Multivariate Nature of Fish Freshness Evaluation by Consumers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Experimental Design
2.2. Consumers’ Sensory Evaluation
2.2.1. Participants
2.2.2. Experimental Procedures
2.3. Freshness Parameters
2.3.1. Bacteriological Analyses
2.3.2. Physicochemical Analyses
Lipid and Protein Oxidation
Torrymeter Readings
Instrumental Color Parameters’ Measurement
Instrumental Texture Profile
2.4. Statistical Analyses
3. Results and Discussion
3.1. Consumers’ Evaluation
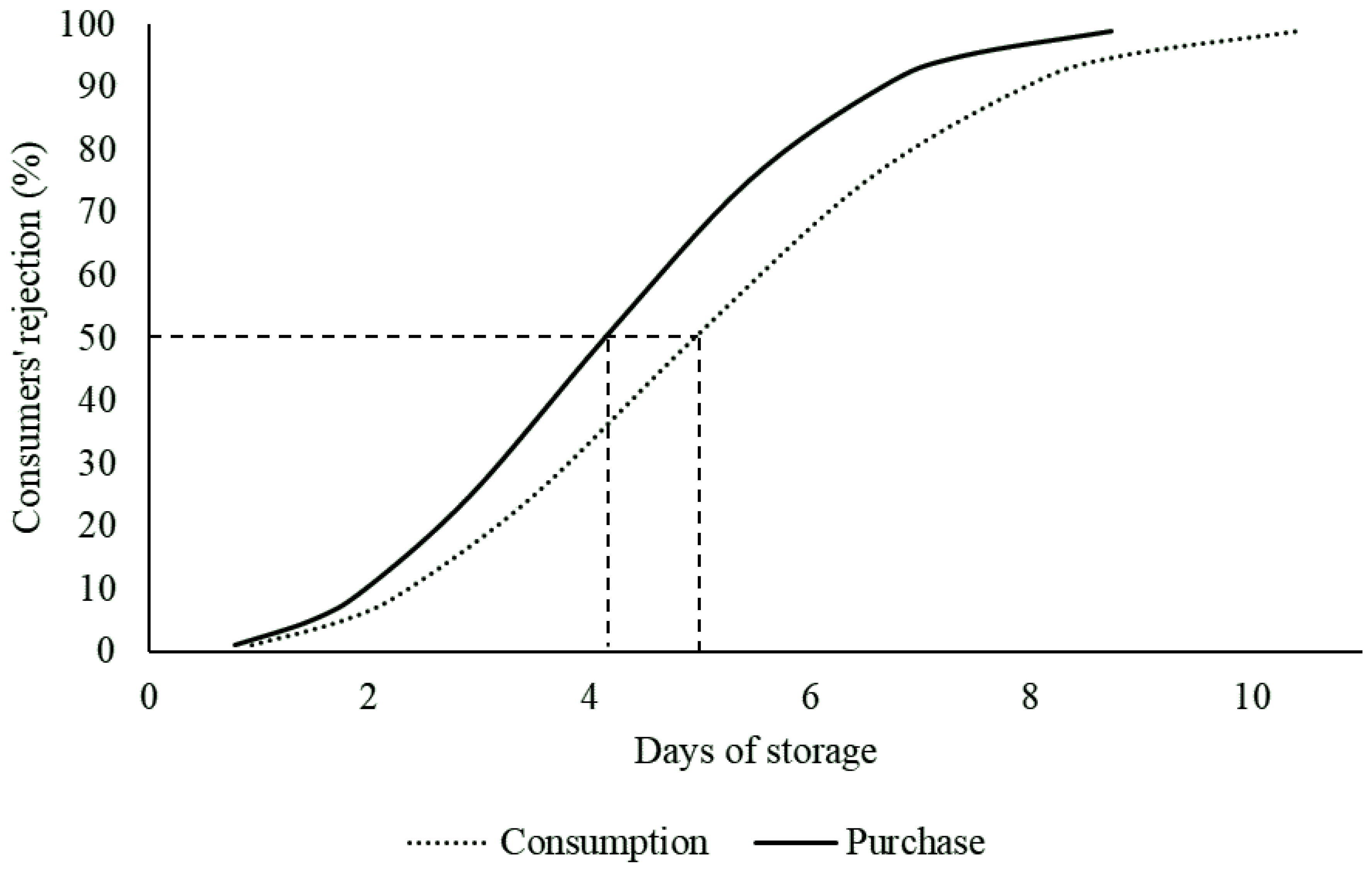
3.1.1. Consumers’ Acceptability Limit Based on Survival Analysis
3.1.2. Survival Analysis Versus Hedonic Scores for Assessing Consumers’ Acceptability Limits
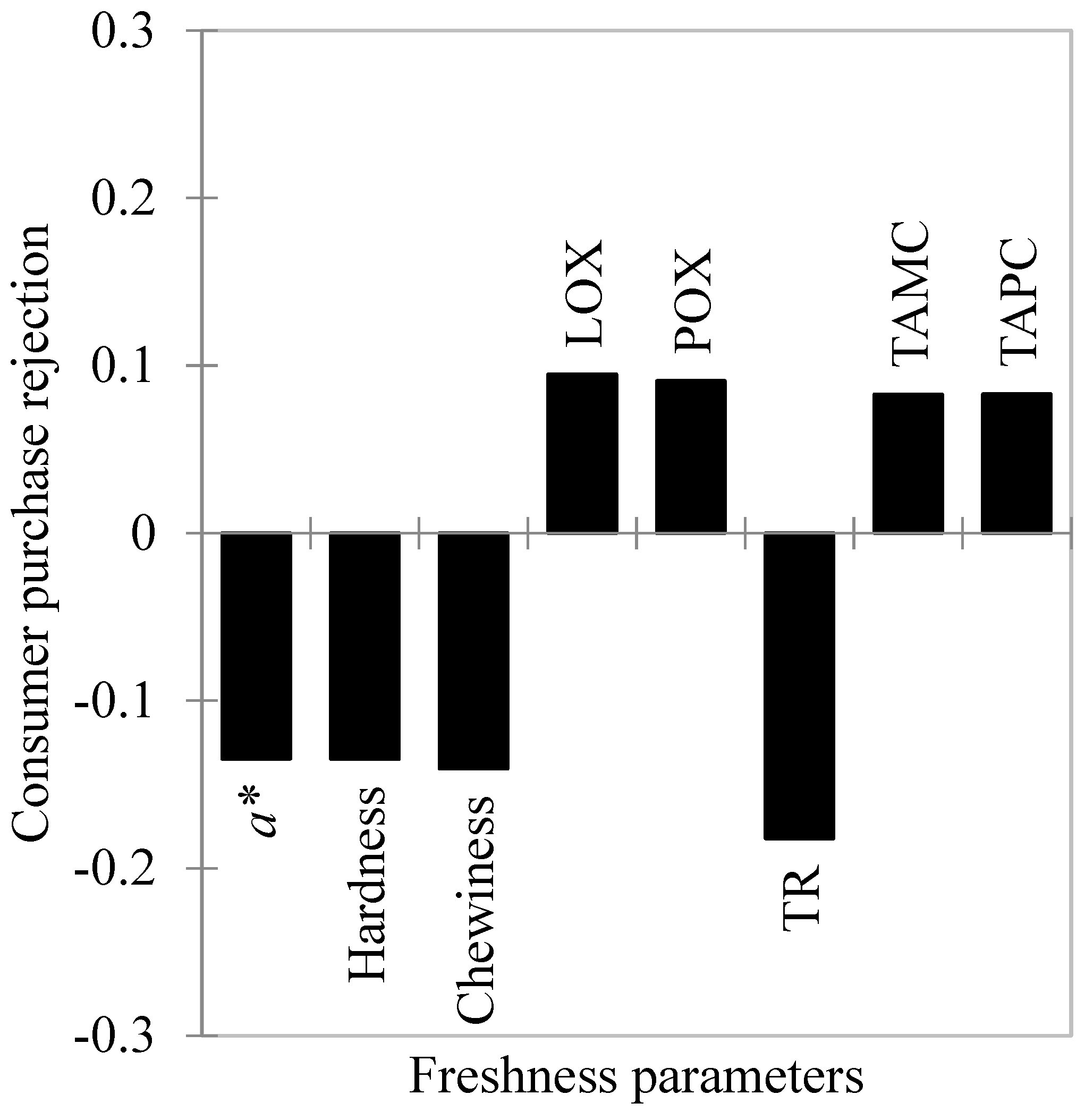
3.2. Freshness Parameters and Their Relationship with Consumers’ Purchase Rejection
3.2.1. Bacteriological Parameters
3.2.2. Lipid and Protein Oxidation
3.2.3. Torrymeter Readings
3.2.4. Instrumental Color Parameters
3.2.5. Texture Profile Analysis
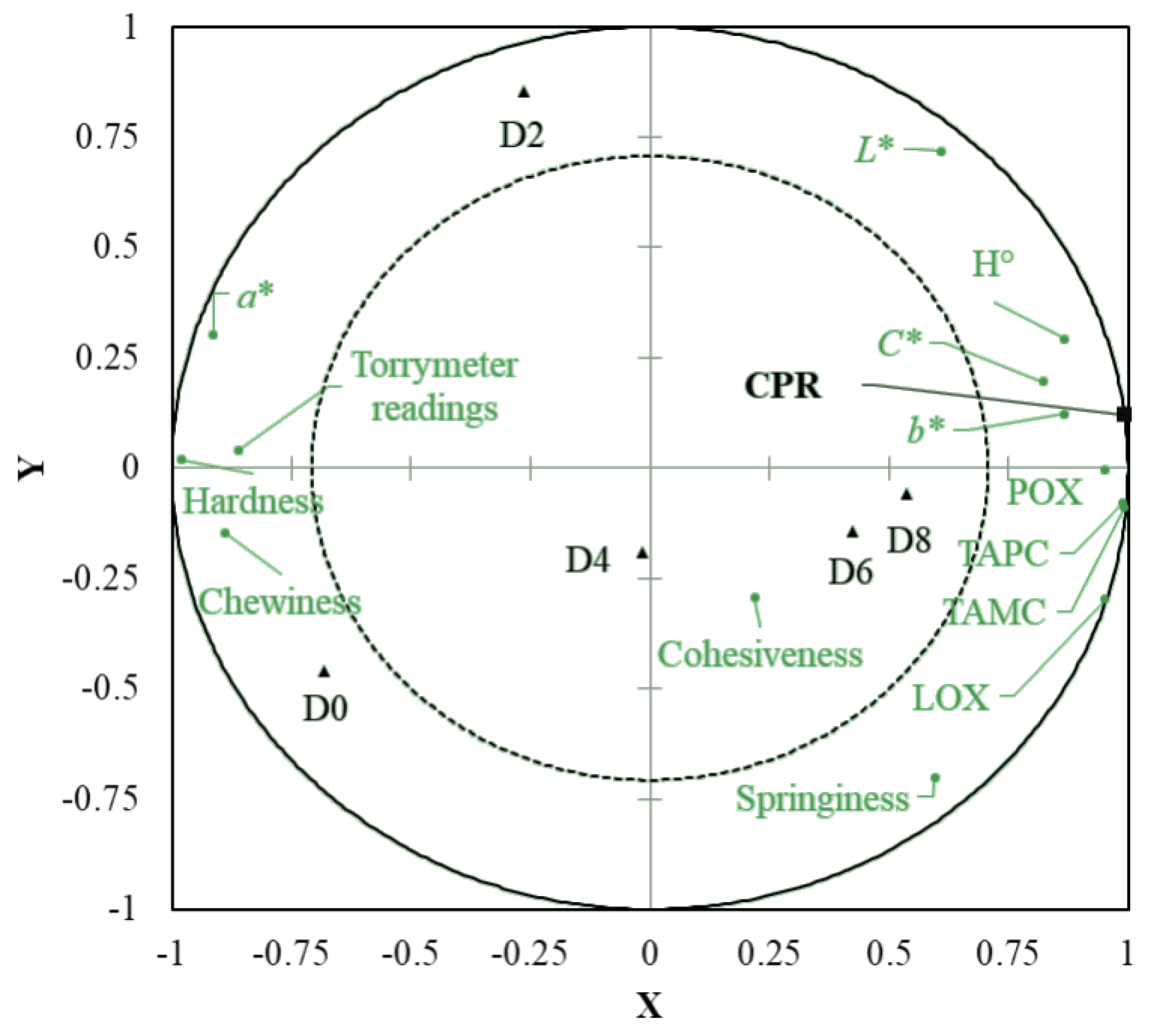
3.3. Multivariate Analysis of Freshness Parameters and Consumers’ Rejection
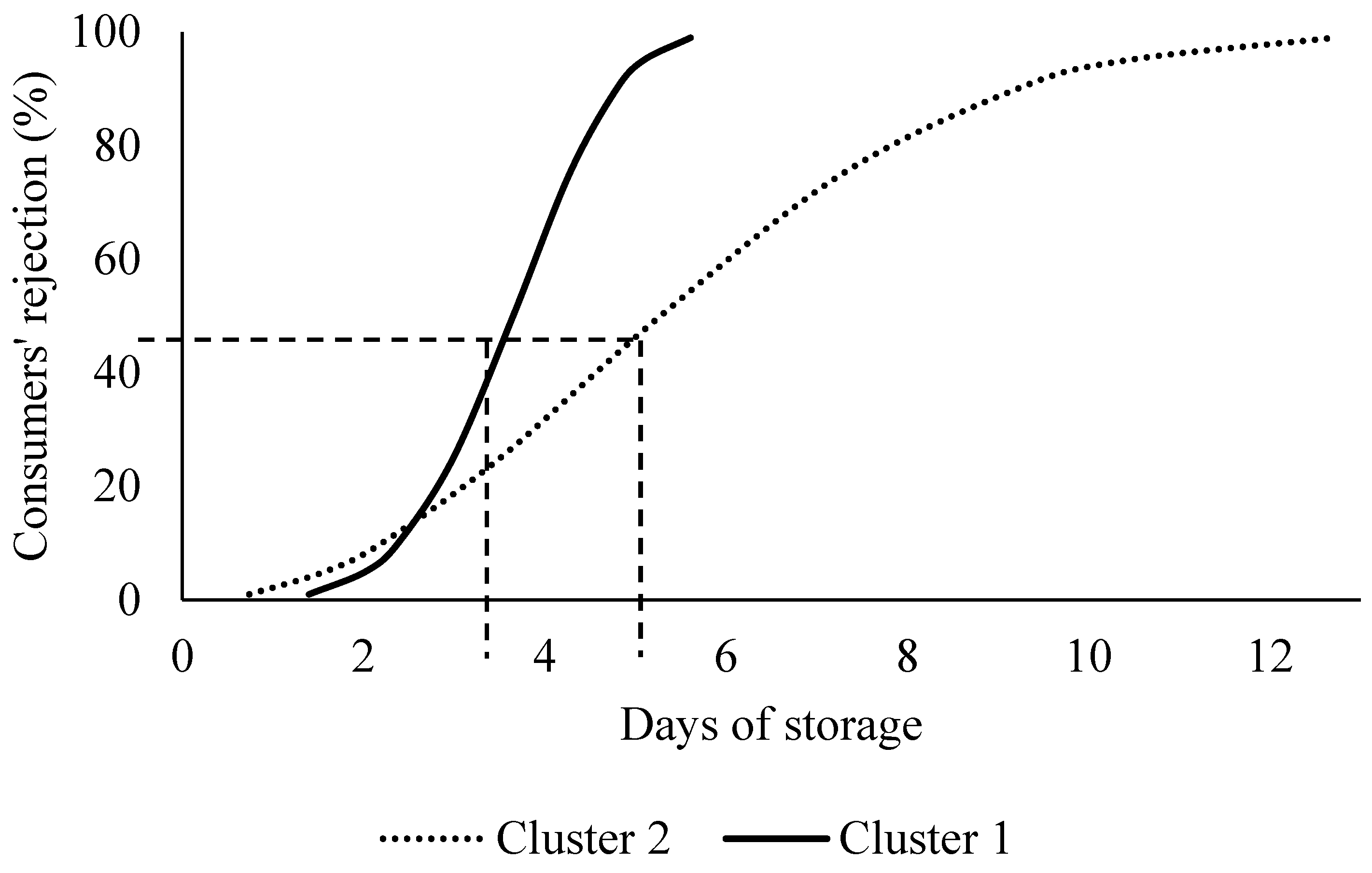
3.4. Consumers’ Acceptability Limits Are Driven by Socioeconomic Characteristics and Fish Consumption Frequency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020; Available online: http://www.fao.org/state-of-fisheries-aquaculture (accessed on 12 November 2021).
