Nonenzymatic Browning of Amorphous Maltose/Whey Protein Isolates Matrix: Effects of Water Sorption and Molecular Mobility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Water Sorption Testing
2.3. Color Changes Measurement
2.4. HPLC Analysis
2.5. NEB Reaction Kinetics
2.6. Molecular Mobility Analysis
2.7. Statistical Analysis
3. Results
3.1. Water Sorption Isotherms
3.2. Color Changes Measurement
3.3. HPLC Measurements
3.4. NEB Reaction Kinetics
3.5. Molecular Mobility and NEB Reactivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, S.; Roos, Y.H. Isothermal study of nonenzymatic browning kinetics in spray-dried and freeze-dried systems at different relative vapor pressure environments. Innov. Food Sci. Emerg. Technol. 2006, 7, 182–194. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; SchmittKopplin, P. Insights into the chemistry of non-enzymatic browning reactions in different ribose-amino acid model systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.N.; Li, Y.; Yang, Y.; Yu, K. The browning kinetics of the nonenzymatic browning reaction in L-ascorbic acid/basic amino acid systems. Food Sci. Technol. 2017, 38, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Wang, J.; Liu, F.; Tang, J.; Bohnet, S. Application of non-enzymatic browning of fructose for heating pattern determination in microwave assisted thermal pasteurization system. J. Food Eng. 2017, 210, 27–34. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; Van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Quevedo, R.; Rojas, R.; Pedreschi, F.; Bastias, J.M.; Siché, R.; Uquiche, E.; Díaz, O. Quantification of the browning kinetic on pita bread using fractal method. Food Bioprocess Technol. 2018, 11, 201–208. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by Maillard conjugation and Maillard reaction products. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Water and the glass transition—Dependence of the glass transition on composition and chemical structure: Special implications for flour functionality in cookie baking. J. Food Eng. 1995, 24, 431–509. [Google Scholar] [CrossRef]
- Rapusas, R.S.; Driscoll, R.H. Kinetics of non-enzymatic browning in onion slices during isothermal heating. J. Food Eng. 1995, 24, 417–429. [Google Scholar] [CrossRef]
