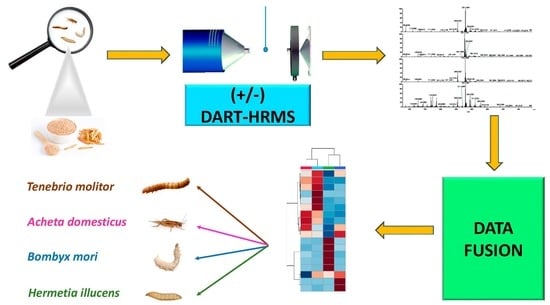
Authentication of Edible Insects’ Powders by the Combination of DART-HRMS Signatures: The First Application of Ambient Mass Spectrometry to Screening of Novel Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Preparation
2.3. DART-HRMS
2.4. Data Fusion and Statistical Analysis
2.5. Creation of the Classifier and Its Validation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; No. 171; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Hayes, B.J.; Lewin, H.A.; Goddard, M.E. The future of livestock breeding: Genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends Genet. 2013, 29, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Regulation (EU) 2015/2283; The European Parliament and The Council of 25 November 2015 on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32015R2283 (accessed on 18 July 2021).
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C. Safety of Dried Yellow Mealworm (Tenebrio molitor Larva) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- EU Implementing Regulation 2017/2470; Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 18 July 2021).
- FAO. Looking at Edible Insects from a Food Safety Perspective. Challenges and Opportunities for the Sector; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- EU Regulation 152/2009; Commission Regulation (EC) No 152/2009 of 27 January 2009 Laying down the Methods of Sampling and Analysis for the Official Control of Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R0152-20170524&qid=1549036706310&from=EN (accessed on 18 July 2021).
- Varunjikar, M.S.; Belghit, I.; Gjerde, J.; Palmblad, M.; Oveland, E.; Rasinger, J.D. Shotgun proteomics approaches for authentication, biological analyses, and allergen detection in feed and food-grade insect species. Food Control 2022, 137, 108888. [Google Scholar] [CrossRef]
- Gowda, S.; Sasaki, Y.; Hasegawa, E.; Chiba, H.; Hui, S. Lipid fingerprinting of yellow mealworm Tenebrio molitor by untargeted liquid chromatography-mass spectrometry. J. Insects Food Feed 2022, 8, 157–168. [Google Scholar] [CrossRef]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Mellado-Carretero, J.; García-Gutiérrez, N.; Ferrando, M.; Güell, C.; García-Gonzalo, D.; De Lamo-Castellví, S. Rapid discrimination and classification of edible insect powders using ATR-FTIR spectroscopy combined with multivariate analysis. J. Insects Food Feed 2020, 6, 141–148. [Google Scholar] [CrossRef]
- Ulrich, S.; Kühn, U.; Biermaier, B.; Piacenza, N.; Schwaiger, K.; Gottschalk, C.; Gareis, M. Direct identification of edible insects by MALDI-TOF mass spectrometry. Food Control 2017, 76, 96–101. [Google Scholar] [CrossRef]
- Gross, J.H. Direct analysis in real time—A critical review on DART-MS. Anal. Bioanal. Chem. 2014, 406, 63–80. [Google Scholar] [CrossRef]
- Birse, N.; Chevallier, O.; Hrbek, V.; Kosek, V.; Hajŝlová, J.; Elliott, C. Ambient mass spectrometry as a tool to determine poultry production system history: A comparison of rapid evaporative ionisation mass spectrometry (REIMS) and direct analysis in real time (DART) ambient mass spectrometry platforms. Food Control 2021, 123, 107740. [Google Scholar] [CrossRef]
