Soybean Oil Bodies as a Milk Fat Substitute Improves Quality, Antioxidant and Digestive Properties of Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of SOBs
2.3. Preparation of SOB Yogurt
2.4. Physicochemical Properties of SOB Yogurt
2.5. Water Holding Capacity of SOB Yogurt
2.6. Tocopherol Content of SOB Yogurt
2.7. Texture Characteristics of SOB Yogurt
2.8. Rheological Properties of SOB Yogurt
2.9. PV and TBARS Values of SOB Yogurt
2.10. Microstructure of SOB Yogurt
2.11. Sensory Evaluation of SOB Yogurt
2.12. In Vitro Simulated Digestion of SOB Yogurt
2.13. Determination of Free Fatty Acids
2.14. DPPH and ABTS Radical Scavenging Rate
2.15. Determination of Protein Digestibility
2.16. Determination of Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.17. Statistical Analysis
3. Results and Analysis
3.1. Physicochemical Properties of SOB Yogurt
3.2. Contents of Fatty Acids and Tocopherol in SOB Yogurt
3.3. Texture Characteristics of SOB Yogurt
3.4. Rheological Properties of SOB Yogurt
3.5. PV and TBARS Values of SOB Yogurt
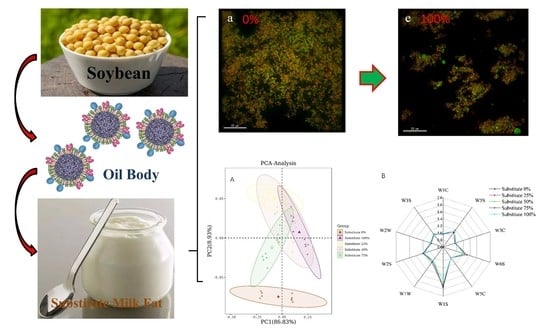
3.6. Microstructure of SOB Yogurt
3.7. Flavor of SOB Yogurt
3.8. Sensory Evaluation of SOB Yogurt
3.9. Release Amount of Free Fatty Acids in SOB Yogurt
3.10. Protein Digestibility of SOB Yogurt
3.11. DPPH and ABTS Free Radical Scavenging Rates of SOB Yogurt
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhou, X.; Wang, W.; Gu, L.Y.; Hu, C.B.; Sun, H.; Xu, C.; Hou, J.C.; Jiang, Z.M. Lactobacillus paracasei 24 Attenuates Lipid Accumulation in HighFat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.J.; Mehrabani, D.; Saberifiroozi, M.; Fattahi, M.R.; Moradi, F.; Najafi, M. The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci. Nutr. 2021, 9, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, Y.Y.; Liu, F.; Liu, Z.J.; Ma, J.G.; Jiang, R.; Song, C.N.; Jiang, Z.M.; Hou, J.C. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. Lwt Food Sci. Technol. 2021, 137, 110338. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef]
- Han, H.T.; Zhao, L.P.; Liu, X.N.; Guo, A.M.; Li, X.Y. Effect of water bath-assisted water extraction on physical and chemical properties of soybean oil body emulsion. Food Sci. Nutr. 2020, 8, 6380–6391. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.L.; Zhao, J.L.; Sun, R.B.; Shang, H.; Sun, C.Q.; Liu, L.W.; Hou, J.C.; Jiang, Z.M. Physical and oxidative stability of astaxanthin microcapsules prepared with liposomes. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.L.; Zhao, X.; Sun, R.B.; Sun, C.Q.; Hou, D.D.; Zhang, X.W.; Jiang, L.Z.; Hou, J.C.; Jiang, Z.M. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 3271–3282. [Google Scholar] [CrossRef]