- Roos, Y.H.; Jouppila, K.; Zielasko, B. Non-enzymatic browning-induced water plasticization: Glass transition temperature depression and reaction kinetics determination using DSC. J. Therm. Anal. Calorim. 1996, 47, 1437–1450. [Google Scholar] [CrossRef]
- Sircar, S. Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2016, 45, 5435–5448. [Google Scholar] [CrossRef]
- Silalai, N.; Roos, Y.H. Coupling of dielectric and mechanical relaxations with glass transition and stickiness of milk solids. J. Food Eng. 2011, 104, 445–454. [Google Scholar] [CrossRef]
- Acevedo, N.C.; Schebor, C.; Buera, P. Non-enzymatic browning kinetics analysed through water–solids interactions and water mobility in dehydrated potato. Food Chem. 2008, 108, 900–906. [Google Scholar] [CrossRef]
- Miao, S.; Roos, Y.H. Nonenzymatic browning kinetics of a carbohydrate based low-moisture food system at temperatures applicable to spray drying. J. Agric. Food Chem. 2004, 52, 5250–5257. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Crystallization and structural relaxation times in structural strength analysis of amorphous sugar/whey protein systems. Food Hydrocoll. 2016, 60, 85–97. [Google Scholar] [CrossRef]
- Hancock, B.C.; Shamblin, S.L.; Zografi, G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 1995, 12, 799–806. [Google Scholar] [CrossRef]
- Liu, W.; Roos, Y.H.; Fan, F. Physicochemical properties and relaxation time in strength analysis of amorphous poly (vinyl-pyrrolidone) and maltodextrin: Effects of water, molecular weight, and lactose addition. J. Food Eng. 2018, 226, 82–95. [Google Scholar] [CrossRef]
- Pham, H.T.; Bazmawe, M.; Kebede, B.; Buvé, C.; Hendrickx, M.E.; Van Loey, A.M. Changes in the soluble and insoluble compounds of shelf-stable orange juice in relation to non-enzymatic browning during storage. J. Agric. Food Chem. 2019, 67, 12854–12862. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Structural strength and crystallization of amorphous lactose in food model solids at various water activities. Innov. Food Sci. Emerg. 2017, 40, 27–34. [Google Scholar] [CrossRef]
- Corey, M.E.; Kerr, W.L.; Mulligan, J.H.; Lavelli, V. Phytochemical stability in dried apple and green tea functional products as related to moisture properties. LWT-Food Sci. Technol. 2011, 44, 67–74. [Google Scholar] [CrossRef]