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. Quality Index Method for fish quality control: Understanding the applications, the appointed limits and the upcoming trends. Trends Food Sci. Technol. 2021, 111, 333–345. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Han, Z.; Zeng, X.-A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Martinsdóttir, E.; Sveinsdóttir, K.; Luten, J.; Schelvis-Smit, R.; Hyldig, G. Reference Manual for the Fish Sector: Sensory Evaluation of Fish Freshness; QIM Eurofish: Ijmuiden, The Netherlands, 2001; 49p. [Google Scholar]
- Hicks, D.; Pivarnik, L.; McDermott, R. Consumer perceptions about seafood—An Internet survey. J. Foodserv. 2008, 19, 213–226. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; de Hooge, I.; Amani, P.; Bech-Larsen, T.; Oostindjer, M. Consumer-Related Food Waste: Causes and Potential for Action. Sustainability 2015, 7, 6457–6477. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.A.; Costa, J.C. Shelf life determination using sensory evaluation scores: A general Weibull modeling approach. Comput. Ind. Eng. 2006, 51, 652–670. [Google Scholar] [CrossRef]
- Hossain, A. Quantity or quality of fish in a developing country: A hedonic analysis. J. Appl. Aquac. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Haider, W.; Solgaard, H.S.; Ravn-Jonsen, L.; Roth, E. Consumer willingness to pay for quality attributes of fresh seafood: A labeled latent class model. Food Qual. Prefer. 2015, 41, 225–236. [Google Scholar] [CrossRef]
- Tomić, M.; Matulić, D.; Jelić, M. What determines fresh fish consumption in Croatia? Appetite 2016, 106, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeke, W.; Vermeir, I.; Brunsø, K. Consumer evaluation of fish quality as basis for fish market segmentation. Food Qual. Prefer. 2007, 18, 651–661. [Google Scholar] [CrossRef]
