Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances
Abstract
:1. Introduction
2. Mechanisms of Anthocyanins Acylation
2.1. Biosynthetic Acylation of Anthocyanins
2.2. Semi-Biosynthetic Acylation of Anthocyanins
2.3. Chemical Acylation of Anthocyanins
2.4. Enzymatic Acylation of Anthocyanins
| Methods | Substrate | Operating Conditions | Result | Reference |
|---|---|---|---|---|
| Semi-biosynthesis | Carrot cell culture; acyl donors: cinnamic and benzoic acid analogues | Concentrations of acids in Me2SO added to cultures at days 4 and 8 at a rate of 0.01 v/v | 14 novel monoacylated anthocyanins | [52] |
| Growing Daucus carota (wild carrot) tissue cultures; acyl donors: selected carboxylic acids | 6-O-acyl-β-D-Glcp-(1 → 6)-β-D-Gal-(1 → O3)-cyanidin | [36] | ||
| Lines of Del/Ros1/At3AT tobacco cells | Del/Ros1/At3AT tobacco cell, aromatic group | Cyanidin 3-O-(6″-O-(coumaroyl)glucoside) | [53] | |
| Tobacco suspension cultures | Nutrient medium (LS supplemented with 1 mg L−1 2.4-D and 100 mg L−1 kanamycin) | Acylated cyanidin 3-O-(coumaroyl) rutinoside | [24] | |
| Chemical acylation | Malvidin-3-glucoside; acyl donors: stearoyl chloride | Anhydrous acetonitrile solution, room temperature, argon atmosphere overnight | Mono- and di-ester derivatives of malvidin-3-glucoside | [43] |
| Cyanidin-3-glucoside; acyl donors: lauric acid | DMF solution, 4 °C, argon atmosphere, 48 h | Acylatedcyanidin-3-glucoside | [40] | |
| Enzymatic acylation | Malvidin-3-glucoside; acyl donors: oleic acid and linoleic acid; enzymes: lipase acrylic resin from Candida antarctica lipase B (CALB) | Anhydrous 2-methyl-2-butanol solution, stirred at 60 °C, argon atmosphere, 48 h | Malvidin-3-glucoside–oleic acid conjugate | [9] |
| Cyanidin 3-glucoside; acyl donors: methyl benzoate, methyl salicylate; enzymes: Novozym 435 | In pyridine solution, stirred at 40 °C, in a vacuum of 900 mbar, 48 h | Cyanidin-3-(6″-benzoyl)-glucoside, cyanidin-3-(6″-salicy-loyl)-glucoside, and cyanidin-3-(6″-cinnamoyl)-glucoside | [12] | |
| Malvidin 3-glucoside; acyl donors: fatty acids (from C4 to C16); enzymes: CALB | In dry 2-methyl-2-butanol solution, stirred, 60 °C, over 24 h | Malvidin-3-glucoside with fatty acid conjugates of different chain lengths | [44] | |
| Delphinidin-3-O-glucoside, cyanidin-3-O-glucoside; acyl donors: octanoic acid; enzymes: CALB | In dry acetonitrile–DMSO 10:1 (v/v) solution, stirred, 60 °C, 9 h | Delphinidin-3-glucoside-6″-O-octanoate, cyanidin-3-glucoside-6″-O-octanoate | [45] | |
| Cyanidin-3-glucoside; acyl donors: fatty acids (from C4 to C12); enzymes: CALB | In 2-methyl-2-butanol solution, stirred, 60 °C | Cyanidin-3-glucoside-fatty acid derivatives | [46] | |
| Cyanidin-3-O-galactoside; acyl donors: saturated fatty acids of different chain lengths; enzymes: (Novozyme 435) | In tertbutanol solution, stirred, 60 °C, 72 h | Cyanidin-3-O-(6″-dodecanoyl)-galactoside | [54] | |
| Delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, acyl donors: lauric acid; enzymes: CALB | In tertbutanol solution, stirred, 60 °C, 72 h | Derivatives of Delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside | [11] |
3. Applications of Acylated Anthocyanins
3.1. Food Colourants
3.2. Functionalizing Agents
3.3. Indicators in Intelligent Packaging
4. Challenges and Future Trends
- (1)
- It is difficult to find suitable plants rich in acylated anthocyanins, and the direct isolation and purification of single acylated anthocyanins from a large variety of anthocyanins in plants is a relatively cumbersome and costly process. Therefore, low valueable food by-products could be used as good sources for the isolation of anthocyanins by countercurrent chromatography (HSCCC) in subsequent studies, which would solve the problem of obtaining raw materials as well as achieve multiple effective uses of resources. In addition, later studies could develop alternative techniques, such as gene editing, gene transfer, and heterologous expression techniques, to construct several acylated anthocyanin bio-factories to obtain highly abundant acylated anthocyanins.
- (2)
- In vitro synthesis of acylated anthocyanins faces challenges of low reaction yields; therefore, subsequent research on the semi-biosynthetic acylation of anthocyanins could focus on investigating more suitable types of plant cells and optimizing the composition of media in order to obtain high yields of acylated anthocyanins. In addition, for the synthesis of acylated anthocyanins using chemical acylation or enzymatic acylation, research into the best reaction conditions, e.g., by optimizing the solvents used in reaction systems, and ways of increasing enzyme activity and developing the hybrid synthesis of acylated anthocyanins is also important.
