Effects of Naringin on Postharvest Storage Quality of Bean Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Vitamin C Analysis
2.3. Polyphenol Composition Analysis
2.4. Polyphenol Oxidase (PPO) and Peroxidase (POD) Analysis
2.5. Oxygen Radical Absorbance Capacity (ORAC) Analysis
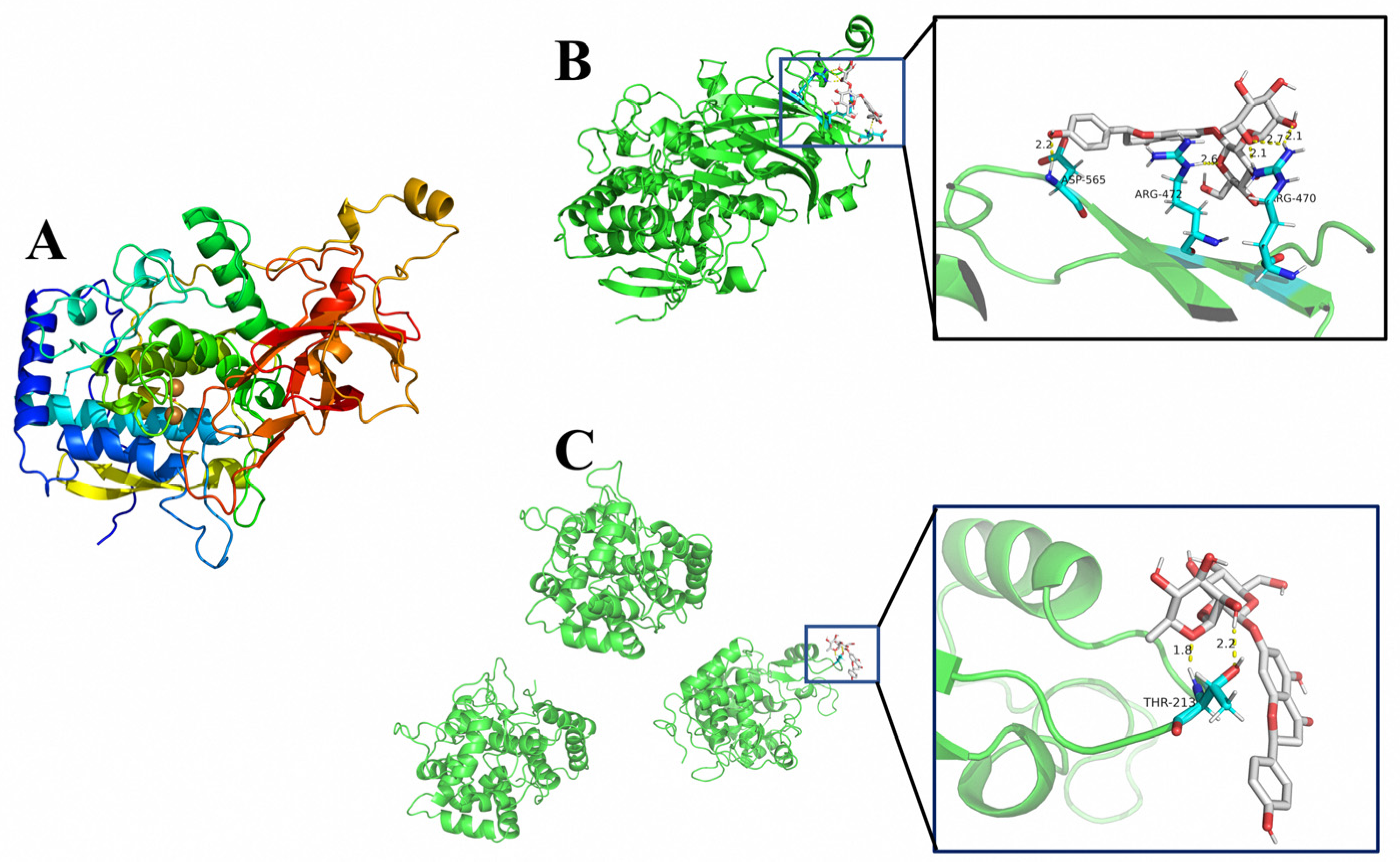
2.6. Polyphenol Oxidase (PPO) 3D Structure Analysis and Molecular Docking
2.7. Statistical Analysis
3. Results
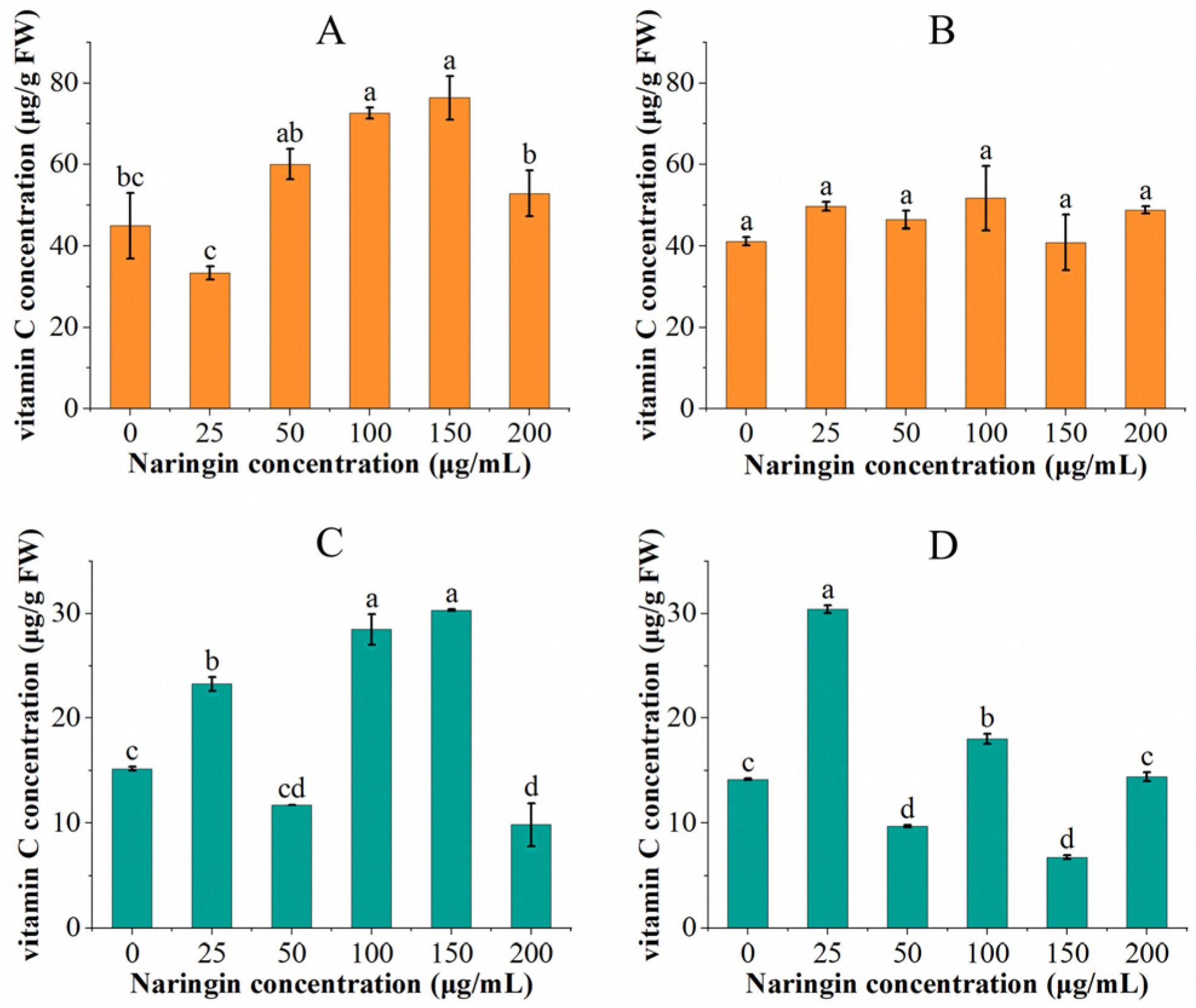
3.1. Effect on Vitamin C Content
3.2. Effect on Polyphenol Composition
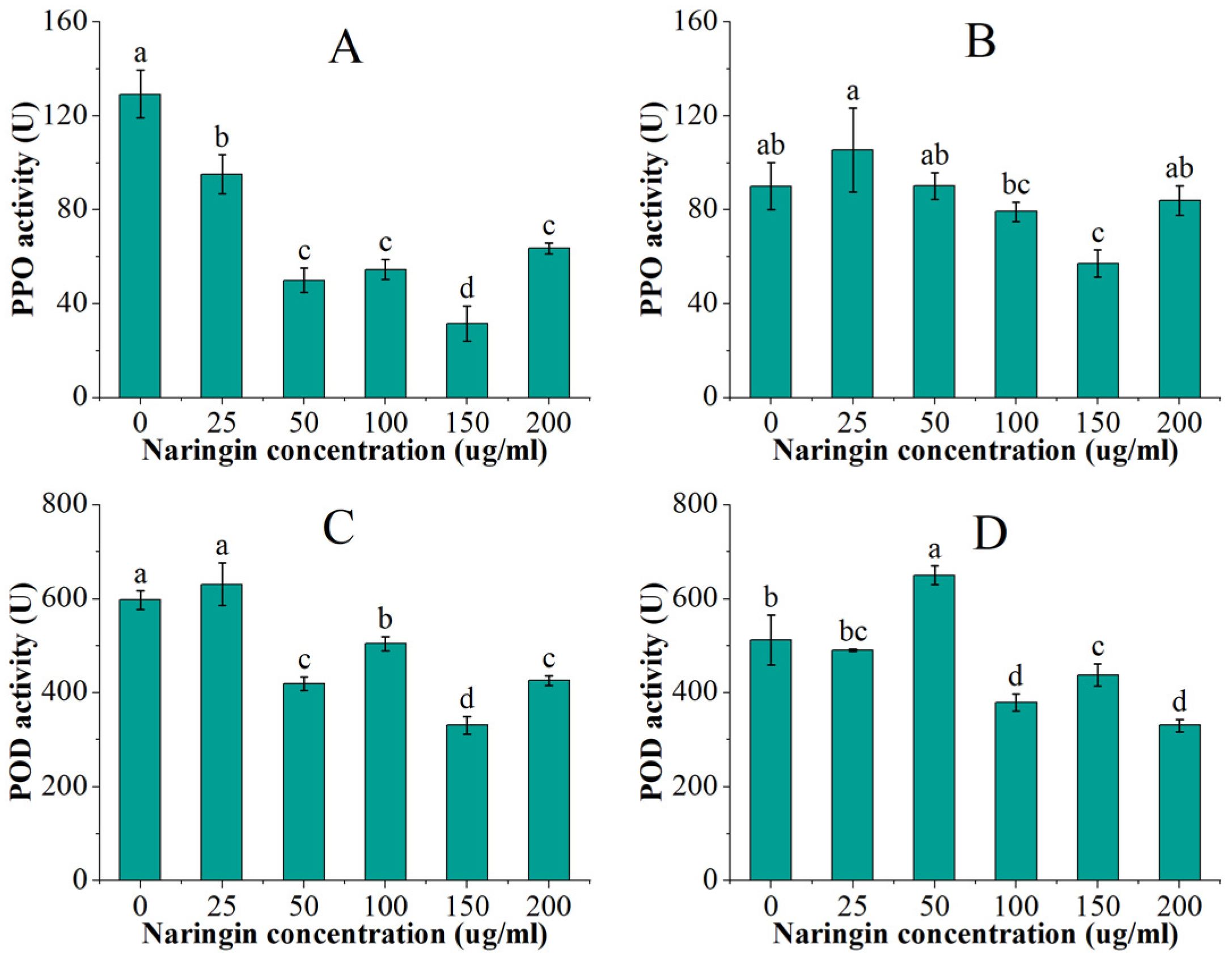
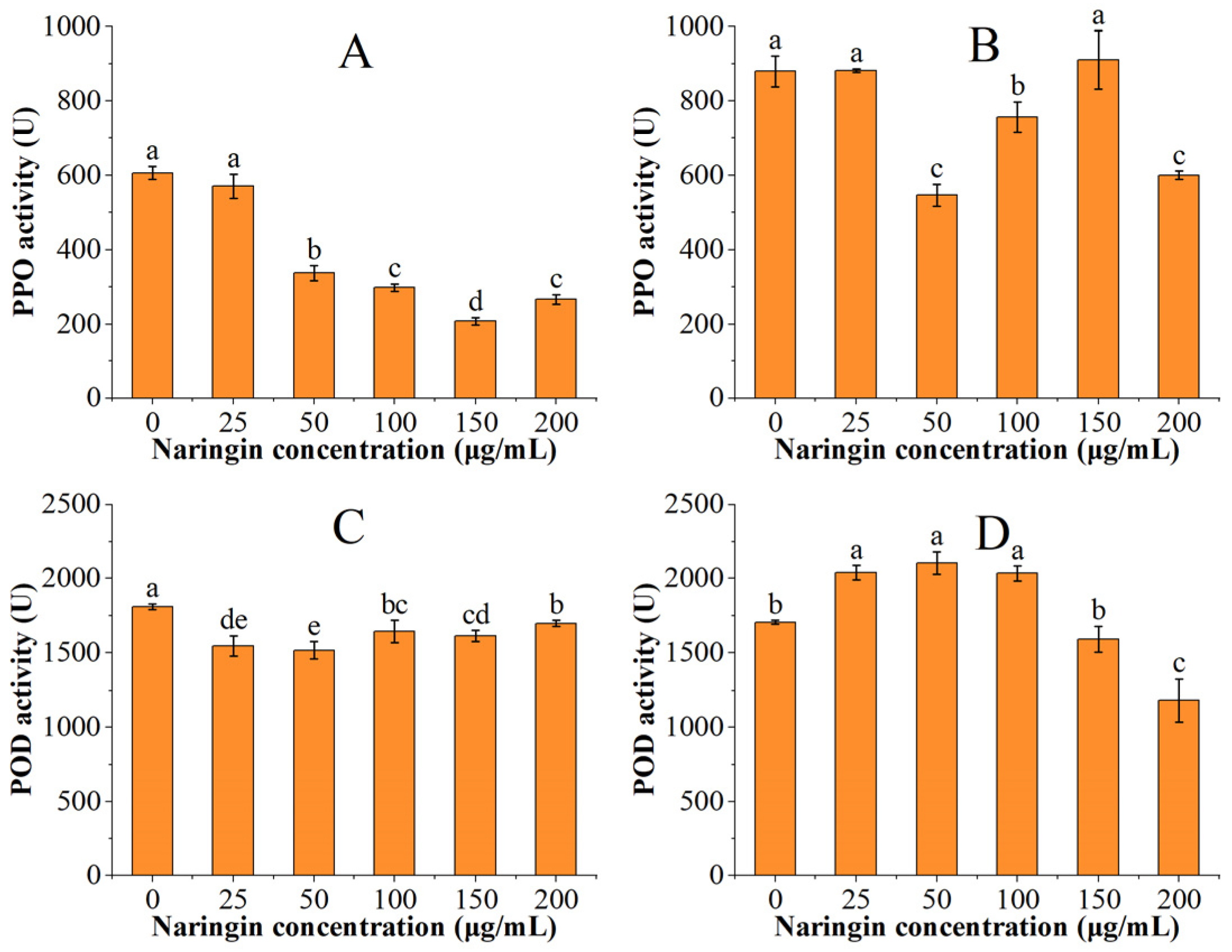
3.3. Effect on Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity
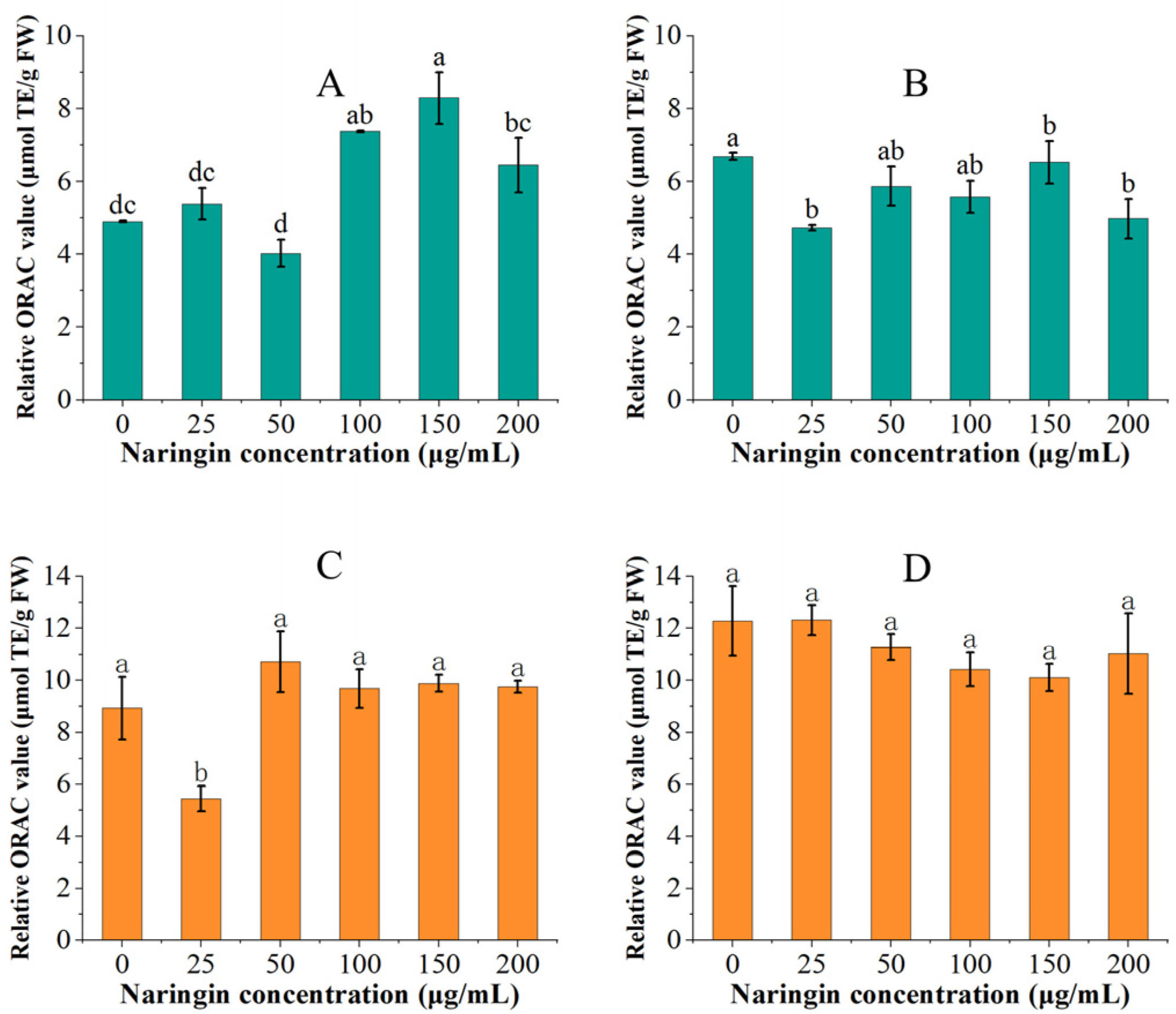
3.4. Effect on Oxygen Radical Absorbance Capacity
3.5. Molecular Docking Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ha, M.C.; Im, D.Y.; Park, H.S.; Dhungana, S.K.; Kim, I.D.; Shin, D.H. Seed Treatment with Illite Enhanced Yield and Nutritional Value of Soybean Sprouts. Molecules 2022, 27, 1152. [Google Scholar] [CrossRef]
- Lv, C.Y.; Zhao, G.H.; Ning, Y. Interactions between plant proteins/enzymes and other food components, and their effects on food quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Swieca, M. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018, 239, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.B.; Chung, H.S.; Moon, K.D. Browning control of fresh-cut lettuce by phytoncide treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Goyal, A.; Siddiqui, S. Effects of ultraviolet irradiation, pulsed electric field, hot water dip and ethanol vapours treatment on keeping and sensory quality of mung bean (Vigna radiata L. Wilczek) sprouts. J. Food Sci. Technol. 2014, 51, 2664–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-H.; Chen, C.-M.; Xu, L.; Cui, Y.; Yu, X.-Y.; Gao, H.-J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.-X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, T.T.; Liu, H.K.; Chen, Y.Y.; Pang, X.; Zheng, L.; Chang, S.; Kang, Y. Moderate vacuum packing and low temperature effects on qualities of harvested mung bean (Vigna radiata L.) sprouts. Postharvest Biol. Technol. 2018, 145, 83–92. [Google Scholar] [CrossRef]
- Gui, M.; He, H.; Li, Y.; Chen, X.; Wang, H.; Wang, T.; Li, J. Effect of UV-B treatment during the growth process on the postharvest quality of mung bean sprouts (Vigna radiata). Int. J. Food Sci. Technol. 2018, 53, 2166–2172. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, L.; Pang, L.; Chen, X.; Jia, X.; Li, X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. RSC Adv. 2020, 10, 9193–9202. [Google Scholar] [CrossRef] [Green Version]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Jam processing and storage effects on β-carotene and flavonoids content in grapefruit. J. Funct. Foods 2013, 5, 736–744. [Google Scholar] [CrossRef]
- Yusof, S.; Ghazali, H.M.; King, G.S. Naringin content in local citrus fruits. Food Chem. 1990, 37, 113–121. [Google Scholar] [CrossRef]
- Li, X.R.; Liu, H.Y.; Wu, X.Z.; Xu, R.N.; Ma, X.Y.; Zhang, C.X.; Song, Z.Z.; Peng, Y.R.; Ni, T.J.; Xu, Y.T. Exploring the interactions of naringenin and naringin with trypsin and pepsin: Experimental and computational modeling approaches. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 258, 119859. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Gao, W.; Guo, Z.; Wang, K.; Liu, S.; Duan, Z.; Chen, Y. Naringin improves lipid metabolism in a tissue-engineered liver model of NAFLD and the underlying mechanisms. Life Sci. 2021, 277, 119487. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacanli, M.; Basaran, A.A.; Basaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, N.; Hu, J.G.; Shijuan, Y.; Xie, L.; Brennan, C.S.; Huang, W.; Guo, X. The manipulation of gene expression and the biosynthesis of Vitamin C, E and folate in light-and dark-germination of sweet corn seeds. Sci. Rep. 2017, 7, 7484. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Chang, X.; Guo, X. Dynamic Changes of Ascorbic Acid, Phenolics Biosynthesis and Antioxidant Activities in Mung Beans (Vigna radiata) until Maturation. Plants 2019, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Xiang, N.; Wen, T.X.; Yu, B.L.; Li, G.K.; Li, C.Y.; Li, W.; Lu, W.J.; Hu, J.G.; Guo, X.B. Dynamic effects of post-harvest preservation on phytochemical profiles and antioxidant activities in sweet corn kernels. Int. J. Food Sci. Technol. 2020, 55, 3111–3122. [Google Scholar] [CrossRef]
- Liao, T.; Zhou, L.; Liu, J.P.; Zou, L.Q.; Dai, T.T.; Liu, W. Inhibitory mechanism of salicylic acid on polyphenol oxidase: A cooperation between acidification and binding effects. Food Chem. 2021, 348, 129100. [Google Scholar] [CrossRef]
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular Modeling of Peroxidase and Polyphenol Oxidase: Substrate Specificity and Active Site Comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Wang, P.; Gu, Z.; Ma, M.; Yang, R. Spermidine improves antioxidant activity and energy metabolism in mung bean sprouts. Food Chem. 2020, 309, 125759. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Huang, Z.; Li, D.; Wang, H.; Xu, X.; Jiang, Y.; Qu, H. Phenolic components, antioxidant enzyme activities and anatomic structure of longan fruit pericarp following treatment with adenylate triphosphate. Sci. Hortic. 2014, 180, 6–13. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Holzwarth, M.; Wittig, J.; Carle, R.; Kammerer, D.R. Influence of putative polyphenoloxidase (PPO) inhibitors on strawberry (Fragaria × ananassa Duch.) PPO, anthocyanin and color stability of stored purées. LWT Food Sci. Technol. 2013, 52, 116–122. [Google Scholar] [CrossRef]
- Sukhonthara, S.; Kaewka, K.; Theerakulkait, C. Inhibitory effect of rice bran extracts and its phenolic compounds on polyphenol oxidase activity and browning in potato and apple puree. Food Chem. 2016, 190, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, Y.; Shi, J.; Xue, S. The purification and characterisation of polyphenol oxidase from green bean (Phaseolus vulgaris L.). Food Chem. 2009, 117, 143–151. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M.; Franczyk, M.; Jakubczyk, A.; Bochnak, J.; Złotek, U. Biochemical Properties of Polyphenol Oxidases from Ready-to-Eat Lentil (Lens culinaris Medik.) Sprouts and Factors Affecting Their Activities: A Search for Potent