Recent Developments in Surface-Enhanced Raman Spectroscopy and Its Application in Food Analysis: Alcoholic Beverages as an Example
Abstract
:1. Introduction
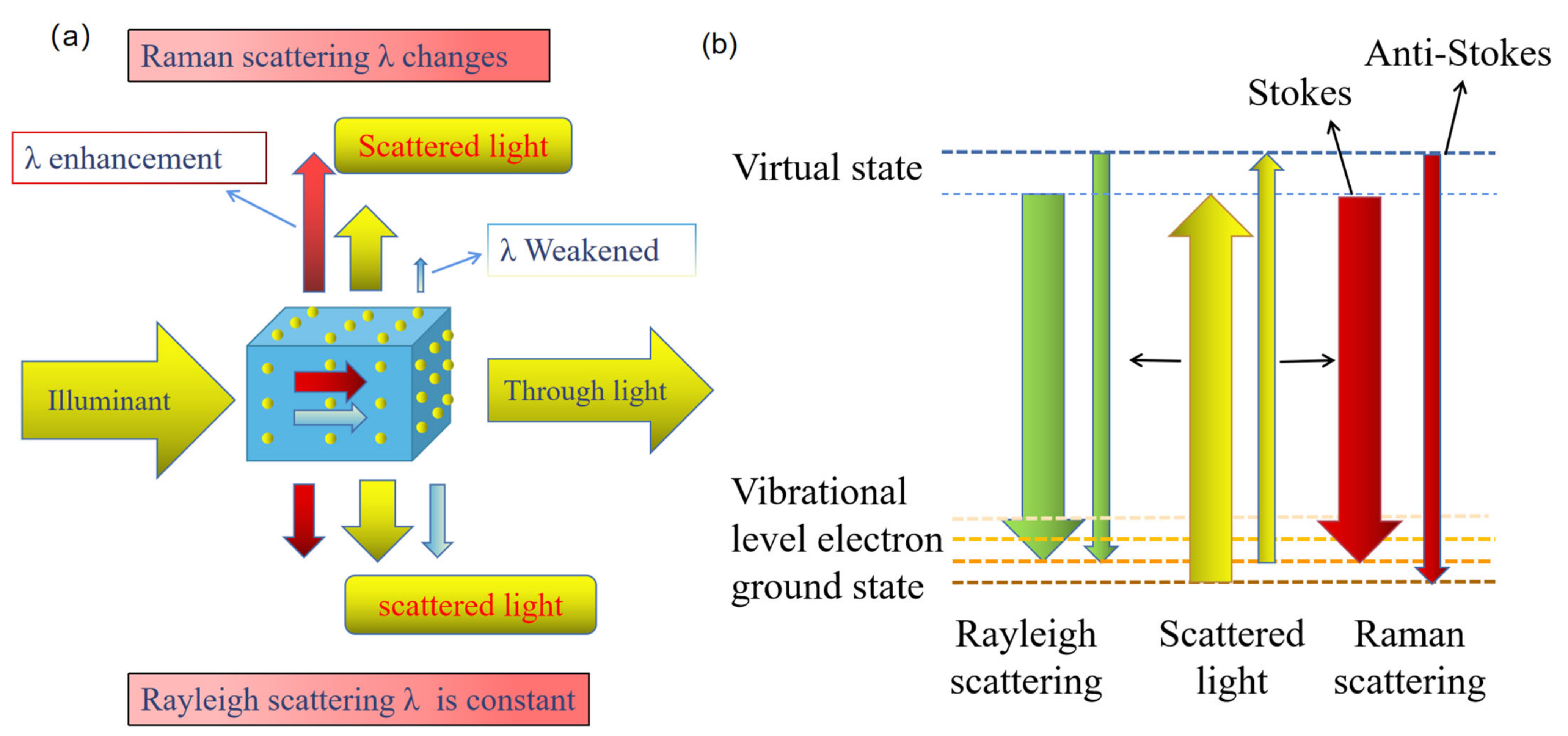
1.1. Basic Principles of Raman Scattering
1.2. Raman Spectroscopy Technology and Its Advantages
1.3. Development and Application Potential of Raman Spectroscopy Technology
1.4. Development History and Principle of Surface-Enhanced Raman Spectroscopy (SERS)
1.5. Application of SERS in Food Safety Detection
1.6. SERS in the Detection of Alcoholic Beverages
2. Latest Development of SERS Technology
2.1. The Development of Substrates in Raman Technology
2.1.1. Colloid Substrates
2.1.2. Solid Substrate
2.1.3. The Flexible Base
2.1.4. Mixed Nanomaterial Substrate
2.1.5. Semiconductor Substrates
2.1.6. Substrate Preparation by the Top-Down Technique
2.1.7. The Bottom-Up Technique Was Used to Prepare the Substrate
2.2. Direct Detection Technology of SERS
2.3. Indirect Detection Techniques for Surface Enhancement
2.4. SERS Combined with Machine Learning and Multivariate Analyses
2.5. SERS Combined with Sample Pretreatment
2.6. SERS in Combination with Other Techniques
3. Ingredients of Alcoholic Beverages
3.1. Basic Ingredients of Alcoholic Beverages
3.2. Beneficial Alcohol Ingredients
3.3. Toxic and Harmful Ingredients
3.4. Microorganisms
4. Application of SERS in the Detection of Alcoholic Beverages
4.1. Characterization and Classification of Alcoholic Beverages
4.2. Detection of Beneficial Ingredients
4.3. Detection of Harmful Ingredients
4.4. Microbiological Detection
5. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, O.; Pissard, A.; Baeten, V. 3-Near-infrared, mid-infrared, and Raman spectroscopy. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 77–134. [Google Scholar]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Gestal, M.; Gómez-Carracedo, M.P.; Andrade, J.M.; Dorado, J.; Fernández, E.; Prada, D.; Pazos, A. Classification of apple beverages using artificial neural networks with previous variable selection. Anal. Chim. Acta 2004, 524, 225–234. [Google Scholar] [CrossRef]
- Sayin, I.; Kahraman, M.; Sahin, F.; Yurdakul, D.; Culha, M. Characterization of Yeast Species Using Surface-Enhanced Raman Scattering. Appl. Spectrosc. 2009, 63, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Feher, I.; Pinzaru, S.C. Wine discrimination based on chemometric analysis of untargeted markers using FT-Raman spectroscopy. Food Control 2018, 85, 385–391. [Google Scholar] [CrossRef]
- Qin, J.W.; Chao, K.L.; Kim, M.; Cho, B.K. Line-Scan Macro-scale Raman Chemical Imaging for Authentication of Powdered Foods and Ingredients. Food Bioprocess Technol. 2016, 9, 113–123. [Google Scholar] [CrossRef]
- Eberhardt, K.; Stiebing, C.; Matthaus, C.; Schmitt, M.; Popp, J. Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Bodelon, G.; Montes-Garcia, V.; Perez-Juste, J.; Pastoriza-Santos, I. Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing. Front. Cell. Infect. Microbiol. 2018, 8, 143. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Ryder, A.G.; Leger, M.N.; Howley, T. Qualitative Analysis Using Raman Spectroscopy and Chemometrics: A Comprehensive Model System for Narcotics Analysis. Appl. Spectrosc. 2010, 64, 1109–1121. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhang, P.; Wang, H.; Zheng, B.; Sun, L.; Zhang, D.H.; Fan, J.H. Raman Spectrum-Based Diagnosis Strategy for Bladder Tumor. Urol. Int. 2022, 106, 109–115. [Google Scholar] [CrossRef]
- Rousaki, A.; Vandenabeele, P. In situ Raman spectroscopy for cultural heritage studies. J. Raman Spectrosc. 2021, 52, 2178–2189 . [Google Scholar] [CrossRef]
- Pozzi, F.; Leona, M. Surface-enhanced Raman spectroscopy in art and archaeology. J. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Chazhengina, S.Y.; Kovalevski, V.V. Raman spectroscopy of weathered shungites. J. Raman Spectrosc. 2017, 48, 1590–1596. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Banholzer, M.J.; Millstone, J.E.; Qin, L.D.; Mirkin, C.A. Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 885–897. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [Green Version]
- McNay, G.; Eustace, D.; Smith, W.E.; Faulds, K.; Graham, D. Surface-Enhanced Raman Scattering (SERS) and Surface-Enhanced Resonance Raman Scattering (SERRS): A Review of Applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef]
- Ai, Y.J.; Liang, P.; Wu, Y.X.; Dong, Q.M.; Li, J.B.; Bai, Y.; Xu, B.J.; Yu, Z.; Ni, D.J. Rapid qualitative and quantitative determination of food colorants by both Raman spectra and Surface-enhanced Raman Scattering (SERS). Food Chem. 2018, 241, 427–433. [Google Scholar] [CrossRef]
- Nishino, T. Surface-enhanced Raman Spectroscopy. Anal. Sci. 2018, 34, 1061–1062. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.; van Duyne, R.; Ren, B. Surface-enhanced Raman scattering. Analyst 2016, 141, 4995. [Google Scholar] [CrossRef] [Green Version]
- Campion, P.K.A. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Marques-Gonzalez, S.; Matsushita, R.; Kiguchi, M. Surface enhanced Raman scattering of molecules in metallic nanogaps. J. Opt. 2015, 17, 114001. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Zhang, J.W.; Yang, Y.; Huang, Z.R.; Long, N.V.; Fu, C.L. Engineering of SERS Substrates Based on Noble Metal Nanomaterials for Chemical and Biomedical Applications. Appl. Spectrosc. Rev. 2015, 50, 499–525. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Rycenga, M.; Au, L.; Xia, Y.N. Isolating and Probing the Hot Spot Formed between Two Silver Nanocubes. Angew. Chem. Int. Ed. 2009, 48, 2180–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.F.; Zou, M.Q.; Qi, X.H.; Liu, F.; Zhu, X.H.; Zhao, B.H. Detection of melamine in liquid milk using surface-enhanced Raman scattering spectroscopy. J. Raman Spectrosc. 2010, 41, 1655–1660. [Google Scholar] [CrossRef]
- Tran, V.; Walkenfort, B.; Konig, M.; Salehi, M.; Schlucker, S. Rapid, Quantitative, and Ultrasensitive Point-of-Care Testing: A Portable SERS Reader for Lateral Flow Assays in Clinical Chemistry. Angew. Chem. Int. Ed. 2019, 58, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Chang, B.Y.; Zhang, H.; Wang, Z.P.; Wu, S.J. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xu, M.X.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.L.; You, T.Y.; Ouyang, X.H.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef]
- Neng, J.; Zhang, Q.; Sun, P. Application of surface-enhanced Raman spectroscopy in fast detection of toxic and harmful substances in food. Biosens. Bioelectron. 2020, 167, 112480. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Li, M.; Liu, W.; Wei, Z.; Zhao, L.; Liu, Y.; Xu, L.; Zhao, G.; Ma, Y. Preparation of an AgNPs@Polydimethylsiloxane (PDMS) multi-hole filter membrane chip for the rapid identification of food-borne pathogens by surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120456. [Google Scholar] [CrossRef]