- (3)
- Acylated anthocyanins usually face the problem of insufficient colour variety when they are used as food colourants. In order to tackle this challenge, on the one hand, innovative food colourants with a wide pH range, which contain acylated anthocyanins and other types of natural dyes, could be developed. When using acylated anthocyanins as food colourants, adjusting the pH values of food products could also be a useful way of enriching the colour of food colourants. On the other hand, natural anthocyanin pigments/dyes and phenolic co-pigments/co-dyes could be used to form non-covalent complexes to enrich the variety of food colourants. Since these non-covalent interactions allow dyes to interact with each other to produce co-pigmentation, the colours in flowers, berries, and foods made from them (including wines, beverages, jams, purees, and syrups) could be stabilized and modulated.
- (4)
- Acylated anthocyanins have a variety of biological properties, such as anti-cancer, anti-inflammatory, anti-aging, anti-cardiovascular, and antioxidant properties, but the current studies on the biological activities of acylated anthocyanins are mostly at the cellular or preliminary mouse level. There are still not enough suitable primate models to validate the unique biological efficacies of acylated anthocyanins. Therefore, more animal models could be constructed, as well as relevant human clinical trials, to validate the biological activities of acylated anthocyanins in subsequent studies. In addition, safety evaluation models and methods for acylated anthocyanins need to be further refined.
- (5)
- Finally, when using acylated anthocyanins as indicators, a slight change in colour may not be observed by the naked eye, and thus their application in intelligent food packaging is challenging. More characterization methods could be introduced to reflect slight changes in colour. For example, traditional physical characteristics, such as CIELAB colour difference calculation, could be combined with molecular spectroscopy techniques, such as visible spectroscopy and near-infrared spectroscopy, to characterize changes in colour. New algorithms could also be developed to establish reliable correlation models to increase sensing accuracy.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 867–901. [Google Scholar]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. 3-Deoxyanthocyanidin Colorant: Nature, Health, Synthesis, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1533–1549. [Google Scholar] [CrossRef] [Green Version]
- Fenger, J.A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Matsufuji, H.; Kido, H.; Misawa, H.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to Light, Heat, and Hydrogen Peroxide at Different pH Values and DPPH Radical Scavenging Activity of Acylated Anthocyanins from Red Radish Extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.J.; Liu, J.H.; Zhao, S.J.; Cai, J.X.; Jing, P. The effects of gallic/ferulic/caffeic acids on colour intensification and anthocyanin stability. Food Chem. 2017, 228, 526–532. [Google Scholar] [CrossRef]
- Tachibana, N.; Kimura, Y.; Ohno, T. Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chem. 2014, 143, 452–458. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, I.; Guimaraes, M.; de Freitas, V.; Mateus, N. Enzymatic synthesis, structural characterization and antioxidant capacity assessment of a new lipophilic malvidin-3-glucoside-oleic acid conjugate. Food Funct. 2016, 7, 2754–2762. [Google Scholar] [CrossRef]