- Zaaboul, F.; Zhao, Q.L.; Xu, Y.J.; Liu, Y.F. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Wu, N.-N.; Huang, X.; Yang, X.-Q.; Guo, J.; Yin, S.-W.; He, X.-T.; Wang, L.-J.; Zhu, J.-H.; Qi, J.-R.; Zheng, E.-L. In vitro assessment of the bioaccessibility of fatty acids and tocopherol from soybean oil body emulsions stabilized with ι-carrageenan. J. Agric. Food Chem. 2012, 60, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Karkani, O.A.; Nenadis, N.; Nikiforidis, C.V.; Kiosseoglou, V. Effect of recovery methods on the oxidative and physical stability of oil body emulsions. Food Chem. 2013, 139, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.T.; Naziri, E.; Kyriakidou, A.; Paraskevopoulou, A.; Tsimidou, M.Z.; Kiosseoglou, V. Oil bodies from dry maize germ as an effective replacer of cow milk fat globules in yogurt-like product formulation. Lwt Food Sci. Technol. 2019, 105, 48–56. [Google Scholar] [CrossRef]
- Wang, W.; Cui, C.L.; Wang, Q.L.; Sun, C.B.; Jiang, L.Z.; Hou, J.C. Effect of pH on physicochemical properties of oil bodies from different oil crops. J. Food Sci. Technol. Mysore 2019, 56, 49–58. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of soybean oil body as a milk fat substitute on ice cream: Physicochemical, sensory and digestive properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef]
- da Silva, J.M.; Klososki, S.J.; Silva, R.; Raices, R.S.L.; Silva, M.C.; Freitas, M.Q.; Barão, C.E.; Pimentel, T.C. Passion fruit-flavored ice cream processed with water-soluble extract of rice by-product: What is the impact of the addition of different prebiotic components? LWT 2020, 128, 109472. [Google Scholar] [CrossRef]
- Ercili-Cura, D.; Lille, M.; Legland, D.; Gaucel, S.; Poutanen, K.; Partanen, R.; Lantto, R. Structural mechanisms leading to improved water retention in acid milk gels by use of transglutaminase. Food Hydrocoll. 2013, 30, 419–427. [Google Scholar] [CrossRef]
- Bertolín, J.; Joy, M.; Rufino-Moya, P.; Lobón, S.; Blanco, M. Simultaneous determination of carotenoids, tocopherols, retinol and cholesterol in ovine lyophilised samples of milk, meat, and liver and in unprocessed/raw samples of fat. Food Chem. 2018, 257, 182–188. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Mu, S.N.; Ma, C.L.; Liu, Y.; Ma, Y.; Zhang, M.H.; Li, H.Y.; Liu, X.Q.; Hou, J.C.; Tian, B. Consequences of ball milling combined with high-pressure homogenization on structure, physicochemical and rheological properties of citrus fiber. Food Hydrocoll. 2022, 127, 107515. [Google Scholar] [CrossRef]
- Su, Y.-R.; Tsai, Y.-C.; Hsu, C.-H.; Chao, A.-C.; Lin, C.-W.; Tsai, M.-L.; Mi, F.-L. Effect of grape seed proanthocyanidin–gelatin colloidal complexes on stability and in vitro digestion of fish oil emulsions. J. Agric. Food Chem. 2015, 63, 10200–10208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Z.J.; Wang, W.; Miao, Y.S.; Gu, L.Y.; Li, Y.A.; Liu, X.; Jiang, L.Z.; Hou, J.C.; Jiang, Z.M. NaCl induces flocculation and lipid oxidation of soybean oil body emulsions recovered by neutral aqueous extraction. J. Sci. Food Agric. 2022, 102, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Marand, M.A.; Amjadi, S.; Marand, M.A.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Yu, H.; Mu, S.; Li, H.; Liu, X.; Zhang, M.; Jiang, Z.; Hou, J. Biological activities and in vitro digestion characteristics of glycosylated α-lactalbumin prepared by microwave heating: Impacts of ultrasonication. LWT 2022, 158, 113141. [Google Scholar] [CrossRef]