- Kashif, M.; Majeed, M.I.; Nawaz, H.; Rashid, N.; Abubakar, M.; Ahmad, S.; Ali, S.; Hyat, H.; Bashir, S.; Batool, F.; et al. Surface-enhanced Raman spectroscopy for identification of food processing bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119989. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yin, L.; Zuo, M.; Chen, Q.; El-Seedi, H.R.; Zou, X. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef]
- Peksa, V.; Jahn, M.; Stolcova, L.; Schulz, V.; Proska, J.; Prochazka, M.; Weber, K.; Cialla-May, D.; Popp, J. Quantitative SERS Analysis of Azorubine (E 122) in Sweet Drinks. Anal. Chem. 2015, 87, 2840–2844. [Google Scholar] [CrossRef]
- Yun, J.; Kim, J.H.; Lee, J.E. Influence of aflatoxin in Nuruk on the safety of starch-based alcoholic beverage. J. Food Sci. 2020, 85, 762–770. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Overah, L.C.; Bassey, F.I.; Martincigh, B.S. Trace metal concentrations in distilled alcoholic beverages and liquors in Nigeria. J. Inst. Brew. 2014, 120, 521–528. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kanteres, F.; Rehm, J. Carcinogenicity of acetaldehyde in alcoholic beverages: Risk assessment outside ethanol metabolism. Addiction 2009, 104, 533–550. [Google Scholar] [CrossRef]
- Arslan, M.; Tahir, H.E.; Zareef, M.; Shi, J.; Rakha, A.; Bilal, M.; Xiaowei, H.; Zhihua, L.; Xiaobo, Z. Recent trends in quality control, discrimination and authentication of alcoholic beverages using nondestructive instrumental techniques. Trends Food Sci. Technol. 2021, 107, 80–113. [Google Scholar] [CrossRef]
- Li, S.H.; An, Y.F.; Fu, W.N.; Sun, X.; Li, W.J.; Li, T.P. Changes in anthocyanins and volatile components of purple sweet potato fermented alcoholic beverage during aging. Food Res. Int. 2017, 100, 235–240. [Google Scholar] [CrossRef]
- Kim, H.M.; Yang, G.; Kim, J.Y.; Yoon, S.J.; Shin, B.K.; Lee, J.; Park, J.H.; Kwon, S.W. Simultaneous Determination of Volatile Organic Compounds in Commercial Alcoholic Beverages by Gas Chromatography with Flame Ionization Detection. J. AOAC Int. 2017, 100, 1492–1499. [Google Scholar] [CrossRef]
- Tubia, I.; Prasad, K.; Perez-Lorenzo, E.; Abadin, C.; Zumarraga, M.; Oyanguren, I.; Barbero, F.; Paredes, J.; Arana, S. Beverage spoilage yeast detection methods and control technologies: A review of Brettanomyces. Int. J. Food Microbiol. 2018, 283, 65–76. [Google Scholar] [CrossRef]
- Sisco, E.; Dake, J. Detection of low molecular weight adulterants in beverages by direct analysis in real time mass spectrometry. Anal. Methods 2016, 8, 2971–2978. [Google Scholar] [CrossRef] [Green Version]
- Debebe, A.; Redi-Abshiro, M.; Chandravanshi, B.S. Non-destructive determination of ethanol levels in fermented alcoholic beverages using Fourier transform mid-infrared spectroscopy. Chem. Cent. J. 2017, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Sliwinska, M.; Wisniewska, P.; Dymerski, T.; Wardencki, W.; Namiesnik, J. Authenticity Assessment of the “Onisiwka” Nalewka Liqueurs Using Two-Dimensional Gas Chromatography and Sensory Evaluation. Food Anal. Methods 2017, 10, 1709–1720. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.A.T.; Pascoa, R.; Lopes, J.A. A review on the application of vibrational spectroscopy in the wine industry: From soil to bottle. Trac-Trends Anal. Chem. 2017, 88, 100–118. [Google Scholar] [CrossRef]
- De Goes, R.E.; Fabris, L.V.M.; Muller, M.; Fabris, J.L. Light-Assisted Detection of Methanol in Contaminated Spirits. J. Lightwave Technol. 2016, 34, 4499–4505. [Google Scholar] [CrossRef]
- Cialla, D.; Marz, A.; Bohme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, I.H.; Genis, H.E.; Guven, B.; Tamer, U.; Alper, N. A novel method for quantification of ethanol and methanol in distilled alcoholic beverages using Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1171–1176. [Google Scholar] [CrossRef]
- Magdas, D.A.; David, M.; Berghian-Grosan, C. Fruit spirits fingerprint pointed out through artificial intelligence and FT-Raman spectroscopy. Food Control 2022, 133, 108630. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Dos Santos, C.A.T.; Pascoa, R.; Sarraguca, M.C.; Porto, P.; Cerdeira, A.L.; Gonzalez-Saiz, J.M.; Pizarro, C.; Lopes, J.A. Merging vibrational spectroscopic data for wine classification according to the geographic origin. Food Res. Int. 2017, 102, 504–510. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Leopold, N.; Franco, R.; Pereiral, E. Expedite SERS Fingerprinting of Portuguese White Wines Using Plasmonic Silver Nanostars. Front. Chem. 2019, 7, 368. [Google Scholar] [CrossRef]
- Dendisova, M.; Palounek, D.; Svecova, M.; Prokopec, V. SERS study of fluorescent and non-fluorescent flavonoids: What is the role of excitation wavelength on SERS optical response? Chem. Pap. 2019, 73, 2945–2953. [Google Scholar] [CrossRef]
- Magdas, D.A.; Pinzaru, S.C.; Guyon, F.; Feher, I.; Cozar, B.I. Application of SERS technique in white wines discrimination. Food Control 2018, 92, 30–36. [Google Scholar] [CrossRef]
- Bettini, S.; Pal, S.; Sawalha, S.; Licciulli, A.; Valli, L.; Giancane, G.; Pagano, R. Cellulose-Based Substrate for SERS-Promoted Histamine Picomolar Detection in Beverages. Chemistryselect 2019, 4, 2968–2975. [Google Scholar] [CrossRef]
- Rojas, L.M.; Qu, Y.Q.; He, L.L. A facile solvent extraction method facilitating surface-enhanced Raman spectroscopic detection of ochratoxin A in wine and wheat. Talanta 2021, 224, 121792. [Google Scholar] [CrossRef]
- Efrima, S.; Zeiri, L. Understanding SERS of bacteria. J. Raman Spectrosc. 2009, 40, 277–288. [Google Scholar] [CrossRef]
- Lemma, T.; Wang, J.; Arstila, K.; Hytonen, V.P.; Toppari, J.J. Identifying yeasts using surface enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 299–307. [Google Scholar] [CrossRef]
- Arroyo-Cerezo, A.; Jimenez-Carvelo, A.M.; González-Casado, A.; Koidis, A.; Cuadros-Rodríguez, L. Deep (offset) non-invasive Raman spectroscopy for the evaluation of food and beverages—A review. LWT 2021, 149, 111822. [Google Scholar] [CrossRef]
- Zeren, C.; Acikgoz, G.; Kahraman, S. Using Raman Spectroscopy for Determination Methanol Quantity in Illegal Alcoholic Beverages. Spectrosc. Spectr. Anal. 2017, 37, 2979–2983. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Hoang, V.D. 9-Alcoholic drink analysis. In Vibrational Spectroscopy Applications in Biomedical, Pharmaceutical and Food Sciences; Bunaciu, A.A., Aboul-Enein, H.Y., Hoang, V.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 205–224. [Google Scholar]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [Google Scholar] [CrossRef]
- Faggio, G.; Grillo, R.; Lisi, N.; Buonocore, F.; Chierchia, R.; Jung Kim, M.; Lee, G.-H.; Capasso, A.; Messina, G. Nanocrystalline graphene for ultrasensitive surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2022, 599, 154035. [Google Scholar] [CrossRef]