- Luo, C.L.; Zhou, Q.; Yang, Z.W.; Wang, R.D.; Zhang, J.L. Evaluation of structure and bioprotective activity of key high molecular weight acylated anthocyanin compounds isolated from the purple sweet potato (Ipomoea batatas L. cultivar Eshu No.8). Food Chem. 2018, 241, 23–31. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef]
- Yan, Z.; Li, C.; Zhang, L.; Liu, Q.; Ou, S.; Zeng, X. Enzymatic Acylation of Anthocyanin Isolated from Black Rice with Methyl Aromatic Acid Ester as Donor: Stability of the Acylated Derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.-d.; Zhang, Y.; Zheng, Z.-d.; Qu, Z.-y.; Liu, M.; Zhu, S.-h.; Liu, S.; Wang, M.; Qu, L. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-G.; Yan, Q.-Q.; Xue, R.-Y.; Zhang, J.; Zhang, Y.-Q. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD-ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-r.; Lu, J.-l.; Wu, Y.-w.; Ouyang, J.; Sun, S.-q. Study on Esterified Modification of Anthocyanins by FTIR. Spectrosc. Spect. Anal. 2010, 30, 38–41. [Google Scholar] [CrossRef]
- Kirby, C.W.; Wu, T.; Tsao, R.; McCallum, J.L. Isolation and structural characterization of unusual pyranoanthocyanins and related anthocyanins from Staghorn sumac (Rhus typhina L.) via UPLC–ESI-MS, 1H, 13C, and 2D NMR spectroscopy. Phytochemistry 2013, 94, 284–293. [Google Scholar] [CrossRef]
- Terahara, N.; Matsui, T.; Minoda, K.; Nasu, K.; Kikuchi, R.; Fukui, K.; Ono, H.; Matsumoto, K. Functional New Acylated Sophoroses and Deglucosylated Anthocyanins in a Fermented Red Vinegar. J. Agric. Food Chem. 2009, 57, 8331–8338. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, O.D.; Edwards, R. Modifying the acylation of flavonols in Petunia hybrida. Phytochemistry 2008, 69, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Wulff-Vester, A.K.; Wendell, M.; Hvoslef-Eide, A.K.; Russell, J.; Oertel, A.; Martens, S.; Mock, H.P.; Martin, C.; Matros, A. Colour bio-factories: Towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 2018, 48, 218–232. [Google Scholar] [CrossRef]
- Shi, M.-Z.; Xie, D.-Y. Biosynthesis and Metabolic Engineering of Anthocyanins in Arabidopsis thaliana. Recent Pat. Biotechnol. 2014, 5, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, N.; Nishizaki, Y.; Ozeki, Y.; Miyahara, T. The role of acyl-glucose in anthocyanin modifications. Molecules 2014, 19, 18747–18766. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant. Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Luo, J.; Nishiyama, Y.; Fuell, C.; Taguchi, G.; Elliott, K.; Hill, L.; Tanaka, Y.; Kitayama, M.; Yamazaki, M.; Bailey, P.; et al. Convergent evolution in the BAHD family of acyl transferases: Identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant. J. 2007, 50, 678–695. [Google Scholar] [CrossRef]
- Miyahara, T.; Sakiyama, R.; Ozeki, Y.; Sasaki, N. Acyl-glucose-dependent glucosyltransferase catalyzes the final step of anthocyanin formation in Arabidopsis. J. Plant. Physiol. 2013, 170, 619–624. [Google Scholar] [CrossRef]
- Xu, Z.S.; Ma, J.; Wang, F.; Ma, H.Y.; Wang, Q.X.; Xiong, A.S. Identification and characterization of DcUCGalT1, a galactosyltransferase responsible for anthocyanin galactosylation in purple carrot (Daucus carota L.) taproots. Sci. Rep. 2016, 6, 27356. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.S.; Yang, Q.Q.; Feng, K.; Xiong, A.S. Changing Carrot Color: Insertions in DcMYB7 Alter the Regulation of Anthocyanin Biosynthesis and Modification. Plant. Physiol. 2019, 181, 195–207. [Google Scholar] [CrossRef]