- Østli, J.; Esaiassen, M.; Garitta, L.; Nøstvold, B.; Hough, G. How fresh is fresh? Perceptions and experience when buying and consuming fresh cod fillets. Food Qual. Prefer. 2013, 27, 26–34. [Google Scholar] [CrossRef]
- Manzocco, L. The Acceptability Limit in Food Shelf Life Studies. Crit. Rev. Food Sci. Nutr. 2015, 56, 1640–1646. [Google Scholar] [CrossRef]
- Costell, E.; Tarrega, A.; Bayarri, S. Food Acceptance: The Role of Consumer Perception and Attitudes. Chemosens. Percept. 2009, 3, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Hough, G.; Garitta, L. Methodology for Sensory Shelf-Life Estimation: A Review. J. Sens. Stud. 2012, 27, 137–147. [Google Scholar] [CrossRef]
- Richards, M.; De Kock, R.; Buys, E.M. Multivariate accelerated shelf-life test of low fat UHT milk. Int. Dairy J. 2014, 36, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Giménez, A.; Varela, P.; Salvador, A.; Ares, G.; Fiszman, S.; Garitta, L. Shelf life estimation of brown pan bread: A consumer approach. Food Qual. Prefer. 2007, 18, 196–204. [Google Scholar] [CrossRef]
- Giménez, A.; Gagliardi, A.; Ares, G. Estimation of failure criteria in multivariate sensory shelf life testing using survival analysis. Food Res. Int. 2017, 99, 542–549. [Google Scholar] [CrossRef]