- Maidannyk, V.A.; Roos, Y.H. Water sorption, glass transition and “strength” of lactose–Whey protein systems. Food Hydrocoll. 2017, 70, 76–87. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, W.; Cui, T.; Fan, F. Crystallization and strength analysis of amorphous maltose and maltose/whey protein isolate mixtures. J. Sci. Food Agric 2021, 101, 2542–2551. [Google Scholar] [CrossRef]
- O’Brien, J.; Bladon, P. Molecular Modelling Study of the Mechanism of Acid-Catalysed Disaccharide Hydrolysis: Implications for Nonenzymatic Browning Reactions. In The Maillard Reaction in Foods and Medicine; Woodhead Publishing: Cambridge, UK, 2005; pp. 147–153. [Google Scholar]
- Burin, L.; Jouppila, K.; Roos, Y.; Kansikas, J.; Buera, M.D.P. Color formation in dehydrated modified whey powder systems as affected by compression and Tg. J. Agric. Food Chem. 2000, 48, 5263–5268. [Google Scholar] [CrossRef]
- Tun, H.; Chen, C.C. Isosteric heat of adsorption from thermodynamic Langmuir isotherm. Adsorption 2021, 27, 979–989. [Google Scholar] [CrossRef]
- Marques, R.C.D.; Oliveira, É.R.; Coutinho, G.S.M.; Ribeiro, A.E.C.; Teixeira, C.S.; Júnior, M.S.S.; Caliari, M. Modeling sorption properties of maize by-products obtained using the Dynamic Dewpoint Isotherm (DDI) method. Food Biosci. 2020, 38, 100738. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Sanders, M.; López, J.; Lemus-Mondaca, R.; Martínez, E.; Di Scala, K. Modelling the water sorption isotherms of quinoa seeds (Chenopodium quinoa Willd.) and determination of sorption heats. Food Bioprocess Technol. 2012, 5, 1686–1693. [Google Scholar] [CrossRef]
- Fan, F.H.; Ma, Q.; Ge, J.; Peng, Q.Y.; Riley, W.W.; Tang, S.Z. Prediction of texture characteristics from extrusion food surface images using a computer vision system and artificial neural networks. J. Food Eng. 2013, 118, 426–433. [Google Scholar] [CrossRef]
- Özhan, B.; Karadeniz, F.; Erge, H.S. Effect of storage on nonenzymatic browning reactions in carob pekmez. Int. J. Food Sci. Technol. 2010, 45, 751–757. [Google Scholar] [CrossRef]
- De Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Gao, Y.; Han, Y.; Wu, X. Path analysis of nonenzymatic browning in Dongbei Suancai during storage caused by different fermentation conditions. Food Chem. 2021, 335, 127620. [Google Scholar] [CrossRef] [PubMed]
