online: https://www.criver.com/sites/default/files/resource-files/SP-DDW-18-optimization-of-a-chronic-colitis-model-in-mice-induced-by-dextran-sulphate-sodium.pdf (accessed on 30 May 2022).
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Ito, R.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Kita, M.; Ueda, Y.; Iwakura, Y.; et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 2006, 146, 330–338. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]
- Tanaka, G.; Hagihara, N.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Alaska pollock protein attenuates the experimental colitis induced by dextran sulfate sodium via regulation of gut microbiota and its metabolites in mice. Metabolites 2022, 12, 44. [Google Scholar] [CrossRef]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary fat influences the expression of genes related to sterol metabolism and the composition of cecal microbiota and its metabolites in rats. J. Oleo Sci. 2019, 68, 1133–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, L.G.; Landström, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Zheng, Y.; Song, Y.; Han, Q.; Liu, W.; Xu, J.; Yu, Z.; Zhang, R.; Li, N. Intestinal epithelial cell-specific IGF1 promotes the expansion of intestinal stem cells during epithelial regeneration and functions on the intestinal immune homeostasis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E638–E649. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediat. Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Miyata, M.; Tanaka, T.; Takahashi, K.; Funaki, A.; Sugiura, Y. Cholesterol-lowering effects of taurine through the reduction of ileal FXR signaling due to the alteration of ileal bile acid composition. Amino Acids 2021, 53, 1523–1532. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 2, 712–715. [Google Scholar] [CrossRef]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Karakoyun, B.; Ertaş, B.; Yüksel, M.; Akakın, D.; Çevik, Ö.; Şener, G. Ameliorative effects of riboflavin on acetic acid-induced colonic injury in rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 563–572. [Google Scholar] [CrossRef]
- Kang, K.; Sun, Y.; Pan, D.; Chang, B.; Sang, L.X. Nicotinamide Ameliorates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice through Its Anti-Inflammatory Properties and Modulates the Gut Microbiota. J. Immunol. Res. 2021, 2021, 5084713. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral administration of compound probiotics ameliorates HFD-induced gut microbe dysbiosis and chronic metabolic inflammation via the G protein-coupled receptor 43 in non-alcoholic fatty liver disease rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef]
- Yang, K.M.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Yang, X.F. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K881. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Maki, J.J.; Looft, T. Turicibacter bilis sp. nov., a novel bacterium isolated from the chicken eggshell and swine ileum. Int. J. Syst. Evol. Microbiol. 2022, 72, 005153. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015, 21, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Jang, M.; Hwang, I.; Cho, J.; Kim, S. Radish sprout alleviates DSS-induced colitis via regulation of NF-kB signaling pathway and modifying gut microbiota. Biomed. Pharmacother. 2021, 144, 112365. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Jiang, Q.; Shao, W.; Li, J.; Zhou, Y.; Chen, L.; Deng, S.; Zheng, B.; Chen, Y. Protective Effects of Shrimp Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Nutr. 2021, 8, 773064. [Google Scholar] [CrossRef]
- Oh, S.Y.; Cho, K.A.; Kang, J.L.; Kim, K.H.; Woo, S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: A pilot study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The administration of Escherichia coli Nissle 1917 ameliorates development of DSS-induced colitis in mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef]
- Long, T.; Yu, Z.-J.; Wang, J.; Liu, J.; He, B.-S. Orally administered chitooligosaccharides modulate colon microbiota in normal and colitis mice. Int. J. Pharmacol. 2018, 14, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The relationship between gut microbiota and inflammatory diseases: The role of macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.; Schmidt, A.; Waldron, C.; Theis, K.; Schmidt, T. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Salem, F.; Kindt, N.; Marchesi, J.R.; Netter, P.; Lopez, A.; Kokten, T.; Danese, S.; Jouzeau, J.Y.; Peyrin-Biroulet, L.; Moulin, D. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: Similarities and differences. United Eur. Gastroenterol. J. 2019, 7, 1008–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharshak, N.; Huh, E.Y.; Paiboonrungruang, C.; Shanahan, M.; Thurlow, L.; Herzog, J.; Djukic, Z.; Orlando, R.; Pawlinski, R.; Ellermann, M.; et al. Enterococcus faecalis gelatinase mediates intestinal permeability via protease-activated receptor 2. Infect. Immun. 2015, 83, 2762–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, Y.; Xu, X.; Guo, L.; Yu, Y.; Li, N.; Xu, C. Characteristics of fecal microbiota and machine learning strategy for fecal invasive biomarkers in pediatric inflammatory bowel disease. Front. Cell Infect. Microbiol. 2021, 11, 711884. [Google Scholar] [CrossRef]
- Furuyashiki, T.; Takata, H.; Kojima, I.; Kuriki, T.; Fukuda, I.; Ashida, H. Metabolic fate of orally administered enzymatically synthesized glycogen in rats. Food Funct. 2011, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Zhao, Z.; Ishimoto, Y.; Satsu, H. Dietary taurine attenuates dextran sulfate sodium (DSS)-induced experimental colitis in mice. Adv. Exp. Med. Biol. 2009, 643, 265–271. [Google Scholar]
- Higashimura, Y.; Takagi, T.; Naito, Y.; Uchiyama, K.; Mizushima, K.; Tanaka, M.; Hamaguchi, M.; Itoh, Y. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J. Crohns Colitis 2020, 14, 856–866. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, T.; Matsui, H.; Matsuda, Y.; Shimono, T.; Kanda, S.; Nishiyama, T.; Hosomi, R.; Fukunaga, K.; Yoshida, M. Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice. Foods 2022, 11, 2032. https://doi.org/10.3390/foods11142032
Ishida T, Matsui H, Matsuda Y, Shimono T, Kanda S, Nishiyama T, Hosomi R, Fukunaga K, Yoshida M. Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice. Foods. 2022; 11(14):2032. https://doi.org/10.3390/foods11142032
Chicago/Turabian StyleIshida, Tatsuya, Hiroyuki Matsui, Yoshikazu Matsuda, Takaki Shimono, Seiji Kanda, Toshimasa Nishiyama, Ryota Hosomi, Kenji Fukunaga, and Munehiro Yoshida. 2022. "Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice" Foods 11, no. 14: 2032. https://doi.org/10.3390/foods11142032
APA StyleIshida, T., Matsui, H., Matsuda, Y., Shimono, T., Kanda, S., Nishiyama, T., Hosomi, R., Fukunaga, K., & Yoshida, M. (2022). Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice. Foods, 11(14), 2032. https://doi.org/10.3390/foods11142032