- Paulsmeyer, M.N.; Brown, P.J.; Juvik, J.A. Discovery of Anthocyanin Acyltransferase1 (AAT1) in Maize Using Genotyping-by-Sequencing (GBS). G3 (Bethesda) 2018, 8, 3669–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, H.; Liu, C. Transcriptome analysis of genes involved in anthocyanins biosynthesis and transport in berries of black and white spine grapes (Vitis davidii). Hereditas 2016, 153, 17. [Google Scholar] [CrossRef] [Green Version]
- Rinaldo, A.R.; Cavallini, E.; Jia, Y.; Moss, S.M.; McDavid, D.A.; Hooper, L.C.; Robinson, S.P.; Tornielli, G.B.; Zenoni, S.; Ford, C.M.; et al. A Grapevine Anthocyanin Acyltransferase, Transcriptionally Regulated by VvMYBA, Can Produce Most Acylated Anthocyanins Present in Grape Skins. Plant. Physiol. 2015, 169, 1897–1916. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.R.; Parvatam, G.; Shetty, N.P. Medium composition potentially regulates the anthocyanin production from suspension culture of Daucus carota. 3 Biotech. 2018, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, N.A.; Welch, K.T.; Cox, J.R.; Dougall, D.K.; Baker, D.C. A quenched molecular dynamics-rotating frame overhauser spectroscopy study of a series of semibiosynthetically monoacylated anthocyanins. J. Org. Chem. 2004, 69, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Andi, S.A.; Gholami, M.; Ford, C.M.; Maskani, F. The effect of light, phenylalanine and methyl jasmonate, alone or in combination, on growth and secondary metabolism in cell suspension cultures of Vitis vinifera. J. Photochem. Photobiol. B 2019, 199, 111625. [Google Scholar] [CrossRef]
- Yoshida, K.; Okuno, R.; Kameda, K.; Kondo, T. Prevention of UV-light induced E,Z-isomerization of caffeoyl residues in the diacylated anthocyanin, gentiodelphin, by intramolecular stacking. Tetrahedron. Lett. 2002, 43, 6181–6184. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcia, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.-y.; Chen, J.; Wang, Z.-q.; Shen, R.-m.; Cui, N.; Sun, A.-d. Direct Acylation of Cyanidin-3-Glucoside with Lauric Acid in Blueberry and Its Stability Analysis. Int. J. Food Prop. 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakajima, N. Structural aspects of acylated plant pigments: Stabilization of flavonoid glucosides and interpretation of their functions. J. Mol. Catal. B Enzym. 2003, 23, 411–417. [Google Scholar] [CrossRef]
- Tommasini, S.; Raneri, D.; Ficarra, R.; Calabrò, M.L.; Stancanelli, R.; Ficarra, P. Improvement in solubility and dissolution rate of flavonoids by complexation with β-cyclodextrin. J. Pharmaceut. Biomed. 2004, 35, 379–387. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, V.C.; Araujo, P.; Mateus, N.; de Freitas, V. Synthesis, characterisation and antioxidant features of procyanidin B4 and malvidin-3-glucoside stearic acid derivatives. Food Chem. 2015, 174, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Guimaraes, M.; Araujo, P.; Evora, A.; de Freitas, V.; Mateus, N. Malvidin 3-Glucoside-Fatty Acid Conjugates: From Hydrophilic toward Novel Lipophilic Derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Benohoud, M.; Rayner, C.M.; Mateus, N.; de Freitas, V.; Blackburn, R.S. Selective enzymatic lipophilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L.) skin extract and characterization of esterified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Barea, B.; Figueroa-Espinoza, M.C. Lipophilization and MS characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.-M.; Ghoul, M. Enzymatic acylation of flavonoids. Process. Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Wibisono, R.; Jensen, D.J.; Stanley, R.A.; Cooney, J.M. Direct acylation of flavonoid glycosides with phenolic acids catalysed by Candida antarctica lipase B (Novozym 435®). Enzym. Microb. Technol. 2006, 39, 1236–1241. [Google Scholar] [CrossRef]