- Coklar, H.; Akbulut, M. The control of Maillard reaction in white grape molasses by the method of reducing reactant concentration. J. Food Sci. Technol. 2019, 40, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.S.; Roos, Y.H. Carotenoids stability in spray dried high solids emulsions using layer-by-layer (LBL) interfacial structure and trehalose-high DE maltodextrin as glass former. J. Funct. Foods 2017, 33, 32–39. [Google Scholar] [CrossRef]
- Choachamnan, J.; Moraru, C.; Lee, S.; Kurosaka, A.; Boonsupthip, W. Multiple-factor mathematical modeling of glycine-glucose browning. J. Food Eng. 2020, 273, 109829. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. Physicochemical properties, structural transformation, and relaxation time in strength analysis for honey powder models. Food Res. Int. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Potes, N.; Kerry, J.P.; Roos, Y.H. Additivity of water sorption, alpha relaxations and crystallization inhibition in lactose–maltodextrin systems. Carbohydr. Polym. 2012, 89, 1050–1059. [Google Scholar] [CrossRef]
- Fan, F.; Roos, Y.H. X-ray diffraction analysis of lactose crystallization in freeze-dried lactose–whey protein systems. Food Res. Int. 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Timmermann, E.O.; Chirife, J.; Iglesias, H.A. Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J. Food Eng. 2001, 48, 19–31. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; Magee, T.R.A. Thermodynamic properties of moisture sorption of potato. J. Food Eng. 2003, 60, 157–165. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, R.R.B.; Singh, A.K.; Patel, A.A.; Patil, G.R. Sorption isotherms and thermodynamics of water sorption of ready-to-use Basundi mix. LWT-Food Sci. Technol. 2009, 42, 441–445. [Google Scholar] [CrossRef]
- Jouppila, K.; Kansikas, J.; Roos, Y.H. Glass transition, water plasticization, and lactose crystallization in skim milk powder. J. Dairy Sci. 1997, 80, 3152–3160. [Google Scholar] [CrossRef]
- Nakagawa, K.; Maeda, H.; Yamaya, Y.; Tonosaki, Y. Maillard reaction intermediates and related phytochemicals in black garlic determined by EPR and HPLC analyses. Molecules 2020, 25, 4578. [Google Scholar] [CrossRef]