- Alongi, M.; Sillani, S.; Lagazio, C.; Manzocco, L. Effect of expiry date communication on acceptability and waste of fresh-cut lettuce during storage at different temperatures. Food Res. Int. 2018, 116, 1121–1125. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Merlino, M.; Giarratana, F.; Verzera, A. A new approach for the shelf-life definition of minimally processed carrots. Postharvest Biol. Technol. 2020, 163, 111138. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Zeng, X.-A.; Liu, D. Recent Advances in Methods and Techniques for Freshness Quality Determination and Evaluation of Fish and Fish Fillets: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1012–1225. [Google Scholar] [CrossRef]
- Gama, L.; Bressan, M.; Rodrigues, E.; Rossato, L.; Moreira, O.; Alves, S.; Bessa, R. Heterosis for meat quality and fatty acid profiles in crosses among Bos indicus and Bos taurus finished on pasture or grain. Meat Sci. 2012, 93, 98–104. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Mano, S.B.; Teixeira, C.E.; Canto, A.C.V.C.S.; Vital, H.C.; Conte-Júnior, C.A. Influence of good manufacturing practices on the shelf life of refrigerated fillets of tilapia (Oreochromis niloticus) packed in modified atmosphere and gamma-irradiated. Food Sci. Nutr. 2013, 1, 298–306. [Google Scholar] [CrossRef]
- Carlucci, D.; Nocella, G.; De Devitiis, B.; Viscecchia, R.; Bimbo, F.; Nardone, G. Consumer purchasing behaviour towards fish and seafood products. Patterns and insights from a sample of international studies. Appetite 2015, 84, 212–227. [Google Scholar] [CrossRef]