- Ding, J.; Xu, Z.J.; Qi, B.K.; Jiang, L.Z.; Sui, X.N. Physicochemical and oxidative stability of a soybean oleosome-based emulsion and its in vitro digestive fate as affected by (-)-epigallocatechin-3-gallate. Food Funct. 2018, 9, 6147–6155. [Google Scholar] [CrossRef]
- Islam, M.; Haque, A.R.; Kabir, M.; Hasan, M.; Khushe, K.J.; Hasan, S. Fruit by-products: The potential natural sources of antioxidants and α-glucosidase inhibitors. J. Food Sci. Technol. 2021, 58, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.-A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-covalent interaction and digestive characteristics between α-lactalbumin and safflower yellow: Impacts of microwave heating temperature. LWT 2022, 159, 113206. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Aqueous Extraction of Oil Bodies from Maize Germ (Zea mays) and Characterization of the Resulting Natural Oil-in-Water Emulsion. J. Agric. Food Chem. 2009, 57, 5591–5596. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an organic yogurt based on sensorial, nutritional, and functional perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef]
- Corekci, B.; Toy, E.; Ozturk, F.; Malkoc, S.; Ozturk, B. Effects of contemporary orthodontic composites on tooth color following short-term fixed orthodontic treatment: A controlled clinical study. Turk. J. Med. Sci. 2015, 45, 1421–1428. [Google Scholar] [CrossRef]
- Kapchie, V.N.; Yao, L.X.; Hauck, C.C.; Wang, T.; Murphy, P.A. Oxidative stability of soybean oil in oleosomes as affected by pH and iron. Food Chem. 2013, 141, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Speziali, G.; Liesinger, L.; Gindlhuber, J.; Leopold, C.; Pucher, B.; Brandi, J.; Castagna, A.; Tomin, T.; Krenn, P.; Thallinger, G.G.; et al. Myristic acid induces proteomic and secretomic changes associated with steatosis, cytoskeleton remodeling, endoplasmic reticulum stress, protein turnover and exosome release in HepG2 cells. J. Proteom. 2018, 181, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Castaner, O.; Fito, M. Response to Letter Regarding Article, “Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial”. Circulation 2017, 136, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Reineccius, G.A.; Milo, C. Effect of type of oil and addition of delta-tocopherol on model flavor compound stability during storage. J. Agric. Food Chem. 2007, 55, 9189–9194. [Google Scholar] [CrossRef] [PubMed]
- Chimi, H.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Klost, M.; Brzeski, C.; Drusch, S. Effect of protein aggregation on rheological properties of pea protein gels. Food Hydrocoll. 2020, 108, 106036. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure formation and rheological properties of pea protein-based gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Player, M.; Kim, H.; Lee, H.; Min, D. Stability of α-, γ-, or δ-tocopherol during soybean oil oxidation. J. Food Sci. 2006, 71, C456–C460. [Google Scholar] [CrossRef]
- Lucey, J.A. ADSA Foundation Scholar Award. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef]