- López-Lorente, Á.I. Recent developments on gold nanostructures for surface enhanced Raman spectroscopy: Particle shape, substrates and analytical applications. A review. Anal. Chim. Acta 2021, 1168, 338474. [Google Scholar] [CrossRef]
- Hossain, M.K.; Drmosh, Q.A. Clusters-based silver nanorings: An active substrate for surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120141. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Nguyen, T.M.A.; Sai, C.D.; Le, T.H.Y.; Anh Tran, T.N.; Bach, T.C.; Le, V.V.; Pham, N.H.; Ngac, A.B.; Nguyen, V.T.; et al. Efficient surface enhanced Raman scattering substrates based on complex gold nanostructures formed by annealing sputtered gold thin films. Opt. Mater. 2021, 121, 111488. [Google Scholar] [CrossRef]
- Subr, M.; Petr, M.; Peksa, V.; Kylian, O.; Hanus, J.; Prochazka, M. Ag Nanorod Arrays for SERS: Aspects of Spectral Reproducibility, Surface Contamination, and Spectral Sensitivity. J. Nanomater. 2015, 2015, 55. [Google Scholar] [CrossRef] [Green Version]
- Bich, H.N.; Van Hieu, N.; Hong, N.T. Rich variety of substrates for surface enhanced Raman spectroscopy. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2016, 7, 033001. [Google Scholar] [CrossRef] [Green Version]
- Cinel, N.A.; Cakmakyapan, S.; Butun, S.; Ertas, G.; Ozbay, E. E-Beam lithography designed substrates for surface enhanced Raman spectroscopy. Photonics Nanostruct. Fundam. Appl. 2015, 15, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.G.; Tang, Y.; Li, S.Q.; Liu, S.; Li, B.X. Hydrothermal Green Synthesis of Nano Silver and Its Application in Surface Enhanced Raman of Organic Dyes in Water. Spectrosc. Spectr. Anal. 2020, 40, 3722–3726. [Google Scholar] [CrossRef]
- Yoon, H.; Suh, J.S. Universal substrates based on Ag colloidal particles for routine surface-enhanced Raman scattering spectral measurements. RSC Adv. 2017, 7, 28573–28579. [Google Scholar] [CrossRef] [Green Version]
- Tay, L.L.; Poirier, S.; Ghaemi, A.; Hulse, J.; Wang, S.L. Paper-based surface-enhanced Raman spectroscopy sensors for field applications. J. Raman Spectrosc. 2021, 52, 563–572. [Google Scholar] [CrossRef]
- Cheng, J.; Su, X.O.; Yao, Y.; Han, C.Q.; Wang, S.; Zhao, Y.P. Highly Sensitive Detection of Melamine Using a One-Step Sample Treatment Combined with a Portable Ag Nanostructure Array SERS Sensor. PLoS ONE 2016, 11, e0154402. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Portaccio, M.; Tate, R.; Lepore, M.; Delfino, I. Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy. Sensors 2017, 17, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.Y.; Liu, X.J.; Ying, Y.B. Facing Challenges in Real-Life Application of Surface-Enhanced Raman Scattering: Design and Nanofabrication of Surface-Enhanced Raman Scattering Substrates for Rapid Field Test of Food Contaminants. J. Agric. Food Chem. 2018, 66, 6525–6543. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, M.; Mullen, E.R.; Korkmaz, A.; Wachsmann-Hogiu, S. Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics 2017, 6, 831–852. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Chua, J.; Tan, E.K.M.; Kah, J.C.Y. Optimizing the SERS enhancement of a facile gold nanostar immobilized paper-based SERS substrate. RSC Adv. 2017, 7, 16264–16272. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Cheng, C.Y.; Lai, Y.S. Paper-based flexible surface enhanced Raman scattering platforms and their applications to food safety. Trends Food Sci. Technol. 2020, 100, 349–358. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.R.; Wang, Q.Q.; Li, M.; Luan, L.L.; Zhang, X.; Xu, W.P. Preparation of Base Membrane of Gold Nanotriangle Filter Paper and Its Application in Rapid Detection of Food Contaminants. Chin. J. Anal. Chem. 2020, 48, 1625–1632. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. An improved surface enhanced Raman spectroscopic method using a paper-based grape skin-gold nanoparticles/graphene oxide substrate for detection of rhodamine 6G in water and food. Chemosphere 2022, 301, 134702. [Google Scholar] [CrossRef]
- Tian, Q.; Cao, S.; He, G.; Long, Y.; Zhou, X.; Zhang, J.; Xie, J.; Zhao, X. Plasmonic Au-Ag alloy nanostars based high sensitivity surface enhanced Raman spectroscopy fiber probes. J. Alloys Compd. 2022, 900, 163345. [Google Scholar] [CrossRef]
- Cheng, J.; Han, C.Q.; Xie, J.C.; Su, X.O.; Wang, P.L. Rapid and Sensitive Detection of Acrylamide in Fried Food Based on Surface-Enhanced Raman Spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 1087–1092. [Google Scholar] [CrossRef]
- Wu, Z.G.; Liu, Y.M.; Wu, M.M.; Chen, Y.; Chen, Y.N.; Xian, H.H.; Wang, X.P.; Wei, H.Y. Preparation and Surface Enhanced Raman Spectroscopy of Au/TiN Composite Films. Spectrosc. Spectr. Anal. 2020, 40, 420–426. [Google Scholar] [CrossRef]
- Xu, W.G.; Mao, N.N.; Zhang, J. Graphene: A Platform for Surface-Enhanced Raman Spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef]
- Kong, L.; Chen, J.; Huang, M. GO/Au@Ag nanobones decorated membrane for simultaneous enrichment and on-site SERS detection of colorants in beverages. Sens. Actuators B Chem. 2021, 344, 130163. [Google Scholar] [CrossRef]
- Yamada, H.; Yamamoto, Y. Surface enhanced Raman scattering (SERS) of chemisorbed species on various kinds of metals and semiconductors. Surf. Sci. 1983, 134, 71–90. [Google Scholar] [CrossRef]
- Bontempi, N.; Salmistraro, M.; Ferroni, M.; Depero, L.E.; Alessandri, I. Probing the spatial extension of light trapping-induced enhanced Raman scattering in high-density Si nanowire arrays. Nanotechnology 2014, 25, 465705. [Google Scholar] [CrossRef] [PubMed]
- Adesoye, S.; Dellinger, K. ZnO and TiO2 nanostructures for surface-enhanced Raman scattering-based bio-sensing: A review. Sens. Bio-Sens. Res. 2022, 37, 100499. [Google Scholar] [CrossRef]
- Yu, L.X.; Lv, R.T. Two-dimensional layer materials for highly efficient molecular sensing based on surface-enhanced Raman scattering. New Carbon Mater. 2021, 36, 995–1015. [Google Scholar] [CrossRef]
- Loo, B.H. Observation of the surface enhanced raman scattering effect from the semiconductor—electrolyte interface. J. Electroanal. Chem. Interfacial Electrochem. 1982, 136, 209–213. [Google Scholar] [CrossRef]
- Yang, L.B.; Jiang, X.; Yang, M. Improvement of surface-enhanced Raman scattering performance for broad band gap semiconductor nanomaterial (TiO2): Strategy of metal doping. Appl. Phys. Lett. 2011, 99, 111114. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.M.; Fang, Y.; Xu, H.; Zhang, H.L.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z.F. Can Graphene be used as a Substrate for Raman Enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.M.; Quan, X.; Chen, S.; Fan, X.F. Distance-independent quenching of quantum dots by nanoscale-graphene in self-assembled sandwich immunoassay. Chem. Commun. 2010, 46, 7909–7911. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, N.; Liu, R.J.; Wang, X.Y.; Zhu, Y.; Zhang, J. SERS and the photo-catalytic performance of Ag/TiO2/graphene composites. Opt. Mater. Express 2018, 8, 704–717. [Google Scholar] [CrossRef]