- Eltholth, M.; Fornace, K.; Grace, D.; Rushton, J.; Haesler, B. Characterization of production, marketing and consumption patterns of farmed tilapia in the Nile Delta of Egypt. Food Policy 2015, 51, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Hough, G.; Langohr, K.; Gomez, G.; Curia, A. Survival Analysis Applied to Sensory Shelf Life of Foods. J. Food Sci. 2003, 68, 359–362. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 387. [Google Scholar]
- ISO 8589; Sensory Analysis: General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 1998.
- APHA. Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association: Washington, DC, USA, 2001.
- Yin, M.C.; Faustman, C.; Riesen, J.W.; Williams, S.N. α-Tocopherol and ascorbate delay oxymyoglobin phospholipid oxidation in vitro. J. Food Sci. 1993, 58, 1273–1276. [Google Scholar] [CrossRef]
- Joseph, P.; Suman, S.; Rentfrow, G.; Li, S.; Beach, C.M. Proteomics of Muscle-Specific Beef Color Stability. J. Agric. Food Chem. 2012, 60, 3196–3203. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.-W.; Moermant, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Armenteros, M.; Heinonen, M.; Ollilainen, V.; Toldrá, F.; Estévez, M. Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS). Meat Sci. 2009, 83, 104–112. [Google Scholar] [CrossRef]
- Mercier, Y.; Gatellier, P.; Viau, M.; Remignon, H.; Renerre, M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in Turkey meat during storage. Meat Sci. 1998, 48, 301–318. [Google Scholar] [CrossRef]
- Duflos, G.; Le Fur, B.; Mulak, V.; Becel, P.; Malle, P. Comparison of methods of differentiating between fresh and frozen-thawed fish or fillets. J. Sci. Food Agric. 2002, 82, 1341–1345. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012.