- Clulow, A.J.; Salim, M.; Hawley, A.; Boyd, B.J. A closer look at the behaviour of milk lipids during digestion. Chem. Phys. Lipids 2018, 211, 107–116. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhao, J.L.; Yu, R.; Hussain, M.A.; Qayum, A.; Jiang, Z.M.; Qu, B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chem. 2022, 383, 132402. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.H.; Zhang, W.W.; He, R.H.; Xiong, F.; Ma, H.L. Protein breakdown and release of antioxidant peptides during simulated gastrointestinal digestion and the absorption by everted intestinal sac of rapeseed proteins. Lwt Food Sci. Technol. 2017, 86, 424–429. [Google Scholar] [CrossRef]
- Chang, M.T.; Tsai, T.R.; Lee, C.Y.; Wei, Y.S.; Chen, Y.J.; Chen, C.R.; Tzen, J.T.C. Elevating Bioavailability of Curcumin via Encapsulation with a Novel Formulation of Artificial Oil Bodies. J. Agric. Food Chem. 2013, 61, 9666–9671. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Days | Substitution Amount | ||||
|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | ||
| pH value | 0 | 4.50 ± 0.02 a | 4.49 ± 0.05 a | 4.48 ± 0.04 a | 4.49 ± 0.03 a | 4.48 ± 0.01 a |
| 7 | 4.43 ± 0.04 a | 4.42 ± 0.03 a | 4.41 ± 0.06 a | 4.42 ± 0.03 a | 4.44 ± 0.02 a | |
| 14 | 4.32 ± 0.04 a | 4.31 ± 0.02 a | 4.34 ± 0.04 a | 4.32 ± 0.02 a | 4.31 ± 0.02 a | |
| 21 | 4.08 ± 0.03 a | 4.06 ± 0.02 a | 4.09 ± 0.04 a | 4.07 ± 0.04 a | 4.05 ± 0.01 a | |
| Titratable acidity (°T) | 0 | 65.62 ± 0.16 a | 67.34 ± 0.13 a | 67.14 ± 0.08 a | 69.16 ± 0.17 a | 65.98 ± 0.13 a |
| 7 | 73.66 ± 0.09 a | 72.83 ± 0.15 a | 74.71 ± 0.11 a | 74.35 ± 0.12 a | 73.63 ± 0.10 a | |
| 14 | 80.15 ± 0.18 a | 81.03 ± 0.14 a | 79.13 ± 0.22 a | 79.75 ± 0.12 a | 80.15 ± 0.21 a | |
| 21 | 90.34 ± 0.16 a | 89.76 ± 0.20 a | 90.98 ± 0.18 a | 90.19 ± 0.14 a | 89.83 ± 0.17 a | |
| water-holding power (%) | 0 | 65.57 ± 0.06 d | 88.57 ± 0.27 a | 76.18 ± 0.17 b | 72.77 ± 0.10 c | 59.41 ± 0.12 e |
| 7 | 56.16 ± 0.11 c | 86.42 ± 0.15 a | 68.11 ± 0.05 b | 66.92 ± 0.03 b | 47.46 ± 0.08 d | |
| 14 | 53.46 ± 0.10 c | 82.60 ± 0.08 a | 63.53 ± 0.14 b | 64.35 ± 0.12 b | 36.67 ± 0.15 d | |
| 21 | 47.67 ± 0.13 c | 78.43 ± 0.15 a | 59.32 ± 0.12 b | 58.76 ± 0.04 b | 32.45 ± 0.08 d | |
| Protein content (%) | 2.97 ± 0.03 e | 3.17 ± 0.03 d | 3.34 ± 0.03 c | 3.58 ± 0.04 b | 3.76 ± 0.04 a | |
| Fat content (%) | 3.54 ± 0.02 a | 3.54 ± 0.03 a | 3.55 ± 0.03 a | 3.56 ± 0.04 a | 3.55 ± 0.04 a | |
| Solid content (%) | 15.47 ± 0.04 e | 15.68 ± 0.03 d | 15.83 ± 0.07 c | 16.10 ± 0.03 b | 16.26 ± 0.05 a | |
| Color | ||||||
| L* | 102.55 ± 0.04 a | 98.28 ± 0.11 b | 94.46 ± 0.06 c | 91.91 ± 0.13 d | 90.73 ± 0.18 d | |
| a* | −1.31 ± 0.06 b | −1.20 ± 0.04 b | −0.82 ± 0.08 a | −0.52 ± 0.08 a | −0.47 ± 0.04 a | |
| b* | 7.62 ± 0.08 e | 9.36 ± 0.01 d | 10.49 ± 0.08 c | 11.41 ± 0.08 b | 12.35 ± 0.05 a | |
| ΔE | 0.11 | 0.76 | 1.52 | 2.33 | ||
| Fatty acids | ||||||
| Cinnamic acid | 12.74 ± 0.05 a | 7.96 ± 0.01 b | 4.22 ± 0.02 c | 2.22 ± 0.00 d | 0.93 ± 0.01 e | |
| Palmitic acid | 43.67 ± 0.14 a | 31.78 ± 0.08 b | 22.33 ± 0.07 c | 17.29 ± 0.04 d | 14.02 ± 0.03 e | |