- Yilmaz, M.; Babur, E.; Ozdemir, M.; Gieseking, R.L.; Dede, Y.; Tamer, U.; Schatz, G.C.; Facchetti, A.; Usta, H.; Demirel, G. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nat. Mater. 2017, 16, 918–924. [Google Scholar] [CrossRef]
- Lombardi, J.R. The theory of surface-enhanced Raman spectroscopy on organic semiconductors: J-aggregates. Chem. Phys. Lett. 2020, 751, 137553. [Google Scholar] [CrossRef]
- Wells, S.M.; Retterer, S.D.; Oran, J.M.; Sepaniak, M.J. Controllable Nanotabrication of Aggregate-like Nanoparticle Substrates and Evaluation for Surface-Enhanced Raman Spectroscopy. ACS Nano 2009, 3, 3845–3853. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.Y.; Yamaguchi, K.; Tanemura, M.; Huang, Z.R.; Jiang, D.L.; Chen, Y.H.; Zhou, F.; Nogami, M. Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale 2012, 4, 2663–2669. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.P.; Wang, Y.; Hou, Y.M.; Chen, T.; Zhang, P.F.; Huang, Q.Y.; Zhao, Q. Fabrication of Gold Nanoparticle Array Substrate by Chemical Replacement and Its Surface Enhanced Raman Scattering Properties. Spectrosc. Spectr. Anal. 2017, 37, 3725–3729. [Google Scholar] [CrossRef]
- Huang, J.; Yao, J.L.; Gu, R.A. Study of Surface Enhanced Raman Spectra of SCN- Adsorbed on the Au-Ag Complex Substrate. Spectrosc. Spectr. Anal. 2009, 29, 2434–2437. [Google Scholar] [CrossRef]
- Ma, J.; Kong, D.D.; Han, X.H.; Guo, W.L.; Shi, X.F. Detection of Antibiotics in Water Using Silver Colloid Films as Substrate of Surface-Enhanced Raman Scattering. Spectrosc. Spectr. Anal. 2013, 33, 2688–2693. [Google Scholar] [CrossRef]
- Han, X.X.; Zhao, B.; Ozaki, Y. Label-free detection in biological applications of surface-enhanced Raman scattering. Trac-Trends Anal. Chem. 2012, 38, 67–78. [Google Scholar] [CrossRef]
- Wang, P.X.; Pang, S.; Chen, J.H.; McLandsborough, L.; Nugen, S.R.; Fan, M.T.; He, L.L. Label-free mapping of single bacterial cells using surface-enhanced Raman spectroscopy. Analyst 2016, 141, 1356–1362. [Google Scholar] [CrossRef]
- Yazgan, N.N.; Boyaci, I.H.; Topcu, A.; Tamer, U. Detection of melamine in milk by surface-enhanced Raman spectroscopy coupled with magnetic and Raman-labeled nanoparticles. Anal. Bioanal. Chem. 2012, 403, 2009–2017. [Google Scholar] [CrossRef]
- Li, X.Z.; Yang, T.Y.; Song, Y.T.; Zhu, J.H.; Wang, D.L.; Li, W. Surface-enhanced Raman spectroscopy (SERS)-based immunochromatographic assay (ICA) for the simultaneous detection of two pyrethroid pesticides. Sens. Actuators B Chem. 2019, 283, 230–238. [Google Scholar] [CrossRef]
- Hughes, J.; Izake, E.L.; Lott, W.B.; Ayoko, G.A.; Sillence, M. Ultra sensitive label free surface enhanced Raman spectroscopy method for the detection of biomolecules. Talanta 2014, 130, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.D.; Dong, X.L. Quantitative Analysis of Dimethoate Pesticide Residues in Honey by Surface-Enhanced Raman Spectroscopy. Spectrosc. Spectr. Anal. 2015, 35, 1572–1576. [Google Scholar] [CrossRef]
- Bao, Q.W.; Zhao, H.; Han, S.; Zhang, C.; Hasi, W. Surface-enhanced Raman spectroscopy for rapid identification and quantification of Flibanserin in different kinds of wine. Anal. Methods 2020, 12, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, S.; Wan, S.; Wang, P.L.; Su, X.O.; Xie, J.C. Rapid and sensitive detection of acrylamide in fried food using dispersive solid-phase extraction combined with surface-enhanced Raman spectroscopy. Food Chem. 2019, 276, 157–163. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.L.; Shi, Y.E.; Liu, Z.; Zhan, J.H. Surface enhanced Raman spectroscopy hyphenated with surface microextraction for in-situ detection of polycyclic aromatic hydrocarbons on food contact materials. Talanta 2016, 158, 322–329. [Google Scholar] [CrossRef]
- Tycova, A.; Gerhardt, R.F.; Belder, D. Surface enhanced Raman spectroscopy in microchip electrophoresis. J. Chromatogr. A 2018, 1541, 39–46. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, X.X.; Wang, Y.R.; Fang, E.H.; Zhang, Z.G.; Chen, X. Headspace Thin-Film Microextraction Coupled with Surface-Enhanced Raman Scattering as a Facile Method for Reproducible and Specific Detection of Sulfur Dioxide in Wine. Anal. Chem. 2015, 87, 633–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.J.; Zheng, J.K.; He, L.L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. Trac-Trends Anal. Chem. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Wu, L.; Yan, H.; Li, G.H.; Xu, X.; Zhu, L.; Chen, X.Q.; Wang, J. Surface-Imprinted Gold Nanoparticle-Based Surface-Enhanced Raman Scattering for Sensitive and Specific Detection of Patulin in Food Samples. Food Anal. Methods 2019, 12, 1648–1657. [Google Scholar] [CrossRef]
- Gao, R.; Li, D.; Zhang, Q.M.; Zheng, S.Q.; Ren, X.G.; Deng, W. GNPs-QDs core-satellites assembly: Trimodal platform for on-site identification and detection of TNT in complex media. Sens. Actuators B Chem. 2021, 328, 128960. [Google Scholar] [CrossRef]
- He, L.L.; Chen, T.; Labuza, T.P. Recovery and quantitative detection of thiabendazole on apples using a surface swab capture method followed by surface-enhanced Raman spectroscopy. Food Chem. 2014, 148, 42–46. [Google Scholar] [CrossRef]
- Shen, Z.D.; Kong, X.M.; Yu, Q.; Yang, Z.X. Research Progress of Thin Layer Chromatography and Surface Enhanced Raman Scattering Spectroscopy. Spectrosc. Spectr. Anal. 2021, 41, 388–394. [Google Scholar] [CrossRef]
- Wardencki, W. Alcoholic Beverages. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 67–76. [Google Scholar]
- Wei, Y.; Zou, W.; Shen, C.H.; Yang, J.G. Basic flavor types and component characteristics of Chinese traditional liquors: A review. J. Food Sci. 2020, 85, 4096–4107. [Google Scholar] [CrossRef]
- Li, H.H.; Qin, D.; Wu, Z.Y.; Sun, B.G.; Sun, X.T.; Huang, M.Q.; Sun, J.Y.; Zheng, F.P. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef]
- Shin, K.S.; Lee, J.H. Acetaldehyde contents and quality characteristics of commercial alcoholic beverages. Food Sci. Biotechnol. 2019, 28, 1027–1036. [Google Scholar] [CrossRef]
- Jackson, R.S. Chapter 6—Chemical constituents of grapes and wine. Food Sci. Technol. 2020, 5, 375–459. [Google Scholar]
- Ljevar, A.; Curko, N.; Tomasevic, M.; Radosevic, K.; Srcek, V.G.; Ganic, K.K. Phenolic Composition, Antioxidant Capacity and in vitro Cytotoxicity Assessment of Fruit Wines. Food Technol. Biotechnol. 2016, 54, 145–155. [Google Scholar] [CrossRef]