- Yu, K.Q.; Li, Z.S.; Sun, J. Polymer structures and glass transition: A molecular dynamics simulation study. Macromol. Theory Simul. 2001, 10, 624–633. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms; McGraw-Hill: New York, NY, USA, 1995; Volume 102, p. 296. [Google Scholar]
- Santagapita, P.R.; Matiacevich, S.B.; del Pilar Buera, M. Non-enzymatic browning kinetics in sucrose-glycine aqueous and dehydrated model systems in presence of MgCl2. Food Res. Int. 2018, 114, 97–103. [Google Scholar] [CrossRef]
- Wu, G.; Hui, X.; Mu, J.; Brennan, M.A.; Brennan, C.S. Functionalization of whey protein isolate fortified with blackcurrant concentrate by spray-drying and freeze-drying strategies. Food Res. Int. 2021, 141, 110025. [Google Scholar] [CrossRef]
- Fan, F.; Xiang, P.; Zhao, L. Vibrational spectra analysis of amorphous lactose in structural transformation: Water/temperature plasticization, crystal formation, and molecular mobility. Food Chem. 2021, 341, 128215. [Google Scholar] [CrossRef]
- Masavang, S.; Roudaut, G.; Champion, D. Identification of complex glass transition phenomena by DSC in expanded cereal-based food extrudates: Impact of plasticization by water and sucrose. J. Food Eng. 2019, 245, 43–52. [Google Scholar] [CrossRef]
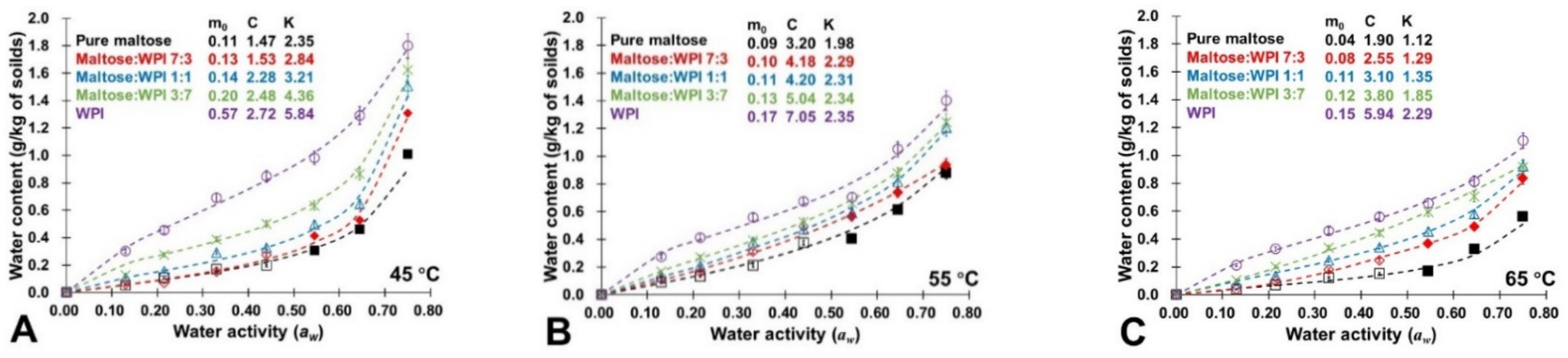
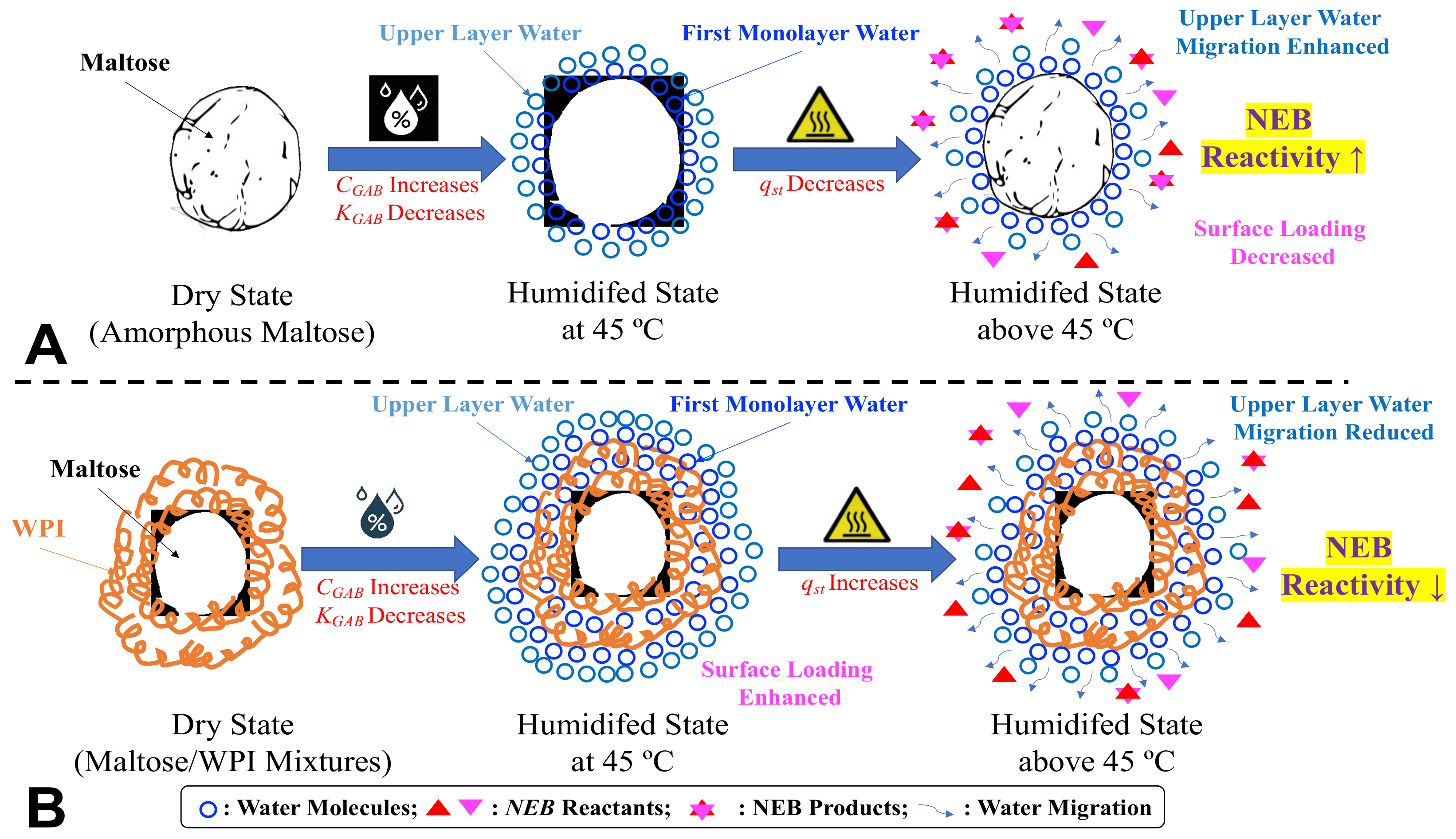



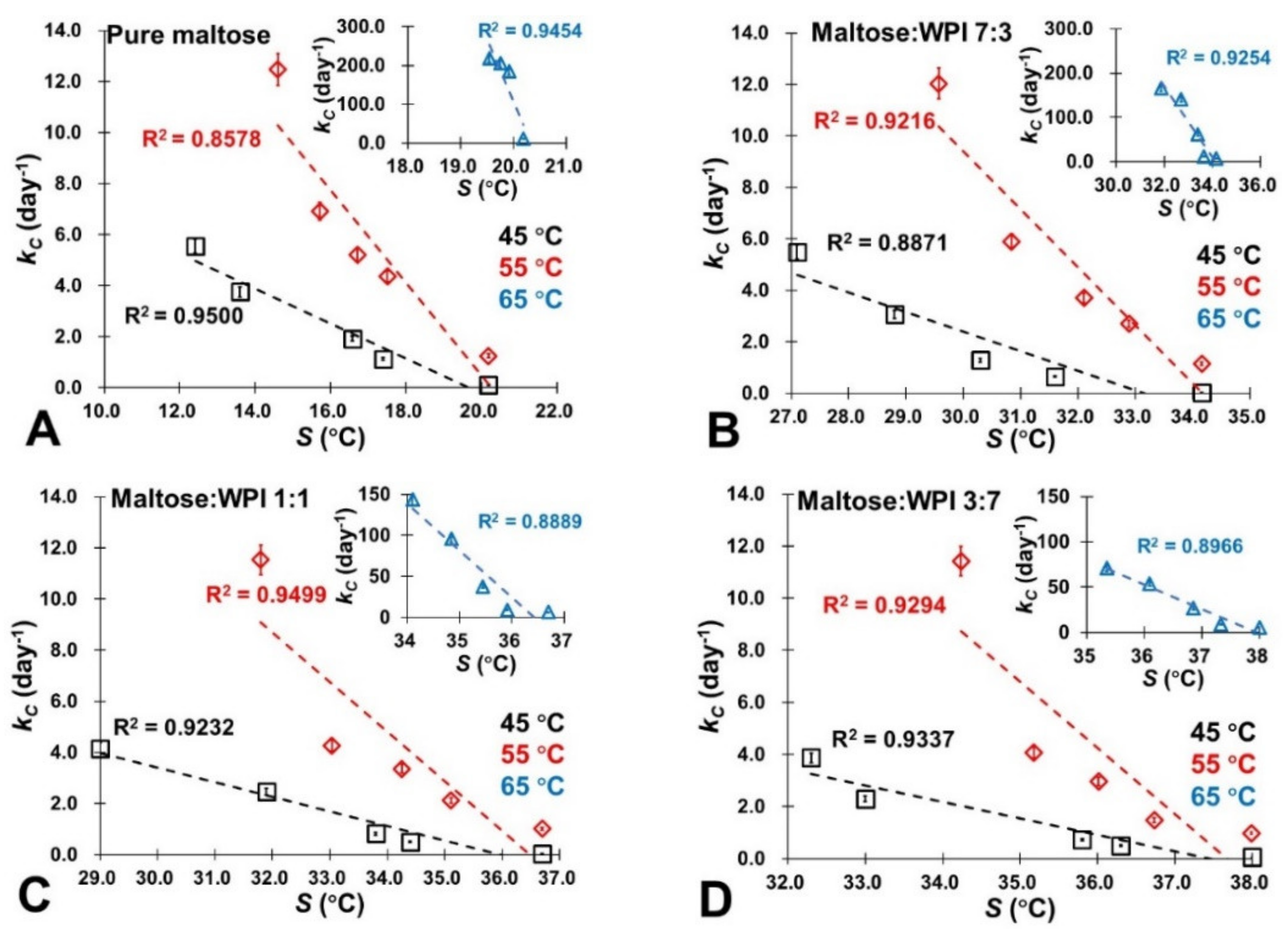


| M | M-W 7:3 | M-W 1:1 | M-W 3:7 | WPI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water Contents (g/kg of Solids) | Qst (kJ∙mol−1) | Water Contents (g/kg of Solids) | Qst (kJ∙mol−1) | Water Contents (g/kg of Solids) | Qst (kJ∙mol−1) | Water Contents (g/kg of Solids) | Qst (kJ∙mol−1) | Water Contents (g/kg of Solids) | Qst (kJ∙mol−1) | |
| 45 °C | ||||||||||
| 0.13 aw | 0.05 ± 0.01 a * | 203.0 ± 2.2 a | 0.06 ± 0.01 a | 108.0 ± 1.2 a | 0.11 ± 0.03 a | 118.3 ± 0.7 a | 0.13 ± 0.03 a | 129.2 ± 2.5 a | 0.30 ± 0.06 a | 124.1 ± 1.7 a |
| 0.22 aw | 0.11 ± 0.03 b ** | 91.9 ± 1.1 b | 0.07 ± 0.02 b | 85.7 ± 0.3 b | 0.16 ± 0.02 b | 116.5 ± 1.6 b | 0.27 ± 0.09 b | 129.0 ± 0.2 b | 0.46 ± 0.07 b | 122.4 ± 1.4 b |
| 0.33 aw | 0.17 ± 0.04 c | 71.5 ± 0.9 c | 0.15 ± 0.05 c | 87.0 ± 1.48 c | 0.29 ± 0.06 c | 113.0 ± 1.7 c | 0.39 ± 0.08 c | 126.8 ± 0.9 c | 0.69 ± 0.02 c | 115.1 ± 1.5 c |
| 0.44 aw | 0.20 ± 0.02 d | 65.0 ± 3.4 d | 0.28 ± 0.05 d | 82.4 ± 2.2 d | 0.33 ± 0.07 d | 108.3 ± 1.4 d | 0.50 ± 0.13 d | 126.8 ± 1.2 d | 0.85 ± 0.08 d | 110.3 ± 1.7 d |