- Musah, R.A.; Espinoza, E.O.; Cody, R.B.; Lesiak, A.D.; Christensen, E.D.; Moore, H.E.; Maleknia, S.; Drijfhout, F.P. A High Throughput Ambient Mass Spectrometric Approach to Species Identification and Classification from Chemical Fingerprint Signatures. Sci. Rep. 2015, 5, 11520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cody, R.B.; McAlpin, C.R.; Cox, C.R.; Jensen, K.R.; Voorhees, K.J. Identification of bacteria by fatty acid profiling with direct analysis in real time mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Marzoli, F.; Massaro, A.; Passabì, E.; Bragolusi, M.; Negro, A.; Cristaudo, I.; Piro, R.; Belluco, S. Assessing direct analysis in real-time mass spectrometry for the identification and serotyping of Legionella pneumophila. J. Appl. Microbiol. 2021, 132, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Giffen, J.E.; Rosati, J.Y.; Longo, C.M.; Musah, R.A. Species identification of necrophagous insect eggs based on amino acid profile differences revealed by direct analysis in real time-high resolution mass spectrometry. Anal. Chem. 2017, 89, 7719–7726. [Google Scholar] [CrossRef] [Green Version]
- Hajeb, P.; Zhu, L.; Bossi, R.; Vorkamp, K. Sample preparation techniques for suspect and non-target screening of emerging contaminants. Chemosphere 2022, 287, 132306. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.; Stella, R.; Negro, A.; Bragolusi, M.; Miano, B.; Arcangeli, G.; Biancotto, G.; Piro, R.; Tata, A. New strategies for the differentiation of fresh and frozen/thawed fish: A rapid and accurate non-targeted method by ambient mass spectrometry and data fusion (part A). Food Control 2021, 130, 108364. [Google Scholar] [CrossRef]
- Tata, A.; Massaro, A.; Riuzzi, G.; Lanza, I.; Bragolusi, M.; Negro, A.; Novelli, E.; Piro, R.; Gottardo, F.; Segato, S. Ambient mass spectrometry for rapid authentication of milk from Alpine or lowland forage. Sci. Rep. 2022, 12, 7360. [Google Scholar] [CrossRef]
- Tata, A.; Massaro, A.; Damiani, T.; Piro, R.; Dall’Asta, C.; Suman, M. Detection of soft-refined oils in extra virgin olive oil using data fusion approaches for LC-MS, GC-IMS and FGC-Enose techniques: The winning synergy of GC-IMS and FGC-Enose. Food Control 2022, 133, 108645. [Google Scholar] [CrossRef]
- Massaro, A.; Negro, A.; Bragolusi, M.; Miano, B.; Tata, A.; Suman, M.; Piro, R. Oregano authentication by mid-level data fusion of chemical fingerprint signatures acquired by ambient mass spectrometry. Food Control 2021, 126, 108058. [Google Scholar] [CrossRef]
- Grapes, M.; Whiting, P.; Dinan, L. Fatty acid and lipid analysis of the house cricket, Acheta domesticus. Insect Biochem. 1989, 19, 767–774. [Google Scholar] [CrossRef]
- Longvah, T.; Manghtya, K.; Qadri, S.S. Eri silkworm: A source of edible oil with a high content of α-linolenic acid and of significant nutritional value. J. Sci. Food Agric. 2012, 92, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.-J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.-L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- EU Implementing Regulation 2022/188; Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Acheta Domesticus as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022R0188 (accessed on 18 July 2021).