- Cakar, U.; Grozdanic, N.; Petrovic, A.; Pejin, B.; Nastasijevic, B.; Markovic, B.; Dordevic, B. Fruit Wines Inhibitory Activity Against alpha-Glucosidase. Curr. Pharm. Biotechnol. 2017, 18, 1264–1272. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, S.; Zhou, T.; Zhang, P.; Li, H.B. Alcoholic Beverage Consumption and Chronic Diseases. Int. J. Environ. Res. Public Health 2016, 13, 522. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Lv, Q.Y.; Liu, A.; Wang, J.R.; Sun, X.Q.; Deng, J.; Chen, Q.F.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Gao, H.Y.; Li, G.Y.; Huang, J.; Han, Y.; Sun, F.Z.; Du, X.W.; An, L.J.; Wang, H.Y.; Wang, J.H. Protective effects of Zhuyeqing liquor on the immune function of normal and immunosuppressed mice in vivo. BMC Complement. Altern. Med. 2013, 13, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koguchi, M.; Saigusa, N.; Teramoto, Y. Antioxidative Activity of Alcoholic Beverages made from Purple Rice (Oryza sativa var. Indica cv. Shiun). Food Sci. Technol. Res. 2010, 16, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Bertelli, A.A.A.; Das, D.K. Grapes, Wines, Resveratrol, and Heart Health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Wine, Resveratrol and Health: A Review. Nat. Prod. Commun. 2009, 4, 635–658. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, M.D.; Samouris, G.; Achilias, D.S. Acetaldehyde contamination of water, alcoholic, and non-alcoholic beverages stored in glass or plastic bottles. Toxicol. Environ. Chem. 2016, 98, 1183–1190. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Gill, J.S.; Chick, J.; Rehm, J. The total margin of exposure of ethanol and acetaldehyde for heavy drinkers consuming cider or vodka. Food Chem. Toxicol. 2015, 83, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Magnusdottir, K.; Kristinsson, J.; Johannesson, P. Adulterated alcoholic beverages. Laeknabladid 2010, 96, 626–628. [Google Scholar]
- Fentie, E.G.; Emire, S.A.; Demsash, H.D.; Dadi, D.W.; Shin, J.H. Cereal- and Fruit-Based Ethiopian Traditional Fermented Alcoholic Beverages. Foods 2020, 9, 1781. [Google Scholar] [CrossRef]
- Tamang, J.P. “Ethno-microbiology” of ethnic Indian fermented foods and alcoholic beverages. J. Appl. Microbiol. 2021, 1–17. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial interactions in alcoholic beverages. Int. Microbiol. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176. [Google Scholar] [CrossRef]
- Zanuttin, F.; Gurian, E.; Ignat, I.; Fornasaro, S.; Calabretti, A.; Bigot, G.; Bonifacio, A. Characterization of white wines from north-eastern Italy with surface-enhanced Raman spectroscopy. Talanta 2019, 203, 99–105. [Google Scholar] [CrossRef]
- Leong, Y.X.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.M.; Phang, I.Y.; Ling, X.Y. Surface-Enhanced Raman Scattering (SERS) Taster: A Machine-Learning-Driven Multireceptor Platform for Multiplex Profiling of Wine Flavors. Nano Lett. 2021, 21, 2642–2649. [Google Scholar] [CrossRef]
- Shukla, S.P.; Roy, M.; Mukherjee, P.; Das, L.; Neogy, S.; Srivastava, D.; Adhikari, S. Size Selective Green Synthesis of Silver and Gold Nanoparticles: Enhanced Antibacterial Efficacy of Resveratrol Capped Silver Sol. J. Nanosci. Nanotechnol. 2016, 16, 2453–2463. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Tian, Y.; Chen, Y.H.; He, L.L. Chemical profiling of red wines using surface-enhanced Raman spectroscopy (SERS). Anal. Methods 2020, 12, 1324–1332. [Google Scholar] [CrossRef]
- De Oliveira, F.S.; Sousa, E.T.; de Andrade, J.B. A sensitive flow analysis system for the fluorimetric determination of low levels of formaldehyde in alcoholic beverages. Talanta 2007, 73, 561–566. [Google Scholar] [CrossRef]
- Pinzaru, S.C.; Magdas, D.A. Ag Nanoparticles Meet Wines: SERS for Wine Analysis. Food Anal. Methods 2018, 11, 892–900. [Google Scholar] [CrossRef]
- Duan, H.Z.; Deng, W.; Gan, Z.F.; Li, D.; Li, D.W. SERS-based chip for discrimination of formaldehyde and acetaldehyde in aqueous solution using silver reduction. Microchim. Acta 2019, 186, 175. [Google Scholar] [CrossRef]
- Mandrile, L.; Cagnasso, I.; Berta, L.; Giovannozzi, A.M.; Petrozziello, M.; Pellegrino, F.; Asproudi, A.; Durbiano, F.; Rossi, A.M. Direct quantification of sulfur dioxide in wine by Surface Enhanced Raman Spectroscopy. Food Chem. 2020, 326, 127009. [Google Scholar] [CrossRef]
- Kong, D.X.; Zhu, W.F.; Li, M. A facile and sensitive SERS-based platform for sulfite residues/SO2 detection in food. Microchem. J. 2021, 165, 106174. [Google Scholar] [CrossRef]
- Yu, S.H.; Liu, Z.G.; Wang, W.X.; Jin, L.; Xu, W.Q.; Wu, Y.Q. Disperse magnetic solid phase microextraction and surface enhanced Raman scattering (Dis-MSPME-SERS) for the rapid detection of trace illegally chemicals. Talanta 2018, 178, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, H.; Rong, L.Y.; Chen, X.Q. A gas-diffusion microfluidic paper-based analytical device (mu PAD) coupled with portable surface-enhanced Raman scattering (SERS): Facile determination of sulphite in wines. Analyst 2016, 141, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, Y.K.; Li, Y.Y.; Fang, X.Q.; Zhai, C.; Wang, W.X.; Zheng, X.C. Calibration Transfer of Surface-Enhanced Raman Spectroscopy Quantitative Prediction Model of Potassium Sorbate in Osmanthus Wine to Other Wine. Spectrosc. Spectr. Anal. 2018, 38, 824–829. [Google Scholar] [CrossRef]
- Xie, Y.F.; Chen, T.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Rapid SERS detection of acid orange II and brilliant blue in food by using Fe3O4@Au core-shell substrate. Food Chem. 2019, 270, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.P.; He, Y. Analysis of Sildenafil in Liquor and Health Wine Using Surface Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2019, 20, 2722. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Hasi, W.; Bao, L.; Han, S.; Sha, X.Y.; Sun, J.; Lou, X.T.; Lin, D.Y.; Lv, Z.W. Rapid Detection of Sildenafil Drugs in Liquid Nutraceuticals Based on Surface-Enhanced Raman Spectroscopy Technology. Chin. J. Chem. 2017, 35, 1522–1528. [Google Scholar] [CrossRef]
- Wu, J.W.; Zhang, L.X.; Bu, X.F.; Li, P.; Zhao, B.; Tian, Y. Determination of the illegal adulteration of natural healthcare products with chemical drugs using surface-enhanced Raman scattering. Analyst 2018, 143, 5202–5209. [Google Scholar] [CrossRef]
- Lin, L.; Qu, F.F.; Nie, P.C.; Zhang, H.; Chu, B.Q.; He, Y. Rapid and Quantitative Determination of Sildenafil in Cocktail Based on Surface Enhanced Raman Spectroscopy. Molecules 2019, 24, 1790. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.R.; Li, J.Y.; Zhang, L.; Ge, Z.P.; Wang, X.R.; Hu, X.Y.; Xu, T.J.; Li, P.; Xu, W.P. HS-beta-cyclodextrin-functionalized Ag@Fe3O4@Ag nanoparticles as a surface-enhanced Raman spectroscopy substrate for the sensitive detection of butyl benzyl phthalate. Anal. Bioanal. Chem. 2019, 411, 5691–5701. [Google Scholar] [CrossRef]
- Li, J.Y.; Hu, X.Y.; Zhou, Y.R.; Zhang, L.; Ge, Z.P.; Wang, X.R.; Xu, W.P. beta-Cyclodextrin-Stabilized Au Nanoparticles for the Detection of Butyl Benzyl Phthalate. ACS Appl. Nano Mater. 2019, 2, 2743–2751. [Google Scholar] [CrossRef]