| 55 °C | ||||||||||
| 0.13 aw | 0.09 ± 0.02 a | 201.7 ± 3.5 a | 0.11 ± 0.07 a | 101.3 ± 3.7 a | 0.17 ± 0.01 a | 116.4 ± 1.4 a | 0.17 ± 0.02 a | 128.7 ± 0.7 a | 0.27 ± 0.02 a | 114.9 ± 2.0 a |
| 0.22 aw | 0.13 ± 0.01 b | 89.9 ± 1.9 b | 0.16 ± 0.02 b | 85.4 ± 0.8 b | 0.26 ± 0.03 b | 114.1 ± 1.2 b | 0.27 ± 0.05 b | 124.8 ± 0.5 b | 0.41 ± 0.04 b | 111.8 ± 1.5 b |
| 0.33 aw | 0.21 ± 0.08 c | 69.2 ± 1.3 c | 0.31 ± 0.10 c | 83.7 ± 2.6 c | 0.43 ± 0.09 c | 112.7 ± 2.2 c | 0.39 ± 0.06 c | 123.2 ± 1.2 c | 0.56 ± 0.08 c | 109.9 ± 1.8 c |
| 0.44 aw | 0.38 ± 0.05 d | 63.1 ± 1.7 d | 0.49 ± 0.07 d | 81.6 ± 1.0 d | 0.47 ± 0.04 d | 107.9 ± 1.0 d | 0.53 ± 0.03 d | 120.4 ± 1.1 d | 0.67 ± 0.12 d | 107.6 ± 1.3 d |
| 65 °C | ||||||||||
| 0.13 aw | 0.04 ± 0.01 a | 20.25 ± 1.0 a | 0.04 ± 0.01 a | 99.5 ± 1.7 a | 0.08 ± 0.02 a | 116.1 ± 0.3 a | 0.11 ± 0.03 a | 128.1 ± 1.5 a | 0.21 ± 0.02 a | 112.4 ± 1.2 a |
| 0.22 aw | 0.07 ± 0.02 b | 83.5 ± 1.8 b | 0.10 ± 0.01 b | 83.8 ± 1.5 b | 0.14 ± 0.05 b | 113.7 ± 1.5 b | 0.20 ± 0.01 b | 124.2 ± 2.5 b | 0.33 ± 0.06 b | 109.4 ± 0.8 b |
| 0.33 aw | 0.12 ± 0.01 c | 59.0 ± 1.6 c | 0.18 ± 0.02 c | 81.3 ± 0.9 c | 0.25 ± 0.02 c | 112.9 ± 3.2 c | 0.34 ± 0.04 c | 121.3 ± 1.0 c | 0.46 ± 0.04 c | 109.2 ± 0.7 c |
| 0.44 aw | 0.15 ± 0.02 d | 51.4 ± 2.2 d | 0.25 ± 0.03 d | 80.0 ± 2.5 d | 0.34 ± 0.01 d | 106.7 ± 1.9 d | 0.44 ± 0.08 d | 118.4 ± 3.4 d | 0.56 ± 0.02 d | 106.4 ± 1.7 d |
| M | M-W 7:3 | M-W 1:1 | M-W 3:7 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔE | kC | kH | R2 | ΔE | kC | kH | R2 | ΔE | kC | kH | R2 | ΔE | kC | kH | R2 | |
| 45 °C | ||||||||||||||||
| Dry | 469.0 ± 1.9 a * | 0.1 ± 0.2 a | 0.2 ± 0.0 a | 0.9994 | 313.3 ± 2.6 a | 0.03 ± 0.24 a | 0.1 ± 0.2 a | 0.9922 | 313.8 ± 3.4 a | 0.04 ± 0.15 a | 0.1 ± 0.1 a | 0.9979 | 258.8 ± 3.1 a | 0.1 ± 0.22 a | 0.1 ± 0.1 a | 0.9970 |
| 0.13 aw | 579.6 ± 2.3 b ** | 1.1 ± 0.1 b | 0.2 ± 0.1 a | 0.9919 | 319.0 ± 1.4 b | 0.7 ± 0.2 b | 0.2 ± 0.2 b | 0.9904 | 329.1 ± 2.0 b | 0.5 ± 0.2 b | 0.1 ± 0.0 a | 0.9894 | 282.2 ± 2.2 b | 0.5 ± 0.2 b | 0.1 ± 0.0 b | 0.9923 |
| 0.22 aw | 668.8 ± 1.4 c | 1.9 ± 0.3 c | 0.4 ± 0.1 b | 0.9988 | 349.6 ± 1.8 c | 1.3 ± 0.2 c | 0.2 ± 0.1 b | 0.9951 | 388.4 ± 2.3 c | 0.8 ± 0.1 c | 0.2 ± 0.1 b | 0.9867 | 284.6 ± 3.5 b | 0.7 ± 0.2 c | 0.1 ± 0.4 c | 0.9935 |