- Monteiro, M.L.G.; Mársico, E.T.; Mutz, Y.D.S.; Castro, V.S.; Moreira, R.V.D.B.P.; Álvares, T.D.S.; Conte-Junior, C.A. Combined effect of oxygen-scavenger packaging and UV-C radiation on shelf life of refrigerated tilapia (Oreochromis niloticus) fillets. Sci. Rep. 2020, 10, 4243. [Google Scholar] [CrossRef] [Green Version]
- Symoneaux, R.; Galmarini, M.V.V.; Mehinagic, E. Comment analysis of consumer’s likes and dislikes as an alternative tool to preference mapping. A case study on apples. Food Qual. Prefer. 2012, 24, 59–66. [Google Scholar] [CrossRef]
- Giménez, A.; Ares, F.; Ares, G. Sensory shelf-life estimation: A review of current methodological approaches. Food Res. Int. 2012, 49, 311–325. [Google Scholar] [CrossRef]
- Eriksson, M.; Strid, I.; Hansson, P.-A. Food waste reduction in supermarkets—Net costs and benefits of reduced storage temperature. Resour. Conserv. Recycl. 2016, 107, 73–81. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: New York, NY, USA, 2010. [Google Scholar]
- Rong, C.; Qi, L.; Chang-Hu, X.; Bang-Zhong, Y. Microbiological, chemical and sensory assessment of (I) whole ungutted, (II) whole gutted and (III) filleted tilapia (Oreochromis niloticus) during refrigerated storage. Int. J. Food Sci. Technol. 2009, 44, 2243–2248. [Google Scholar] [CrossRef]
- Santos, J.D.S.L.; Mársico, E.T.; Cinquini, M.A.; Silva, F.; Junior, C.A.C.; Monteiro, M.L.G. Physicochemical and sensory characterization of three different portions from commercial pirarucu (Arapaima gigas) fillets. Braz. J. Food Technol. 2018, 21, e2017178. [Google Scholar] [CrossRef]
- Corrigan, V.; Hedderley, D.; Harvey, W. Modeling the Shelf Life of Fruit-Filled Snack Bars Using Survival Analysis and Sensory Profiling Techniques. J. Sens. Stud. 2012, 27, 403–416. [Google Scholar] [CrossRef]
- Moawad, R.K.; Mohamed, G.F.; Hanna; El-Banna, A.; Bareh, G.F.; Mahmoud, K.F. Assessment of hurdle technology to preserve Nile tilapia fillets during refrigeration with the application of marjoram oil/polyphosphates dipping. Asian J. Sci. Res. 2017, 10, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Khalafalla, F.A.; Ali, F.H.; Hassan, A.-R.H. Quality improvement and shelf-life extension of refrigerated Nile tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 33–40. [Google Scholar] [CrossRef] [Green Version]
- ICMSF. Microorganisms in Foods, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Fraser, O.P.; Sumar, S. Compositional changes and spoilage in fish (part II)—Microbiological induced deterioration. Nutr. Food Sci. 1998, 98, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Baron, C.P. Protein oxidation in aquatic foods. In Antioxidants and Functional Components in Aquatic Foods, 1st ed.; Kristinsson, H.G., Ed.; John Wiley & Sons: Chichester, UK, 2014; pp. 23–42. [Google Scholar]
- Baron, C.P.; Andersen, H.J. Myoglobin-Induced Lipid Oxidation. A Review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Dergal, N.B.; Degand, G.; Douny, C.; Brose, F.; Daube, G.; Rodrigues, A.; Scippo, M.L. Microbial, biochemical and sensorial quality assessment of Algerian farmed tilapia (Oreochromis niloticus) stored at 4 and 30 °C. Afr. J. Food Sci. 2013, 7, 498–507. [Google Scholar]
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comparison of selected methods of assessing freshness quality and remaining storage life of iced gilthead sea bream (Sparus aurata). Food Res. Int. 2003, 36, 551–560. [Google Scholar] [CrossRef]