Tools Limiting Enzymatic Browning. Foods 2019, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Zhou, L.; Sun, Y.; Zeng, Z.; Chen, H.; Liu, J.; Zou, L.; Liu, W. Anti-browning effect of Rosa roxburghii on apple juice and identification of polyphenol oxidase inhibitors. Food Chem. 2021, 359, 129855. [Google Scholar] [CrossRef]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol Oxidase: Characteristics and Mechanisms of Browning Control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Adams, J.B.; Brown, H.M. Discoloration in Raw and Processed Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2007, 47, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Uribe, C.E.; Vallejo, W.; Oliveros, G.; Muñoz, A. Study of scavenging capacity of naringin extracted from Citrus uranium peel against free radicals. Prospectiva 2016, 14, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Lin, M.; Katsumura, Y.; Muroya, Y. Free-Radical Scavenging Activities of Silybin and Its Analogues: A Pulse Radiolysis Study. Int. J. Chem. Kinet. 2011, 43, 590–597. [Google Scholar] [CrossRef]
- Burguieres, E.; McCue, P.; Kwon, Y.-I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Wang, H.; Gui, M.; Tian, X.; Xin, X.; Wang, T.; Li, J. Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata). Int. J. Food Sci. Technol. 2017, 52, 827–833. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Chan, C.-L.; Corke, H. Hot Air Drying Induces Browning and Enhances Phenolic Content and Antioxidant Capacity in Mung Bean (Vigna radiata L.) Sprouts. J. Food Process. Preserv. 2017, 41, e12846. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Wang, M.-F.; Lui, W.-Y.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Jang, J.-H.; Moon, K.-D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
| Naringin Concentration (μg/mL) | 0 | 25 | 50 | 100 | 150 | 200 |
|---|---|---|---|---|---|---|
| Soybean sprouts treatment abbreviations | S1 | S2 | S3 | S4 | S5 | S6 |
| Mung bean sprouts treatment abbreviations | M1 | M2 | M3 | M4 | M5 | M6 |
| Polyphenols (μg/g FW) | Processing Time | M1 | M2 | M3 | M4 | M5 | M6 |
|---|---|---|---|---|---|---|---|
| gallic acid | 3 d | 11.40 ± 0.38 a | 14.27 ± 0.53 a | 11.70 ± 1.13 a | 14.07 ± 2.21 a | 12.50 ± 0.76 a | 11.95 ± 0.36 a |
| 6 d | 14.12 ± 0.15 a | 11.39 ± 0.17 cd | 12.42 ± 0.05 bc | 12.81 ± 0.36 b | 11.08 ± 0.24 d | 10.81 ± 0.52 d | |
| caffeic acid | 3 d | 19.40 ± 0.17 b | 17.71 ± 0.11 bc | 15.77 ± 0.14 cd | 16.93 ± 0.70 cd | 15.26 ± 0.04 d | 24.53 ± 1.35 a |