- De Castro, V.C.; da Silva, P.H.A.; de Oliveira, E.B.; Desobry, S.; Humeau, C. Extraction, identification and enzymatic synthesis of acylated derivatives of anthocyanins from jaboticaba (Myrciaria cauliflora) fruits. Int. J. Food Sci. Technol. 2014, 49, 196–204. [Google Scholar] [CrossRef]
- Salem, J.H.; Humeau, C.; Chevalot, I.; Harscoat-Schiavo, C.; Vanderesse, R.; Blanchard, F.; Fick, M. Effect of acyl donor chain length on isoquercitrin acylation and biological activities of corresponding esters. Process. Biochem. 2010, 45, 382–389. [Google Scholar] [CrossRef]
- Dougall, D.K.; Baker, D.C.; Gakh, E.G.; Redus, M.A.; Whittemore, N.A. Studies on the stability and conformation of monoacylated anthocyanins part 2—Anthocyanins from wild carrot suspension cultures acylated with supplied carboxylic acids. Carbohyd. Res. 1998, 310, 177–189. [Google Scholar] [CrossRef]
- Kallam, K.; Appelhagen, I.; Luo, J.; Albert, N.; Zhang, H.; Deroles, S.; Hill, L.; Findlay, K.; Andersen, O.M.; Davies, K.; et al. Aromatic Decoration Determines the Formation of Anthocyanic Vacuolar Inclusions. Curr. Biol. 2017, 27, 945–957. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Afzal, M.; Redha, A.; AlHasan, R. Anthocyanins Potentially Contribute to Defense against Alzheimer’s Disease. Molecules 2019, 24, 4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Zhou, Q.; Yang, Y.; Wang, Y.; Zhang, J.L. Highly Acylated Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) Alleviate Hyperuricemia and Kidney Inflammation in Hyperuricemic Mice: Possible Attenuation Effects on Allopurinol. J. Agric. Food Chem. 2019, 67, 6202–6211. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocolloid 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Terahara, N.; Callebaut, A.; Ohba, R.; Nagata, T.; Ohnishi-Kameyama, M.; Suzuki, M. Acylated anthocyanidin 3-sophoroside-5-glucosides from Ajuga reptans flowers and the corresponding cell cultures. Phytochemistry 2001, 58, 493–500. [Google Scholar] [CrossRef]
- Mane, C.; Chanforan, C.; Lemmonier, P.; Jouenne, E. Composition used as food colorant for e.g. beverages, is anthocyanin-based colorant composition comprising anthocyanins and pelargonidin-based anthocyanins, and has red color with specified hue value. Patent WO2013079518-A1, 6 June 2013. [Google Scholar]
- Diaz-Garcia, M.C.; Castellar, M.R.; Obon, J.M.; Obon, C.; Alcaraz, F.; Rivera, D. Production of an anthocyanin-rich food colourant from Thymus moroderi and its application in foods. J. Sci. Food Agric. 2015, 95, 1283–1293. [Google Scholar] [CrossRef]
- Giusti, M.M.; Tang, P. Uses of Acylated Anthocyanins Extracted from Black Goji (Lycium ruthenicum Murr.) as a Source of Natural Color. Patent WO2017205173-A1, 30 November 2017. [Google Scholar]
- Oliveira, H.; Basilio, N.; Pina, F.; Fernandes, I.; de Freitas, V.; Mateus, N. Purple-fleshed sweet potato acylated anthocyanins: Equilibrium network and photophysical properties. Food Chem. 2019, 288, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; He, W.; Lu, M.; Yuan, B.; Zeng, M.; Gao, D.; Qin, F.; Chen, J.; He, Z. Anthocyanin composition and storage degradation kinetics of anthocyanins-based natural food colourant from purple-fleshed sweet potato. Int. J. Food Sci. Technol. 2019, 54, 2529–2539. [Google Scholar] [CrossRef]