- Liu, J.N.; Li, J.Y.; Li, F.; Zhou, Y.R.; Hu, X.Y.; Xu, T.J.; Xu, W.P. Liquid-liquid interfacial self-assembled Au NP arrays for the rapid and sensitive detection of butyl benzyl phthalate (BBP) by surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2018, 410, 5277–5285. [Google Scholar] [CrossRef]
- Wu, Y.P.; Yu, W.F.; Yang, B.H.; Li, P. Self-assembled two-dimensional gold nanoparticle film for sensitive nontargeted analysis of food additives with surface-enhanced Raman spectroscopy. Analyst 2018, 143, 2363–2368. [Google Scholar] [CrossRef]
- Chen, S.; Guo, P.; Wan, J.C.; Luo, P.J.; Wu, R.M.; Wang, W.J. Rapid Detecting Study of Sodium Saccharin Additive in Spirit with SERS. Spectrosc. Spectr. Anal. 2017, 37, 1412–1417. [Google Scholar] [CrossRef]
- Velez-Escamilla, L.Y.; Contreras-Torres, F.F. Latest Advances and Developments to Detection of Micro- and Nanoplastics Using Surface-Enhanced Raman Spectroscopy. Part. Part. Syst. Charact. 2022, 39, 2100217. [Google Scholar] [CrossRef]
- Song, L.C.; Li, J.L.; Li, H.; Chang, Y.W.; Dai, S.J.; Xu, R.M.; Dou, M.H.; Li, Q.J.; Lv, G.P.; Zheng, T.S. Highly sensitive SERS detection for Aflatoxin B1 and Ochratoxin A based on aptamer-functionalized photonic crystal microsphere array. Sens. Actuators B Chem. 2022, 364, 131778. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Fang, S.Y.; Liu, Y.P.; Long, F.; Zhu, A.N. SERS based aptasensor for ochratoxin A by combining Fe3O4@Au magnetic nanoparticles and Au-DTNB@Ag nanoprobes with multiple signal enhancement. Microchim. Acta 2018, 185, 491. [Google Scholar] [CrossRef]
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of Ethyl Carbamate in Foods and Beverages: Review of the Formation Mechanisms, Advances in Analytical Methods, and Mitigation Strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef]
- Ajtony, Z.; Szoboszlai, N.; Bencs, L.; Viszket, E.; Mihucz, V.G. Determination of ethyl carbamate in wine by high performance liquid chromatography. Food Chem. 2013, 141, 1301–1305. [Google Scholar] [CrossRef]
- Alberts, P.; Stander, M.A.; De Villiers, A. Development of a novel solid-phase extraction, LC-MS/MS method for the analysis of ethyl carbamate in alcoholic beverages: Application to South African wine and spirits. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 826–839. [Google Scholar] [CrossRef]
- Yang, D.T.; Zhou, H.B.; Ying, Y.B.; Niessner, R.; Haisch, C. Surface-enhanced Raman scattering for quantitative detection of ethyl carbamate in alcoholic beverages. Anal. Bioanal. Chem. 2013, 405, 9419–9425. [Google Scholar] [CrossRef]
- Qi, H.Y.; Chen, H.C.; Wang, Y.; Jiang, L. Detection of ethyl carbamate in liquors using surface-enhanced Raman spectroscopy. R. Soc. Open Sci. 2018, 5, 181539. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.Z.; Xu, E.B.; Li, J.P.; Long, J.; Jiao, A.Q.; Jin, Z.Y. Highly sensitive determination of ethyl carbamate in alcoholic beverages by surface-enhanced Raman spectroscopy combined with a molecular imprinting polymer. RSC Adv. 2016, 6, 109442–109452. [Google Scholar] [CrossRef]
- Rostami, S.; Zor, K.; Zhai, D.S.; Viehrig, M.; Morelli, L.; Mehdinia, A.; Smedsgaard, J.; Rindzevicius, T.; Boisen, A. High-throughput label-free detection of Ochratoxin A in wine using supported liquid membrane extraction and Ag-capped silicon nanopillar SERS substrates. Food Control 2020, 113, 107183. [Google Scholar] [CrossRef]
- Huo, N.; Li, D.; Zheng, S.; Deng, W. MOF-based hybrid film for multiphase detection of sulfur dioxide with colorimetric and surface-enhanced Raman scattering readout. Chem. Eng. J. 2022, 432, 134317. [Google Scholar] [CrossRef]
- Agnolucci, M.; Tirelli, A.; Cocolin, L.; Toffanin, A. Brettanomyces bruxellensis yeasts: Impact on wine and winemaking. World J. Microbiol. Biotechnol. 2017, 33, 1217–1225. [Google Scholar] [CrossRef]
- Witkowska, E.; Jagielski, T.; Kamińska, A. Genus- and species-level identification of dermatophyte fungi by surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Wang, J.; Li, H.; Jiao, T.; Chen, Q. Trends in the bacterial recognition patterns used in surface enhanced Raman spectroscopy. TrAC Trends Anal. Chem. 2021, 142, 116310. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Poggesi, S.; Zhou, L.; Bariani, G.C.; Mittapalli, R.; Adam, P.M.; Manzano, M. Surface enhanced Raman spectroscopy phylogenetic tree for genosensing of Brettanomyces bruxellensis yeast on nanostructured ultrafine glass supports. Optik 2020, 203, 163956. [Google Scholar] [CrossRef]
- Rodriguez, S.B.; Thornton, M.A.; Thornton, R.J. Raman Spectroscopy and Chemometrics for Identification and Strain Discrimination of the Wine Spoilage Yeasts Saccharomyces cerevisiae, Zygosaccharomyces bailii, and Brettanomyces bruxellensis. Appl. Environ. Microbiol. 2013, 79, 6264–6270. [Google Scholar] [CrossRef] [Green Version]






| Alcoholic Beverages | Composition | Content (%) | Impact |
|---|---|---|---|
| Main ingredients | Water and alcohol | 85–95 | An essential ingredient in alcoholic beverages |
| Basic ingredients | Higher alcohols, lipids, aldehydes and ketones, furans, aromatic compounds, pyrazines, acids, etc. | 3–9 | Various taste |
| Active ingredients | Pyrazines, terpenes, polyphenols, flavonoids, amino acids, etc. | 1–3 | (1) Characteristics of different liquor components; (2) Endow alcoholic drinks with healthy elements; (3) Beneficial to human health, etc. |
| Toxic and harmful ingredient | Food additives, adulterants, methanol, formaldehyde, cyanide, metal ions, pesticide residues, mycotoxins, etc. | 0.5–1.5 | (1) Increase the shelf life; (2) Improve the taste or color; (3) Affect the quality of wine, etc. |
| Microorganism | Saccharomyces cerevisiae, beneficial microorganisms, etc. | 1–1.5 | (1) Fermented alcoholic beverages are essential; (2) The source of aroma and taste substances; (3) Affect the quality of wine, etc. |
| Types of Alcoholic Beverages | SERS and Related Parameters Adjustment | Conclusion |
|---|---|---|
| white wine [145] | Unlabeled SERS spectra were combined with multivariate data analysis (principal component analysis SIMCA prediction model), with colloidal dispersions of Ag nanoparticles as substrates Instrument and parameters: Portable I-Raman Plus 785S Spectrometer (equipped with a 785 nm emitting 300 mW diode laser and a spectrometer with a high quantum efficiency CCD array (cooled at 2 °C)) with laser power of 320 mW. The total collection time was 10 s (5 s for two accumulative times) | (1) Accurate prediction of which wine producer it was, with a sensitivity of 91% and a specificity of 95% (2) Excellent efficiency (87.93%) in distinguishing specific wines or specific producers (3) The main spectral differences were caused by adenine, carboxylic acid, and glutathione |
| white wine [59] | Silver Nanostars (AgNSs), SERS combined with data analysis software (SPSS program, stepwise linear discriminant analysis (SLDA)) Instrument and parameter: compact DeltaNU532 Raman system equipped with a frequency doubled Nd: YAG laser operating at 532 nm line (output power 100 mW). Five seconds of five accumulations have been set for each spectral acquisition in the 200–3350 cm−1 spectral range. | (1) Successfully distinguished three different white wines (2) The method is proved to be simple, fast, and cheap |