- Ochrem, A.S.; Zapletal, P.; Maj, D.; Gil, Z.; Zychlińska-Buczek, J. Changes in physical and dielectrical properties of carp meat (Cyprinus carpio) during cold storage. J. Food Process. Eng. 2014, 37, 177–184. [Google Scholar] [CrossRef]
- Sant’Ana, L.S.; Soares, S.; Vaz-Pires, P. Development of a quality index method (QIM) sensory scheme and study of shelf-life of ice-stored blackspot seabream (Pagellus bogaraveo). LWT 2011, 44, 2253–2259. [Google Scholar] [CrossRef] [Green Version]
- Badiani, A.; Bonaldo, A.; Testi, S.; Rotolo, M.; Serratore, P.; Giulini, G.; Pagliuca, G.; Gatta, P.P. Good handling practices of the catch: The effect of early icing on the freshness quality of cuttlefish (Sepia officinalis L.). Food Control 2012, 32, 327–333. [Google Scholar] [CrossRef]
- Pérez-Alvarez, J.A.; Fernández-López, J. Chemical and Biochemical Aspects of Color in Muscle Foods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 25–44. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Chen, Z.; Jia, Y.; Han, P.; Cheng, C. Effect of pullulan hydrolysates on the quality of Nile tilapia (Oreochromis niloticus) fillets during ice storage. J. Food Process. Preserv. 2019, 43, e14043. [Google Scholar] [CrossRef]
- Joseph, P.; Suman, S.; Li, S.; Xiong, Y.; Webster, C.; Thompson, K.; Metts, L. Fishmeal-based diet decreases the redness of sunshine bass (Morone chrysops × Morone saxatilis) fillets. LWT 2008, 42, 730–734. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 873–880. [Google Scholar] [CrossRef]
- Robb, D.; Kestin, S.; Warriss, P. Muscle activity at slaughter: I. Changes in flesh colour and gaping in rainbow trout. Aquaculture 2000, 182, 261–269. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Møller, J.K.; Skibsted, L.H. Heme-iron in lipid oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W.; Li, X. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2012, 138, 821–826. [Google Scholar] [CrossRef]
- Sáez, M.; Suárez, M.; Martínez, T. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT 2019, 118, 108767. [Google Scholar] [CrossRef]
- Lougovois, V.P.; Kyrana, V.R. Freshness quality and spoilage of chill-stored fish. In Food Policy, Control and Research; Riley, A.P., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2005; pp. 35–86. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Pieniak, Z.; Verbeke, W.; Scholderer, J.; Brunsø, K.; Olsen, S.O. European consumers’ use of and trust in information sources about fish. Food Qual. Prefer. 2007, 18, 1050–1063. [Google Scholar] [CrossRef]
- Birch, D.; Lawley, M. Buying seafood: Understanding barriers to purchase across consumption segments. Food Qual. Prefer. 2012, 26, 12–21. [Google Scholar] [CrossRef]
- Pieniak, Z.; Verbeke, W.; Olsen, S.O.; Hansen, K.B.; Brunsø, K. Health-related attitudes as a basis for segmenting European fish consumers. Food Policy 2010, 35, 448–455. [Google Scholar] [CrossRef]
- Thong, N.T.; Solgaard, H.S. Consumer’s food motives and seafood consumption. Food Qual. Prefer. 2016, 56, 181–188. [Google Scholar] [CrossRef]

| Consumers | |
|---|---|
| N | 104 |
| Gender (%) | |
| Female | 54.8 |
| Male | 45.2 |
| Age (years) (%) | |
| 18–25 | 9.6 |
| 26–35 | 31.7 |
| 36–45 | 36.5 |
| 46–55 | 10.6 |
| 56–65 | 9.6 |
| >65 | 1.9 |
| Education (%) | |
| Primary school | 1.9 |
| Secondary school | 18.3 |
| Under graduation | 16.3 |
| Graduation | 63.5 |
| Income—minimum Brazilian wage (BRL 998.0)a (%) | |
| 1 to 5 | 26.0 |
| >5 to 10 | 27.9 |