- Swer, T.L.; Chauhan, K.; Mukhim, C.; Bashir, K.; Kumar, A. Application of anthocyanins extracted from Sohiong (Prunus nepalensis L.) in food processing. LWT—Food Sci. Technol. 2019, 114, 108360. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Horikoshi, Y.; Fushimi, T.; Saito, A.; Koizumi, R.; Fujii, Y.; Hu, Q.Q.; Hirota, Y.; Aizawa, K.; Osakabe, N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct. 2019, 10, 1726–1735. [Google Scholar] [CrossRef]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative Study on the Chemical Structure and In Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-H.; Yoon, H.-S.; Park, H.-J.; Kim, M.-Y.; Shin, H.-K.; Park, K.-Y.; Yang, J.-O.; Sohn, M.-S.; Do, M.-S. Anti-Obesity and Antioxidative Effects of Purple Sweet Potato Extract in 3T3-L1 Adipocytes In Vitro. J. Med. Food 2011, 14, 1097–1106. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Quynhchi, P.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red Cabbage Microgreens Lower Circulating Low-Density Lipoprotein (LDL), Liver Cholesterol, and Inflammatory Cytokines in Mice Fed a High-Fat Diet. J. Agr. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT—Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R.; Soni, A. Anthocyanins Based Indicator Sensor for Intelligent Packaging Application. Agr. Res. 2016, 5, 205–209. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-z.; Zhang, M.; Bhandari, B.; Yang, C.-h. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocolloid 2020, 100, 105438. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Vijaeeswarri, J.; Anna, J.L. Effective natural dye extraction from different plant materials using ultrasound. Ind. Crop. Prod. 2011, 33, 116–122. [Google Scholar] [CrossRef]
- Song, B.J.; Sapper, T.N.; Burtch, C.E.; Brimmer, K.; Goldschmidt, M.; Ferruzzi, M.G. Photo- and thermodegradation of anthocyanins from grape and purple sweet potato in model beverage systems. J. Agric. Food Chem. 2013, 61, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Pina, F.; Basílio, N.; Guimarães, M.; de Freitas, V.; Cruz, L. Extending the stability of red and blue colors of malvidin-3-glucoside-lipophilic derivatives in the presence of SDS micelles. Dyes Pigment. 2018, 151, 321–326. [Google Scholar] [CrossRef]
- Venter, A.; Fisher, H.; Stafford, G.I.; Duodu, K.G. Pigmented flower extracts of plant species from the Geraniaceae and Lamiaceae families as natural food colourants: Anthocyanin composition, thermal and oxidative stability. Int. J. Food Sci. Technol. 2022, 57, 4347–4355. [Google Scholar] [CrossRef]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure−Function Relationships of Anthocyanins from Various Anthocyanin-Rich Extracts on the Inhibition of Colon Cancer Cell Growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems-An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.W.; Kawakishi, S.; Osawa, T. Antioxidative activity of the anthocyanin pigments cyanidin 3-O-beta-D-glucoside and cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Brauch, J.E.; Kroner, M.; Schweiggert, R.M.; Carle, R. Studies into the Stability of 3-O-Glycosylated and 3,5-O-Diglycosylated Anthocyanins in Differently Purified Liquid and Dried Maqui (Aristotelia chilensis (Mol.) Stuntz) Preparations during Storage and Thermal Treatment. J. Agric. Food Chem. 2015, 63, 8705–8714. [Google Scholar] [CrossRef]