| 0.33 aw | 1216.0 ± 4.3 d | 3.8 ± 0.1 d | 4.2 ± 0.4 b | 0.9885 | 579.6 ± 2.2 d | 3.1 ± 0.1 d | 3.6 ± 0.4 b | 0.9807 | 576.8 ± 1.9 d | 2.5 ± 0.2 d | 2.3 ± 0.5 c | 0.9626 | 419.5 ± 1.7 c | 2.3 ± 0.2 d | 1.7 ± 1.1 d | 0.9650 |
| 0.44 aw | 1551.0 ± 3.0 e | 5.6 ± 0.1 e | 4.7 ± 0.9 d | 0.9895 | 1264.4 ± 3.5 e | 5.5 ± 0.1 e | 6.5 ± 0.8 d | 0.9757 | 437.3 ± 2.8 e | 4.2 ± 0.1 e | 5.1 ± 0.3 d | 0.9575 | 618.4 ± 1.9 d | 3.9 ± 0.1 e | 3.4 ± 1.5 e | 0.9872 |
| 55 °C | ||||||||||||||||
| Dry | 615.8 ± 1.0 a | 1.3 ± 0.2 a | 0.4 ± 0.5 a | 0.9994 | 616.5 ± 1.6 a | 1.2 ± 0.1 a | 0.3 ± 0.4 a | 0.9922 | 723.5 ± 3.2 a | 1.0 ± 0.1 a | 0.2 ± 0.1 a | 0.9979 | 599.4 ± 2.6 a | 1.0 ± 0.2 a | 0.2 ± 0.0 a | 0.9970 |
| 0.13 aw | 675.9 ± 1.3 b | 4.4 ± 0.3 b | 0.7 ± 0.2 b | 0.9919 | 631.5 ± 2.1 b | 2.7 ± 0.2 b | 0.5 ± 0.4 b | 0.9904 | 808.2 ± 1.1 b | 2.1 ± 0.1 b | 0.4 ± 0.1 b | 0.9894 | 675.0 ± 1.0 b | 1.5 ± 0.2 b | 0.4 ± 0.1 b | 0.9923 |
| 0.22 aw | 763.5 ± 2.7 c | 5.2 ± 0.1 c | 3.1 ± 0.7 c | 0.9988 | 775.5 ± 1.8 c | 3.7 ± 0.2 c | 1.3 ± 0.7 c | 0.9951 | 881.8 ± 1.9 c | 3.3 ± 0.2 c | 0.9 ± 0.5 c | 0.9867 | 710.2 ± 1.6 c | 3.0 ± 0.1 c | 0.7 ± 0.0 c | 0.9935 |
| 0.33 aw | 1092.7 ± 3.9 d | 6.9 ± 0.2 d | 4.6 ± 0.9 d | 0.9885 | 865.9 ± 2.6 d | 5.9 ± 0.2 d | 3.2 ± 0.2 d | 0.9807 | 982.2 ± 2.7 d | 4.3 ± 0.1 d | 2.0 ± 0.9 d | 0.9626 | 742.9 ± 1.4 d | 4.1 ± 0.1 d | 1.6 ± 0.3 d | 0.9650 |
| 0.44 aw | 1201.0 ± 4.2 e | 12.5 ± 0.1 e | 5.4 ± 1.4 e | 0.9895 | 902.2 ± 1.0 e | 12.0 ± 0.2 e | 4.4 ± 0.9 e | 0.9757 | 895.9 ± 1.5 e | 11.5 ± 0.2 e | 3.5 ± 0.7 e | 0.9575 | 892.6 ± 1.4 e | 11.4 ± 0.1 e | 3.4 ± 0.7 e | 0.9872 |
| 65 °C | ||||||||||||||||
| Dry | 748.8 ± 2.3 a | 11.6 ± 1.0 a | 0.8 ± 0.7 a | 0.9994 | 481.5 ± 1.9 a | 7.2 ± 0.1 a | 0.7 ± 0.3 a | 0.9922 | 512.1 ± 2.7 a | 6.5 ± 0.2 a | 0.5 ± 0.7 a | 0.9979 | 482.8 ± 1.7 a | 5.5 ± 0.2 a | 0.5 ± 0.1 a | 0.9970 |
| 0.13 aw | 821.7 ± 1.2 b | 14.9 ± 0.1 b | 1.5 ± 0.8 b | 0.9919 | 509.4 ± 2.7 b | 11.6 ± 0.2 b | 1.3 ± 0.6 b | 0.9904 | 526.8 ± 1.6 b | 9.4 ± 0.1 b | 1.1 ± 0.8 b | 0.9894 | 625.9 ± 2.8 b | 9.0 ± 0.2 b | 1.0 ± 0.7 b | 0.9923 |
| 0.22 aw | 1293.5 ± 2.7 c | 185.0 ± 1.2 c | 6.8 ± 0.5 c | 0.9988 | 541.8 ± 1.6 c | 62.0 ± 4.9 c | 5.0 ± 1.0 c | 0.9951 | 578.2 ± 1.3 c | 37.8 ± 1.1 c | 2.5 ± 0.3 c | 0.9867 | 631.3 ± 0.8 c | 23.5 ± 1.0 c | 2.0 ± 0.2 c | 0.9935 |