| >10 to 20 | 28.8 |
| >20 to 30 | 11.5 |
| >30 | 5.8 |
| Freshness Parameters | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| Bacteriological€ | |||||
| Total aerobic mesophilic count (TAMC) | 4.94 ± 0.47 e | 5.82 ± 0.32 d | 6.92 ± 0.24c | 7.93 ± 0.23b | 8.60 ± 0.21 a |
| Total aerobic psychrotrophic count (TAPC) | 5.5 ± 0.17 e | 6.41 ± 0.44 d | 7.47 ± 0.53c | 8.43 ± 0.24b | 9.24 ± 0.46 a |
| Physicochemical | |||||
| Lipid oxidation * | 0.03 ± 0.00 c | 0.03 ± 0.01 c | 0.04 ± 0.01b | 0.05 ± 0.01ab | 0.05 ± 0.01 a |
| Protein oxidation # | 5.52 ± 0.92 d | 6.76 ± 1.28 c | 7.57 ± 1.25bc | 9.15 ± 1.84a | 8.27 ± 1.03 ab |
| Torrymeter readings | 11.14 ± 0.92 a | 10.64 ± 0.93 a | 11.33 ± 1.05a | 8.34 ± 1.64b | 7.73 ± 1.11 b |
| Color | |||||
| Lightness (L*) | 47.35 ± 2.31 b | 51.69 ± 1.30 a | 50.72 ± 1.15a | 50.57 ± 1.64a | 50.82 ± 1.28 a |
| Redness (a*) | 2.10 ± 0.38 ab | 2.19 ± 0.39 a | 1.81 ± 0.37b | 0.89 ± 0.10c | 1.00 ± 0.32 c |
| Yellowness (b*) | 1.64 ± 0.33 c | 2.65 ± 0.71 b | 3.02 ± 0.69b | 2.90 ± 0.90b | 4.51 ± 1.37 a |
| Chroma (C*) | 5.08 ± 0.87 c | 8.31 ± 1.66 b | 8.29 ± 0.47b | 8.30 ± 0.93b | 13.56 ± 2.62 a |
| Hue angle (H°) | 0.57 ± 0.09 c | 1.07 ± 0.20 b | 1.18 ± 0.19ab | 1.31 ± 0.15a | 1.18 ± 0.14 ab |
| Texture | |||||
| Hardness (N) | 10.33 ± 2.48 a | 8.97 ± 2.71 a | 8.60 ± 2.79a | 5.80 ± 1.38b | 5.88 ± 1.27 b |
| Chewiness (N × mm) | 5.45 ± 1.58 a | 4.50 ± 1.44 abc | 4.66 ± 1.16ab | 3.19 ± 0.72c | 3.95 ± 0.92 bc |
| Cohesiveness (ratio) | 0.69 ± 0.02 a | 0.69 ± 0.02 a | 0.71 ± 0.03a | 0.70 ± 0.04a | 0.69 ± 0.03 a |
| Springiness (ratio) | 0.89 ± 0.08 a | 0.87 ± 0.07 a | 0.91 ± 0.05a | 0.90 ± 0.06a | 0.91 ± 0.04 a |
| Socioeconomic Characteristics | Consumers | |
|---|---|---|
| Cluster 1 | Cluster 2 | |
| N | 62 | 30 |
| Gender (%) | ||
| Female | 65 (+) ** | 33 (−) ** |
| Male | 35 (−) ** | 67 (+) ** |
| Education (%) | ||
| Primary school | 0 (−) * | 7 (+) * |
| Secondary school | 0 (−) *** | 53 (+) *** |
| Undergraduate | 10 (−) * | 30 (+) * |
| Graduation | 90 (+) *** | 10 (−) *** |
| Income—minimum Brazilian wage (BRL 998.00)a (%) | ||
| 1 to 5 | 16 (−) ** | 47 (+) ** |
| >5 to 10 | 27 | 30 |
| >10 to 20 | 35 (+) * | 13 (−) * |
| >20 to 30 | 15 | 7 |
| >30 | 6 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, F.M.; Monteiro, M.L.G.; Ferrari, R.G.; Mutz, Y.S.; Martins, I.B.A.; Salim, A.P.A.A.; De Alcantara, M.; Deliza, R.; Mano, S.B.; Conte-Junior, C.A. Multivariate Nature of Fish Freshness Evaluation by Consumers. Foods 2022, 11, 2144. https://doi.org/10.3390/foods11142144
Viana FM, Monteiro MLG, Ferrari RG, Mutz YS, Martins IBA, Salim APAA, De Alcantara M, Deliza R, Mano SB, Conte-Junior CA. Multivariate Nature of Fish Freshness Evaluation by Consumers. Foods. 2022; 11(14):2144. https://doi.org/10.3390/foods11142144
Chicago/Turabian StyleViana, Fernanda M., Maria Lucia G. Monteiro, Rafaela G. Ferrari, Yhan S. Mutz, Inayara B. A. Martins, Ana Paula A. A. Salim, Marcela De Alcantara, Rosires Deliza, Sérgio B. Mano, and Carlos A. Conte-Junior. 2022. "Multivariate Nature of Fish Freshness Evaluation by Consumers" Foods 11, no. 14: 2144. https://doi.org/10.3390/foods11142144
APA StyleViana, F. M., Monteiro, M. L. G., Ferrari, R. G., Mutz, Y. S., Martins, I. B. A., Salim, A. P. A. A., De Alcantara, M., Deliza, R., Mano, S. B., & Conte-Junior, C. A. (2022). Multivariate Nature of Fish Freshness Evaluation by Consumers. Foods, 11(14), 2144. https://doi.org/10.3390/foods11142144