- Hernandez-Herrero, J.A.; Frutos, M.J. Colour and antioxidant capacity stability in grape, strawberry and plum peel model juices at different pHs and temperatures. Food Chem. 2014, 154, 199–204. [Google Scholar] [CrossRef]
- Ercoli, S.; Cartes, J.; Cornejo, P.; Tereucán, G.; Winterhalter, P.; Contreras, B.; Ruiz, A. Stability of phenolic compounds, antioxidant activity and colour parameters of a coloured extract obtained from coloured-flesh potatoes. LWT 2021, 136, 110370. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Lee, P.-H.; Lei, C.-C.; Hsu, Y.-C.; Chang, H.-H.; Tung, C.-W.; Lin, C.-L.; Yang, H.-F.; Lu, L.-C.; Jong, M.-C.; et al. Hyperuricemia as an Independent Risk Factor of Chronic Kidney Disease in Middle-Aged and Elderly Population. Am. J. Med. Sci. 2010, 339, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Kydd, A.S.R.; Buchbinder, R.; Bombardier, C.; Edwards, C.J. Allopurinol for chronic gout. Cochrane Database Syst. Rev. 2014, CD006077. [Google Scholar] [CrossRef]
- Ramasamy, S.N.; Korb-Wells, C.S.; Kannangara, D.R.W.; Smith, M.W.H.; Wang, N.; Roberts, D.M.; Graham, G.G.; Williams, K.M.; Day, R.O. Allopurinol Hypersensitivity: A Systematic Review of All Published Cases, 1950–2012. Drug Saf. 2013, 36, 953–980. [Google Scholar] [CrossRef]
- Stamp, L.K.; Taylor, W.J.; Jones, P.B.; Dockerty, J.L.; Drake, J.; Frampton, C.; Dalbeth, N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: A proposed safe starting dose of allopurinol. Arthritis Rheum. 2012, 64, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Fama, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Eskandarabadi, S.M.; Mahmoudian, M.; Farah, K.R.; Abdali, A.; Nozad, E.; Enayati, M. Active intelligent packaging film based on ethylene vinyl acetate nanocomposite containing extracted anthocyanin, rosemary extract and ZnO/Fe-MMT nanoparticles. Food Packag. Shelf. 2019, 22, 100389. [Google Scholar] [CrossRef]
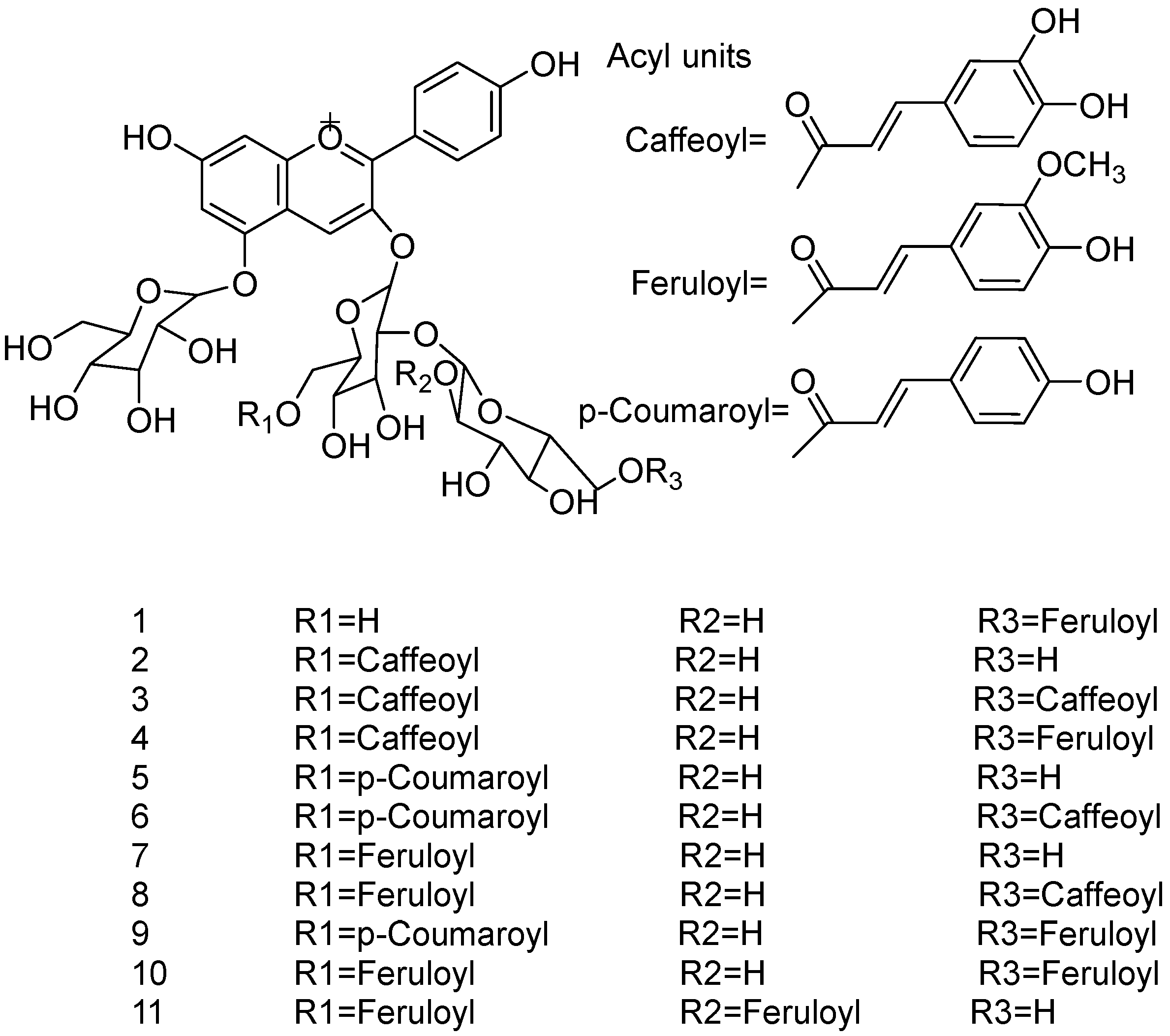
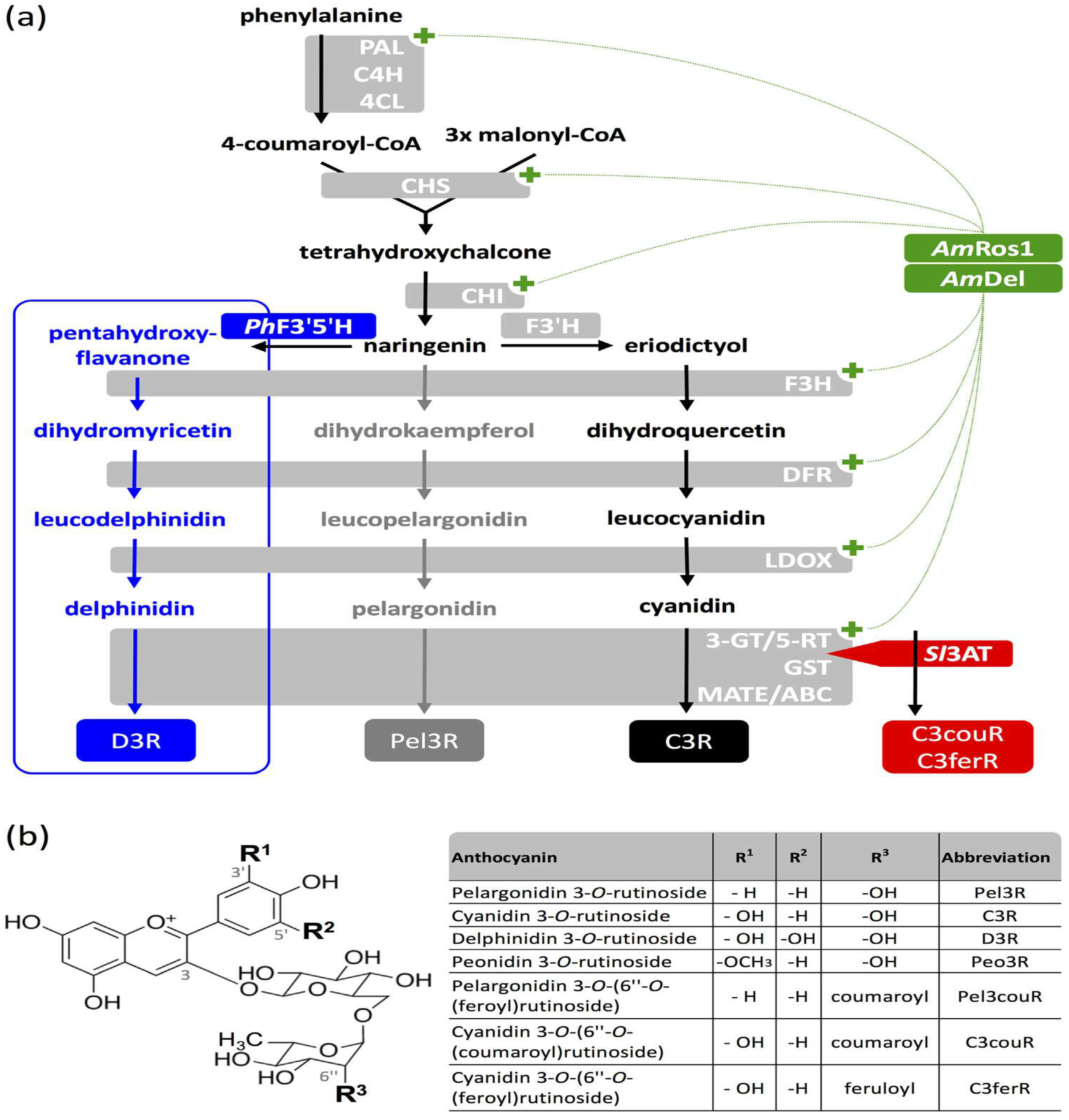
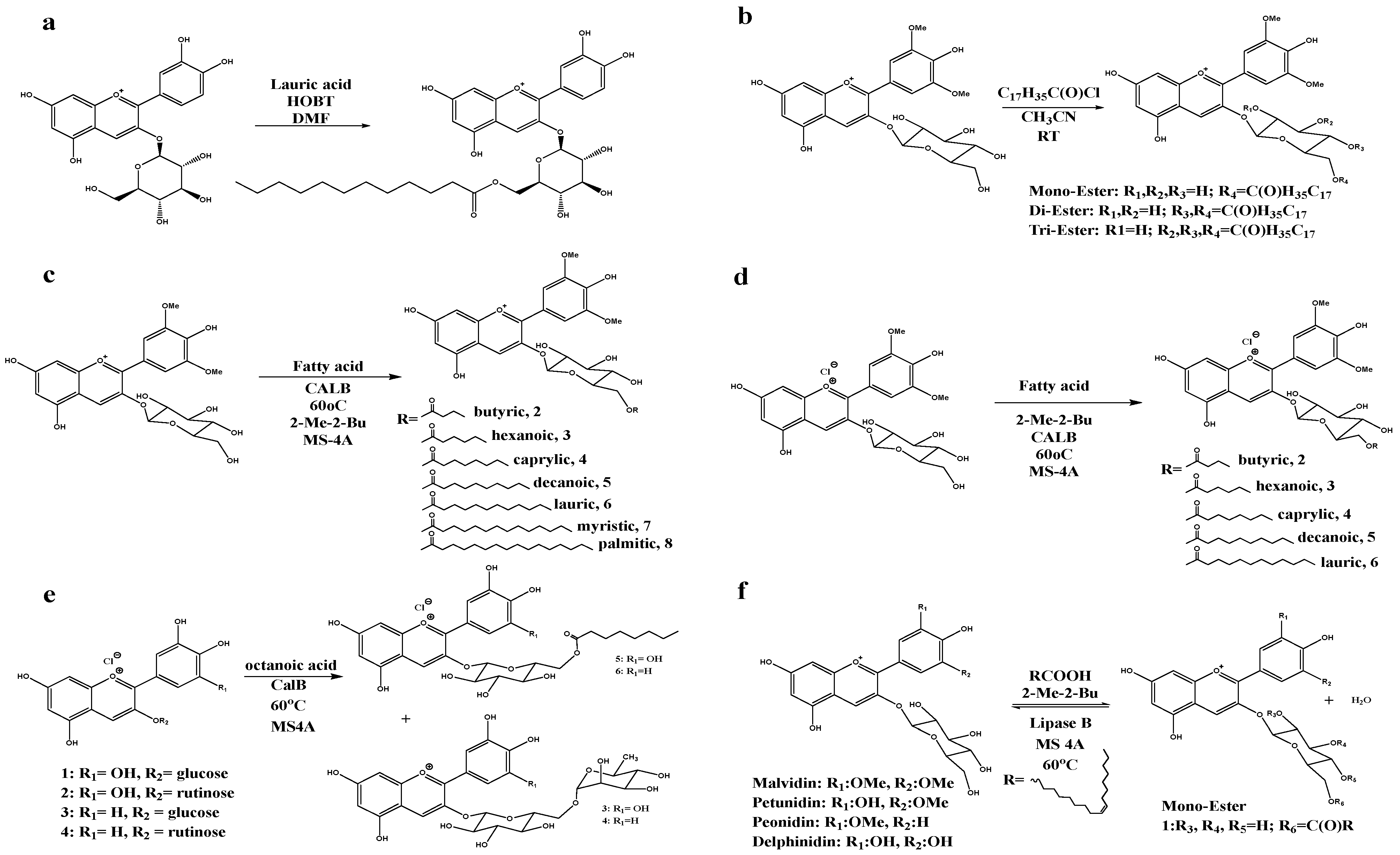


| Application Categories | Sources | Major Acylated Anthocyanins | Specific Functions or Application Fields | Reference |
|---|---|---|---|---|
| Food colourants | Ajuga reptans flowers and corresponding cell cultures | Delphinidin 3-(p-coumaroylferuloyl) sophoroside-5-malonylglucoside, delphinidin 3-(diferuloyl) sophoroside-5-malonylglucoside, and cyanidin 3-(di-p-coumaroyl) sophoroside-5-glucoside | Food colourants in the food industry | [60] |
| Anthocyanins and pelargonidin-based anthocyanins | Beverages, fruit preparations, dairy products, ice cream, and confectionary | [61] | ||
| Thymus moroderi and another five Thymus spp. | Cyanidin dimalonyldiglucoside, cyanidin 3-malonyl-glucoside, cyanidin 3-malonyl-acetylglucoside, peonidin dimalonyl-diglucoside, and pelargonidin 3-malonyl-acetyl-glucoside. | Food colourants in yoghurts | [62] | |
| Black goji berry (Lycium ruthenicum Murr.) | Acylated anthocyanins | Food colourants in food products | [63] | |
| Purple-fleshed sweet potato | Peonidin-3-(6′-hydroxybenzoyl)-sophoroside-5-glucoside, peonidin-3-(6′-hydroxybenzoyl-6″-caffeoyl)-sophoroside-5-glucoside. | A higher capability in retaining red and blue colours | [64] | |
| Purple-fleshed sweet potato | Peonidin 3-p-hydroxybenzoyl sophoroside-5-glucoside, peonidin 3-feruloyl sophoroside-5-glucoside, peonidin 3-caffeoyl sophoroside-5-glucoside, peonidin dicaffeoyl sophoroside-5-glucoside, peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside and peonidin caffeoyl-feruloyl sophoroside-5-glucoside. | Natural colourant in food industry | [65] | |