| 0.33 aw | 1348.6 ± 3.8 d | 206.2 ± 3.1d | 12.4 ± 2.3 d | 0.9885 | 729.3 ± 3.2 d | 141.4 ± 3.2 d | 11.8 ± 1.5 d | 0.9807 | 1019.7 ± 3.9 d | 96.2 ± 1.3 d | 9.5 ± 1.5 d | 0.9626 | 639.71 ± 0.66 d | 53.7 ± 2.6 d | 6.5 ± 0.9 d | 0.9650 |
| 0.44 aw | 1495.4 ± 1.7 e | 218.1 ± 2.3e | 17.0 ± 2.1 e | 0.9895 | 1075.3 ± 3.0 e | 166.5 ± 3.2 e | 13.3 ± 3.1 e | 0.9757 | 1007.8 ± 2.6 e | 143.6 ± 3.7 e | 10.5 ± 1.9 e | 0.9575 | 754.4 ± 1.6 e | 88.8 ± 1.4 e | 9.0 ± 0.6 e | 0.9872 |
| M | M-W 7:3 | M-W 1:1 | M-W 3:7 | ||
|---|---|---|---|---|---|
| 45 °C | Dry | 20.2 ± 4.6 a * | 34.2 ± 3.9 a | 36.7 ± 1.5 a | 38.0 ± 1.3 a |
| 0.13 aw | 18.8 ± 0.1 b ** | 31.6 ± 0.1 b | 34.4 ± 0.1 b | 36.3 ± 0.1 b | |
| 0.22 aw | 17.5 ± 0.2 c | 30.3 ± 0.1 c | 33.8 ± 0.2 c | 35.8 ± 0.1 c | |
| 0.33 aw | 16.0 ± 0.1 d | 28.8 ± 0.1 d | 31.9 ± 0.1 d | 33.0 ± 0.1 d | |
| 0.44 aw | 15.5 ± 0.2 e | 27.1 ± 0.1 d | 29.0 ± 0.1 e | 32.3 ± 0.2 e | |
| 55 °C | Dry | 20.2 ± 4.6 a | 34.2 ± 3.9 a | 36.7 ± 1.5 a | 38.0 ± 1.3 a |
| 0.13 aw | 19.4 ± 0.1 b | 32.9 ± 0.1 b | 35.1 ± 0.1 b | 36.7 ± 0 b | |
| 0.22 aw | 18.8 ± 0.2 c | 32.1 ± 0.1 c | 34.3 ± 0.1 c | 36.0 ± 0.2 c | |
| 0.33 aw | 18.0 ± 0.1 d | 30.8 ± 0.1 d | 33.0 ± 0.2 d | 35.2 ± 0 d | |
| 0.44 aw | 17.1 ± 0.1 e | 29.6 ± 0.3 e | 31.8 ± 0.2 e | 34.2 ± 0 e | |
| 65 °C | Dry | 20.2 ± 4.6 a | 34.2 ± 3.9 a | 36.7 ± 1.5 a | 38.0 ± 1.3 a |
| 0.13 aw | 20.2 ± 0.1b | 33.7 ± 0.1 b | 35.9 ± 0.1 b | 37.3 ± 0.1 b | |
| 0.22 aw | 19.9 ± 0.1 c | 33.4 ± 0.2 c | 35.4 ± 0.1 c | 36.9 ± 0.1 c | |
| 0.33 aw | 19.7 ± 0.1 d | 32.7 ± 0.2 d | 34.8 ± 0.1 d | 36.1 ± 0.1 d | |
| 0.44 aw | 19.5 ± 0.1 e | 31.7 ± 0.1 e | 34.1 ± 0.2 e | 35.3 ± 0.0 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Ye, H.; Fan, F. Nonenzymatic Browning of Amorphous Maltose/Whey Protein Isolates Matrix: Effects of Water Sorption and Molecular Mobility. Foods 2022, 11, 2128. https://doi.org/10.3390/foods11142128
Wu Y, Ye H, Fan F. Nonenzymatic Browning of Amorphous Maltose/Whey Protein Isolates Matrix: Effects of Water Sorption and Molecular Mobility. Foods. 2022; 11(14):2128. https://doi.org/10.3390/foods11142128
Chicago/Turabian StyleWu, Yaowen, Haoxuan Ye, and Fanghui Fan. 2022. "Nonenzymatic Browning of Amorphous Maltose/Whey Protein Isolates Matrix: Effects of Water Sorption and Molecular Mobility" Foods 11, no. 14: 2128. https://doi.org/10.3390/foods11142128