- Virgilio, M.; Backeljau, T.; Nevado, B.; De Meyer, M. Comparative performances of DNA barcoding across insect orders. BMC Bioinform. 2010, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanna, D.; Righetti, L.; Elliott, C.; Suman, M. The scientific challenges in moving from targeted to non-targeted mass spectrometric methods for food fraud analysis: A proposed validation workflow to bring about a harmonized approach. Trends Food Sci. Technol. 2018, 80, 223–241. [Google Scholar] [CrossRef]
- Katz, L.; Tata, A.; Woolman, M.; Zarrine-Afsar, A. Lipid Profiling in Cancer Diagnosis with Hand-Held Ambient Mass Spectrometry Probes: Addressing the Late-Stage Performance Concerns. Metabolites 2021, 11, 660. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
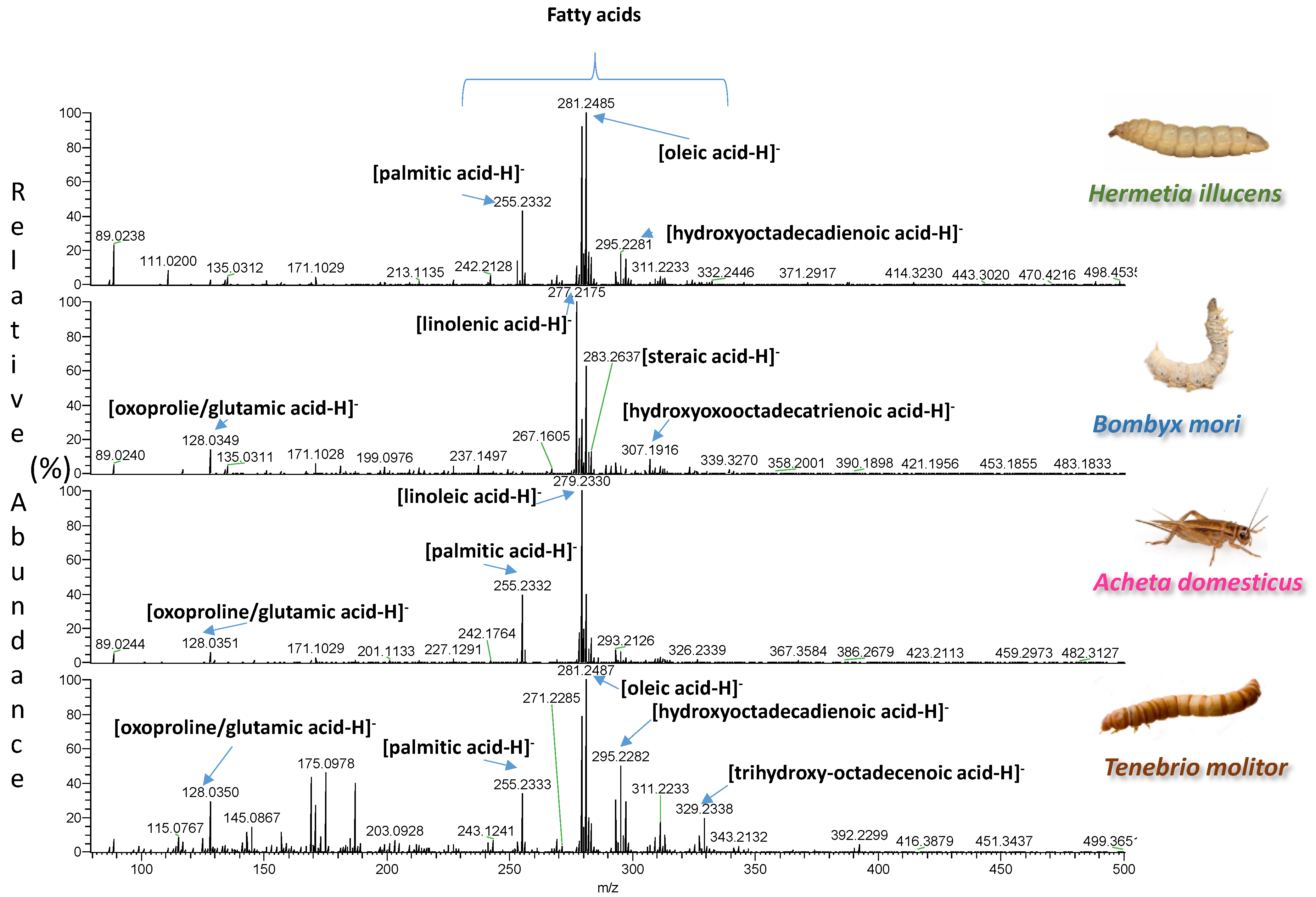

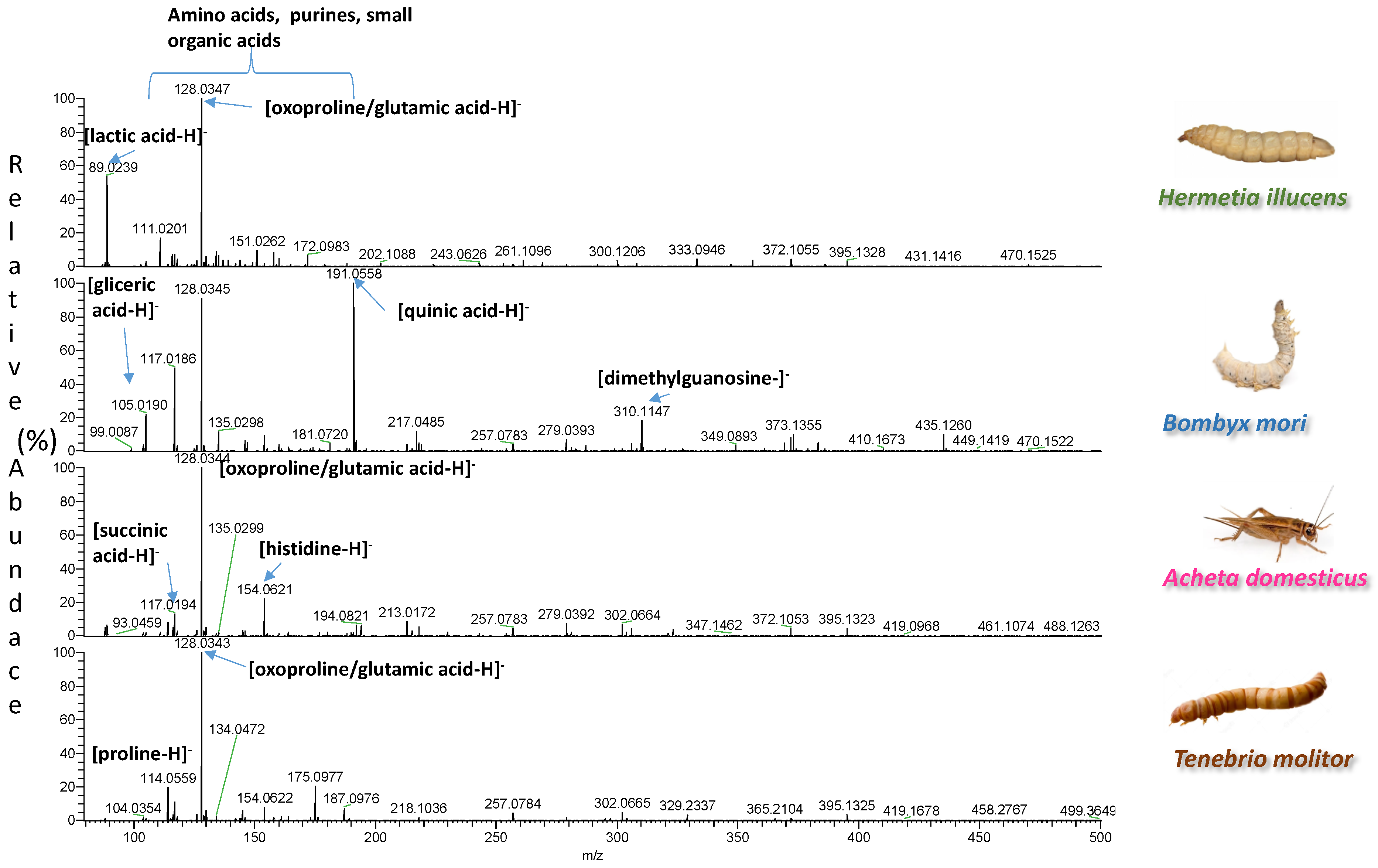
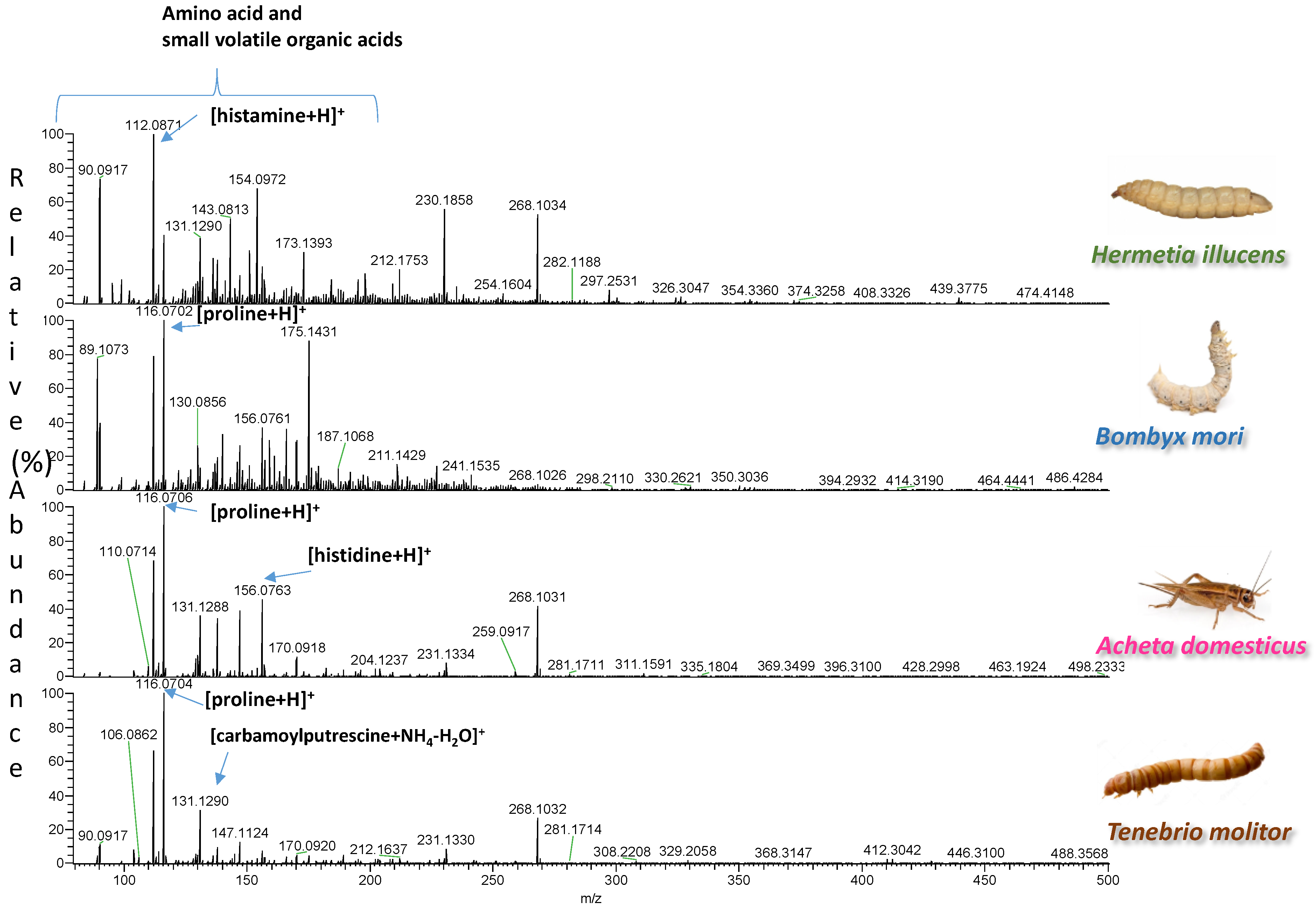
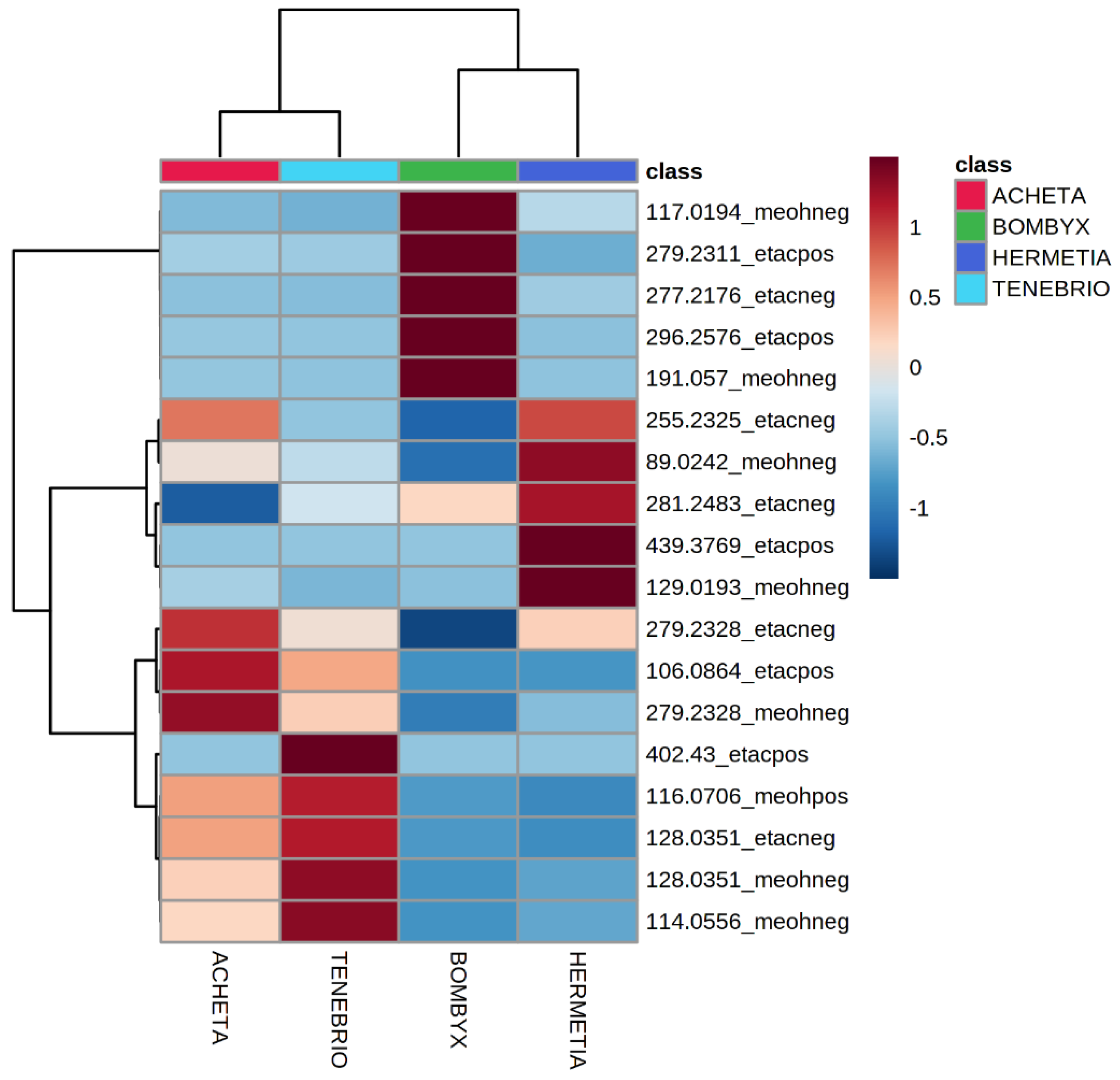
| Insect | Observed m/z | Theoretical m/z | Error (ppm) | Elemental Formula | Type of Ion | Tentative Assignment | References |
|---|---|---|---|---|---|---|---|
| Tenebrio molitor | 114.0556 | 114.0561 | −4.4 | C5H9NO2 | [M-H]− | proline | [5] |
| 116.0706 | 116.0706 | 0 | C5H9NO2 | [M+H]+ | proline | [5] | |
| 128.0351 | 128.0348 | 2.4 | C5H9NO4 | [M-H]− | glutamic acid/oxoproline | ||
| 402.4300 | 402.4306 | −1.5 | C25H52O2 | [M+H]+ | erythro-6,8-pentacosanediol | ||
| Acheta domesticus | 106.0864 | 106.0863 | 0.9 | C4H8O2 | [M+NH4]+ | FA C4:0 (butyric acid) | |