| Stearic acid | 13.76 ± 0.08 a | 10.31 ± 0.02 b | 7.44 ± 0.02 c | 5.95 ± 0.03 d | 5.06 ± 0.01 e | |
| Oleic acid | 25.36 ± 0.13 e | 26.32 ± 0.12 d | 26.95 ± 0.08 c | 27.60 ± 0.04 b | 28.01 ± 0.11 a | |
| Linoleic acid | 4.08 ± 0.03 e | 20.68 ± 0.07 d | 34.30 ± 0.10 c | 41.30 ± 0.12 b | 45.78 ± 0.08 a | |
| Linolenic acid | 0.39 ± 0.01 e | 2.95 ± 0.01 d | 4.76 ± 0.03 c | 5.64 ± 0.02 b | 6.20 ± 0.02 a | |
| Saturated fatty acid | 70.17 ± 0.06 a | 50.05 ± 0.07 b | 33.99 ± 0.03 c | 25.46 ± 0.08 d | 20.01 ± 0.05 e | |
| Unsaturated fatty acid | 29.83 ± 0.02 e | 49.95 ± 0.12 d | 66.01 ± 0.11 c | 74.54 ± 0.12 b | 79.99 ± 0.17 a | |
| Tocopherol (µg/kg) | ||||||
| δ-tocopherol | - | 517.11 ± 1.47 d | 1203.32 ± 2.41 c | 1558.99 ± 7.16 b | 2223.31 ± 10.61 a | |
| γ-tocopherol | - | 201.68 ± 1.29 d | 275.64 ± 1.68 c | 326.42 ± 1.57 b | 450.66 ± 1.72 a | |
| α-tocopherol | - | 862.16 ± 1.45 d | 1415.29 ± 4.13 c | 1986.62 ± 7.89 b | 2829.54 ± 9.71 a | |
| Indicators | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| Hardness (g) | 119.20 ± 0.83 a | 113.56 ± 0.49 b | 87.24 ± 0.39 c | 63.02 ± 0.34 d | 48.37 ± 0.96 e |
| Consistency (g·s) | 1060.04 ± 1.54 a | 1027.31 ± 2.26 a | 733.43 ± 1.68 b | 543.60 ± 1.33 c | 413.94 ± 0.69 d |
| Cohesion (g) | 50.11 ± 0.98 a | 48.65 ± 0.98 a | 38.41 ± 0.71 b | 30.17 ± 0.75 c | 22.23 ± 0.70 d |
| Viscosity index (g·s) | 500.41 ± 0.83 a | 472.76 ± 0.20 a | 359.46 ± 0.29 b | 250.98 ± 0.14 c | 143.97 ± 0.36 d |
| Indicators | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| apparent | 8.42 ± 0.23 a | 8.33 ± 0.20 a | 7.79 ± 0.18 b | 7.40 ± 0.22 c | 7.23 ± 0.23 d |
| taste | 8.17 ± 0.16 b | 8.41 ± 0.15 a | 7.60 ± 0.23 c | 6.77 ± 0.14 d | 6.37 ± 0.21 e |
| bean smell | 0.00 ± 0.00 e | 0.31 ± 0.02 d | 0.45 ± 0.03 c | 0.65 ± 0.04 b | 1.01 ± 0.04 a |
| acidity | 8.36 ± 0.23 a | 8.38 ± 0.16 a | 8.31 ± 0.21 a | 8.37 ± 0.22 a | 8.35 ± 0.26 a |
| overall evaluation | 8.27 ± 0.14 b | 8.54 ± 0.29 a | 7.77 ± 0.31 c | 7.23 ± 0.27 d | 6.78 ± 0.16 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, N.; Sun, R.; Su, C.; Ma, Y.; Zhang, X.; Wu, M.; Hou, J. Soybean Oil Bodies as a Milk Fat Substitute Improves Quality, Antioxidant and Digestive Properties of Yogurt. Foods 2022, 11, 2088. https://doi.org/10.3390/foods11142088
Dou N, Sun R, Su C, Ma Y, Zhang X, Wu M, Hou J. Soybean Oil Bodies as a Milk Fat Substitute Improves Quality, Antioxidant and Digestive Properties of Yogurt. Foods. 2022; 11(14):2088. https://doi.org/10.3390/foods11142088
Chicago/Turabian StyleDou, Nianxu, Rongbo Sun, Chengcheng Su, Yue Ma, Xuewei Zhang, Mengguo Wu, and Juncai Hou. 2022. "Soybean Oil Bodies as a Milk Fat Substitute Improves Quality, Antioxidant and Digestive Properties of Yogurt" Foods 11, no. 14: 2088. https://doi.org/10.3390/foods11142088
APA StyleDou, N., Sun, R., Su, C., Ma, Y., Zhang, X., Wu, M., & Hou, J. (2022). Soybean Oil Bodies as a Milk Fat Substitute Improves Quality, Antioxidant and Digestive Properties of Yogurt. Foods, 11(14), 2088. https://doi.org/10.3390/foods11142088