| wine [61] | FT-Raman and chemometrics, Silver Nanoparticles (AgNPs) Instrument and parameter: compact DeltaNU532 Raman system equipped with a frequency doubled Nd: YAG laser operating at 532 nm line (output power 100 mW). Five seconds of five accumulations have been set for each spectral acquisition in the 200–3350 cm−1 spectral range. | (1) Some wines have a geographical differentiation rate of more than 90%, while Romania’s 372 varieties have a geographical differentiation rate of 83.3%. |
| wine [147] | Silver Nanoparticles (AgNPs) and FT-Raman spectra (Bruker Equinox 55 FT-IR spectrometer with an integrated FRA 106S Raman module) Instrument and parameter: Detection was achieved with a liquid nitrogen-cooled Ge detector. Spectral resolution was 4 cm−1. Laser power was set to 350 mW and 500 scans were co-added. | (1) Ag NPs had a strong interaction with wine components, and showed induced aggregation. The main SERS signal characteristics for anthocyanins were under 532 nm excitation. |
| Red wine [148] | Method: direct analysis of red wine by Raman spectroscopy; mixed with silver nanoparticles, known as AgNPs; a reproducible SERS substrate, AgNPs mirror; SERS was combined with solvent extraction | (1) AgNPs images can reduce fluorescence (2) An innovative method based on extraction successfully suppressed the dominance of adenine in SERS spectroscopy |
| Instrument and parameter: ChemLogix EZRaman-I Series High Performance Portable Raman Analyzer with a 785 nm laser source, under following conditions: 170 mW laser power, 5 times integration, and 2 s exposure time. | (3) These chemicals form a barcode that could potentially be used to determine the classification and authenticity of a wine |
| Composition | SERS Platform and Related Parameters | Limit of Detection (LOD) | Conclusion |
|---|---|---|---|
| Sulfur dioxide (wine) | (1) Active substrate: silver nanoparticle (AuNPs) Instrument and parameter: Thin-film microextraction (TFME) + SERS (2) Active substrate: The preferred combination of silver nanoparticles (AgNPs) with sulfur-containing substances (ratio 1:1) Instrument and parameter: Thermo Fisher Scientific DXRxi Raman Spectrometer (Waltham, USA), with a 532 nm excitation laser source and a charge coupled device (CCD) detector Spectra were collected with a 10-mW laser power and a 50 μm rectangular aperture in the spectral range of 50 cm−1 to 3400 cm−1. Each spectrum was collected for 1 s and exposed 50 times. Repeat each measurement at least three times | (1) SERS signal intensity at 600 cm−1 had a good linear relationship with SO2 concentration in the range of 1–200 ug/mL, and the linear correlation coefficient was 99.2%. The detection limit of SO2 was 0.1 ug/mL [119]. | Both studies showed that SERS could be a simple, rapid, and selective method for the determination of SO2 content in wine. In the first study, the detection limit of SO2 was reduced by combining the pretreatment method. It shows that SERS technology combined with the pretreatment method is more advantageous. |
| (2) Regulation (EU) sets the legal limit for the amount of SO2 in red wine at 150 mg/L and white wine at 200 mg/L.& The detection limit (LOD) was 0.6 mg/L to 9.6 mg/L [152]. | |||
| Sulfur dioxid(SO2), & sulfite [154] (wine) | Active substrate: combined with surface-enhanced Raman Spectroscopy (SERS), AuNPs dispersed on the substrate of sea urchin-like ZnO nanowire. Instrument and parameter: Gas-diffusion microfluidic paper-based analytical device (PAD) + SERS. A portable Raman instrument (I-Raman, B&W Tek Inc., USA) with a microscope (20 objective) is used for acquisition. The laser excitation wavelength is 785 nm. | The SERS signal displacement was 620 cm−1, and the SO2 concentration showed a good linear relationship in the range of 5–300 μg mL−1. The linear correlation coefficient was 0.995 and the detection limit for sulfite was found to be 2 μg mL−1. | (1) This method is endowed with portability, minimal reagent consumption, and operational simplicity; (2) This method has good selectivity in sulfite analysis of wine samples. |
| Sulfite [155] (liquor) | Method: A novel paper-based analysis device for gas diffusion microfluidics (PAD) combined with surface-enhanced Raman spectroscopy (SERS) Active substrate: Sea urchin-like nano ZnO-paper Instrument and parameter: Portable Raman instrument with microscope (20 objective) (I-Raman, B&W Tek Inc., USA). The laser excitation wavelength is 785 nm. | The SERS signal displacement was 620 cm−1, and the SO2 concentration showed a good linear relationship in the range of 5–300 g mL−1. The linear correlation coefficient was 0.995, and the detection limit was 2 g mL−1. | (1) This method would permit a fast, disposable, and economical routine on-site monitoring of sulfite. |
| Sildenafil [161] (cocktail) | Active substrate: Opto Trace Raman 202 (OTR 202) colloidal activity Instrument and parameter: (1) RmTracer-200-HS portable Raman spectrometer combined with a 785 nm excitation wavelength diode-stabilized stimulator (Opto Trace Technologies, Inc., Mountain View, CA, USA); (2) FEI Tecnai G2 F20 S-TWIN transmission electron microscope. (3) Density functional theory (DFT). A 785 nm excitation wavelength, a power of 200 mw, a scanning range of 200–3300 cm−1, an optical resolution of 2 cm−1, an integration time of 10 s, and an average spectral value of three times. | The detection limit (LOD) was as low as 0.1 mg/L | (1) SERS technology can quickly and quantitatively determine SD in cocktails; (2) It is conducive to providing a fast and accurate scheme for the detection of SD in alcoholic drinks. |
| Sildenafil [158] (white wine, wine, health wine, etc.) | Active substrate: Opto Trace Raman 202 (OTR 202) active colloids Instrument and parameter: (1) RmTracer-200-HS portable Raman spectrometer combined with a 785 nm excitation wavelength diode-stabilized stimulator (Opto Trace Technologies, Inc., Mountain View, CA, USA); (2) FEI Tecnai G2 F20 S-TWIN transmission electron microscope. a 785 nm excitation wavelength, a power of 200 mw, a scanning range of 200–3300 cm−1, an optical resolution of 2 cm−1, an integration time of 10 s, and an average spectral value of three times. | There was a good linear relationship between the intensity of Raman peak and the concentration of sildenafil in health wine and liquor. The Raman enhancement factor (EF) of OTR 202 colloids reached 1.84 × 107 and the limits of detection (LODs) of sildenafil in health wine and liquor were found to be as low as 0.1 mg/L. | (1) The Raman EF of OTR 202 colloids could reach 1.84 × 107 (2) The proposed method showed good performance |