| Sohiong (Prunus nepalensis L.) | Anthocyanins | Yoghurt, syrup, and hard-boiled candy | [66] | |
| Functionalizing agents | Purple sweet potato (Ipomoea batatas L.) | Peonidin 3-(6′,6″-dicaffeoyl sophoroside)-5-glucoside, peonidin 3-(6′-caffeoyl-6″-p-hydroxybenzoyl sophoroside)-5-glucoside, peonidin 3-(6′-caffeoyl-6″-feruloyl sophoroside)-5glucoside | Alleviating hyperuricemia and kidney inflammation | [58] |
| Purple sweet potato (Ipomoea batatas L. cultivar Eshu No.8) | Cyanidin-3-caffeoyl-feruloyl sophoroside-5-glucoside and peonidin-3-dicaffeoyl sophoroside-5-glucoside | Bioprotective activity and antioxidant capacity | [10] | |
| Purple carrot (Daucus carota L.) | Cyanidin-3-(2″-xylose-6″-sinapoyl-glucose-galactoside), cyanidin-3-(2″-xylose-6″-feruloyl-glucose-galactoside), cyanidin-3-(2″-xylose-6″(4-coumaroyl) glucose-galactoside) | Anthocyanins might possess adrenomimetic properties and be applied in wound recovery | [67] | |
| Purple root tubers and leaves of sweet potato (Ipomoea batatas) | Peonidin derivatives and cyanidin derivatives | Anti-proliferative activity | [68] | |
| Blackcurrant (Ribes nigrum) | Delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside derivatives | Antioxidant capacity and applied in inhibiting lipid peroxidation | [11] | |
| Alpine bearberry (Arctostaphylos alpina) | Cyanidin-3-O-(6″-dodecanoyl) galactoside | Antioxidant capacity applied in lipophilic food, cosmetic, and pharmaceutical products | [54] | |
| Purple sweet potato | Cyanidnin-(3-caffeylferulysophoroside-5-glucoside), peonidin-(3-caffeylferulysophoroside-5-glucoside) | Anti-obesity and antioxidative effects | [69] | |
| Red cabbage microgreens | Cyanidin(3-(glucosyl) (sinapoyl), (p-coumaroyl) sophorside-5-glucoside), cyanidin-(3-(glucosyl) (sinapoyl) (feruloyl)sophorside-5-glucoside) | Anti-obesity effect | [70] | |
| Intelligent packaging | Grapes (Christian Hansen) | Anthocyanin extracted from grapes | Monitoring pH variations | [71] |
| Red cabbage (Brassica oleracea var capitata) | Anthocyanins from red cabbage (Brassica oleracea var capitata) | Time–temperature indicators to detect pasteurized milk | [59] | |
| The flowers of rose and red cabbage | Anthocyanins from the flowers of rose and red cabbage | pH indicators to detect the freshness of buffalo meat | [72] | |
| Purple sweet potato | Purple sweet potato anthocyanins | pH indicators for the quality of pork | [73] | |
| Black rice | Black rice bran anthocyanins | Intelligent film for seafood spoilage monitoring | [74] | |
| Black carrot | Black carrot anthocyanins | pH-sensing indicator for monitoring fish freshness | [75] | |
| Purple sweet potato | Purple sweet potato anthocyanins | pH-sensitive films to monitor fish freshness | [76] | |
| Purple sweet potato and red cabbage | Anthocyanins from purple sweet potato and red cabbage | pH-sensitive films for the detection of shrimp deterioration | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Chen, S.; Zeng, X.; Han, Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods 2022, 11, 2166. https://doi.org/10.3390/foods11142166
Luo X, Wang R, Wang J, Li Y, Luo H, Chen S, Zeng X, Han Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods. 2022; 11(14):2166. https://doi.org/10.3390/foods11142166
Chicago/Turabian StyleLuo, Xiu’er, Ruoyong Wang, Jinhua Wang, Ying Li, Huainan Luo, Shi Chen, Xin’an Zeng, and Zhong Han. 2022. "Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances" Foods 11, no. 14: 2166. https://doi.org/10.3390/foods11142166