| 255.2325 | 255.2330 | −1.9 | C16H32O2 | [M-H]− | FA C16:0 (palmitic acid) | [25] | |
| 279.2328 | 279.2330 | −0.7 | C18H32O2 | [M-H]− | FA C18:2 (linoleic acid) | [25] | |
| Bombyx mori | 117.0194 | 117.0193 | 0.9 | C4H6O4 | [M-H]− | succinic acid | |
| 191.0570 | 191.0561 | −4.7 | C7H12O6 | [M-H]− | quinic acid | ||
| 277.2176 | 277.2173 | 1.1 | C18H30O2 | [M-H]− | FA C18:3 (linolenic acid) | [26] | |
| 279.2311 | 279.2319 | −2.9 | C18H30O2 | [M+H]+ | FA C18:3 (linolenic acid) | [26] | |
| 296.2576 | 296.2584 | −2.7 | C18H30O2 | [M+NH4]+ | FA C18:3 (linolenic acid) | [26] | |
| Hermetia illucens | 89.0242 | 89.0244 | −2.2 | C3H6O3 | [M-H]− | lactic acid | |
| 129.0193 | 129.0193 | 0 | C5H6O4 | [M-H]− | N/A | ||
| 255.2325 | 255.2330 | −1.9 | C16H32O2 | [M-H]− | FA C16:0 (palmitic acid) | [27,28] | |
| 281.2483 | 281.2486 | −1.1 | C18H34O2 | [M-H]− | FA C18:1 (oleic acid) | [27,28] | |
| 439.3769 | 439.3788 | −4.2 | C27H52O4 | [M-H2O+H]+ | diacylglycerol DG(24:0) |
| Merged Dataset | Sensitivity | Specificity | Accuracy | Samples Correctly Classified |
|---|---|---|---|---|
| Training set | 100% | 100% | 100% | 25/25 |
| Test set | 100% | 100% | 100% | 8/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tata, A.; Massaro, A.; Marzoli, F.; Miano, B.; Bragolusi, M.; Piro, R.; Belluco, S. Authentication of Edible Insects’ Powders by the Combination of DART-HRMS Signatures: The First Application of Ambient Mass Spectrometry to Screening of Novel Food. Foods 2022, 11, 2264. https://doi.org/10.3390/foods11152264
Tata A, Massaro A, Marzoli F, Miano B, Bragolusi M, Piro R, Belluco S. Authentication of Edible Insects’ Powders by the Combination of DART-HRMS Signatures: The First Application of Ambient Mass Spectrometry to Screening of Novel Food. Foods. 2022; 11(15):2264. https://doi.org/10.3390/foods11152264
Chicago/Turabian StyleTata, Alessandra, Andrea Massaro, Filippo Marzoli, Brunella Miano, Marco Bragolusi, Roberto Piro, and Simone Belluco. 2022. "Authentication of Edible Insects’ Powders by the Combination of DART-HRMS Signatures: The First Application of Ambient Mass Spectrometry to Screening of Novel Food" Foods 11, no. 15: 2264. https://doi.org/10.3390/foods11152264