| Flibanserin [115] (white wine, beer, and wine) | Active substrate: AgNPs Instrument and parameter: SERS technology was combined with a machine learning algorithm (PCA, SVM). Portable Raman spectrometer BWS415-785H (B&W Tek, Inc.) with a wavelength of 785 nm. The spectrometer provided a 175–2000 cm−1 range of spectral measurement and the spectral resolution of the spectrometer was better than 3 cm−1. The output power was fixed at 150 mW and the integration time was 20 s. | The limit of detection of 1 g/mL for Flibanserin in liquor | (1) The results showed that this method can quickly and accurately detect Flibanserin in different wine solutions |
| Ethyl carbamate [173] (vodka, obstler, and white rum) | Active substrate: Individual silver-coated gold nanoparticle colloids (Au@Ag NPs) Instrument and parameter: Raman microscope (LabRAM HR, HORIBA Jobin Yvon, Germany), using a × 10 objectives. The 633-nm line of HE-NE laser was used as the excitation source. The laser power at the sample site was set to 14 mW. The spectral range is from 200 to 2000 cm−1 and the recorded resolution is 1 cm−1. | Control Board of Ontario (Canada) established the upper limits for EC in alcoholic beverages as ranging between 30 and 400 μg/L. The detection limits were 9.0 × 10−9 M (0.8 μg·L−1), 1.3 × 10−7 M (11.6 μg·L−1) and 7.8 × 10−8 M (6.9 μg·L−1), respectively, and the SNR was 3. | (1) The characteristic band at 1003 cm−1 was the strongest peak with the best reproducibility in SERS spectrum, which could be used for quantitative evaluation of ethyl carbamate |
| Sulfur dioxide (SO2) [174] (wine) | Active substrate: Porous NU-901 wrapping on thiol-magenta modified Ag nanoparticles (TM-Ag@NU-901) Instrument and parameter: Portable Raman spectrometers. | The detection limit is 1 μM, far lower than the acceptable limit of SO2 in wine (2.5 mm) stipulated by the European Union. | (1) It has the advantages of visual visualization, specificity, sensitivity, low cost, and time |
| Ochratoxin A(OTA) (wine) | Active substrate: AgNPs Instrument and parameter: Solvent-mediated liquid-liquid extraction (LLE) + Thermal science DXRxi Raman spectroscopic microscopy 20× objective, 780 nm excitation wavelength, 5 mW laser power, 50 μm slit aperture and 0.01 s collection time for an area of 2.8 mm × 2.8 mm. Active substrate: Silver (Ag)-capped silicon nanopillars substrate Method: SERS + high throughput supported liquid membrane (SLM) extraction. Instrument and parameter: DXRxi Raman Imaging Microscope, 10× objective lens, 50 μm slit, and an estimated laser spot of 3.6 μm diameter. All the spectra were collected three times for 0.05 s in each spot. | There is a good linear correlation between Raman intensity and OTA concentration; the correlation coefficient R = 0.9938, which is within the range of 0.01–1 ppm [63]. | Both methods can detect Ochratoxin A in wine quickly and without damage. The detection limit of the first method was lower, up to 0.01 PPM. |
| The detection limit is 115 PPB [176]. | |||
| Ethyl carbamate [175] (wine, yellow wine and fruit brandy) | Active substrate: Silver dendrite nanostructure Instrument and parameter: Molecularly imprinted polymers and SERS (MIPs SERS) (with an air-cooled He–Ne laser for 785 nm excitation, a motorized microscope, and a CCD array detector with 1024 × 256 pixels was used to record the SERS spectra). | ~ | (1) The MIPs-SPE can successfully separate EC and other components from wine samples. (2) SERS spectroscopic collection can be completed within 10 s, greatly shortening the detection time. |
| Histamine [62] (wine) | Active substrate: Silver nanoparticles (AgNPs)-cellulose hybrid substrate Instrument and parameter: Thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and Raman spectroscopy (Raman). | The high sensitivity of the promoted SERS phenomenon allows detecting histamine 10−12 M concentrate | The cellulose device is sensitive to histamine detection |
| Butyl Benzyl Phthalate [163] (liquor, wine) | Active substrate: AuNPs (AuNPs@β-CD) Instrument and parameter: The LAB-RAM HR800 spectrometer is equipped with a 633 nm laser excitation source and a laser power of 0.9 mW. | Detection limit as low as 14.9 nM | (1) A novel and effective solvent discoloration and SERS sensor system has opened up a new way. |
| Phthalate esters [162] (PAEs) (liquor) | Active substrate: Ag@Fe3O4@Ag/β-cyclodextrin (CD) nanoparticles Instrument and parameter: LabRAM HR800 confocal microscope and a 1-mW, 633-nm He-Ne laser (with a LWD 50/0.5 NA objective lens, and the laser spots had a diameter of about 2.0 μm; the acquisition time was 1 s for MG and 5 s for the other analytes.) | The detection limit was 1 ppb | (1) This substrate could detect that the level of BBP in liquor was reduced to 1.3 mg/kg, which was low enough to detect BBP in liquor samples |
| Formaldehyde(FA) and acetaldehyde(AA) [151] | Active substrate: Silver nanoparticle (AgNPs), RGO/[Ag(NH3)2] + (rGO/[Ag(NH3)2] + /Atp) paper Instrument and parameter: Portable Raman spectrometers. | The detection limits were 0.15 and 1.3 ng·L−1 | (1) The establishment of sample pretreatment method is a potential way to improve SERS detection of samples |
| Acid orange II and brilliant blue [157] (Red wine) | Active substrate: Fe3O4 @Au nanoshells Instrument and parameter: Portable RamTracer-200-HS Raman spectrometer (An excitation light source of 785 nm for an average scan time of 20 s, the number of integrals was two times, a scanning power of 300 mW, a sweep range of 100–3300 cm−1). | The detection limit of lime II was 1 μg/mL. The detection limit of brilliant blue was 0.5 μg/mL | (1) The method was verified by HPLC, and the results showed that the determination of pigments in wine was effective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Cao, X.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Zou, L.; Li, Q. Recent Developments in Surface-Enhanced Raman Spectroscopy and Its Application in Food Analysis: Alcoholic Beverages as an Example. Foods 2022, 11, 2165. https://doi.org/10.3390/foods11142165
Li L, Cao X, Zhang T, Wu Q, Xiang P, Shen C, Zou L, Li Q. Recent Developments in Surface-Enhanced Raman Spectroscopy and Its Application in Food Analysis: Alcoholic Beverages as an Example. Foods. 2022; 11(14):2165. https://doi.org/10.3390/foods11142165
Chicago/Turabian StyleLi, Lijiao, Xiaonian Cao, Ting Zhang, Qian Wu, Peng Xiang, Caihong Shen, Liang Zou, and Qiang Li. 2022. "Recent Developments in Surface-Enhanced Raman Spectroscopy and Its Application in Food Analysis: Alcoholic Beverages as an Example" Foods 11, no. 14: 2165. https://doi.org/10.3390/foods11142165
APA StyleLi, L., Cao, X., Zhang, T., Wu, Q., Xiang, P., Shen, C., Zou, L., & Li, Q. (2022). Recent Developments in Surface-Enhanced Raman Spectroscopy and Its Application in Food Analysis: Alcoholic Beverages as an Example. Foods, 11(14), 2165. https://doi.org/10.3390/foods11142165