| 6 d | 16.09 ± 0.16 a | 17.47 ± 2.45 a | 18.81 ± 0.19 a | 18.45 ± 2.01 a | 18.03 ± 1.97 a | 14.07 ± 5.47 a | |
| p-coumaric acid | 3 d | 20.88 ± 0.64 c | 25.14 ± 0.53 abc | 26.42 ± 1.45 bc | 24.27 ± 0.65 bc | 24.84 ± 1.46 bc | 29.36 ± 1.86 a |
| 6 d | 31.76 ± 0.04 a | 23.93 ± 0.05 d | 25.55 ± 0.18 cd | 32.24 ± 2.34 a | 27.80 ± 0.79 bc | 30.78 ± 0.03 ab | |
| ferulic acid | 3 d | 5.43 ± 1.58 a | 4.63 ± 1.12 a | 3.52 ± 0.10 a | 4.12 ± 0.47 a | 3.88 ± 0.09 a | 3.53 ± 0.20 a |
| 6 d | 3.52 ± 0.05 ab | 3.55 ± 0.20 ab | 3.71 ± 0.20 a | 3.67 ± 0.34 a | 2.96 ± 0.06 b | 4.01 ± 0.03 a |
| Polyphenols (μg/g FW) | Processing Time | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|
| gallic acid | 3 d | 19.29 ± 0.09 a | 18.06 ± 0.36 ab | 18.00 ± 0.01 ab | 19.25 ± 0.62 a | 17.77 ± 0.55 b | 19.13 ± 0.11 ab |
| 6 d | 21.54 ± 0.51 a | 20.27 ± 0.19 a | 21.78 ± 0.08 a | 20.20 ± 1.31 a | 19.72 ± 2.06 a | 19.74 ± 0.01 a | |
| daidzin | 3 d | 17.07 ± 1.15 b | 19.49 ± 2.17 ab | 17.91 ± 1.28 ab | 19.74 ± 1.83 ab | 22.80 ± 2.72 ab | 26.06 ± 1.30 a |
| 6 d | 16.28 ± 2.64 a | 17.38 ± 1.39 a | 18.48 ± 0.71 a | 20.46 ± 1.38 a | 23.67 ± 2.50 a | 23.48 ± 3.06 a | |
| rutin | 3 d | 27.44 ± 1.44 a | 31.82 ± 2.35 a | 35.88 ± 2.39 a | 30.00 ± 2.58 a | 35.01 ± 4.79 a | 33.63 ± 0.56 a |
| 6 d | 26.50 ± 5.24 a | 27.95 ± 3.95 a | 32.28 ± 5.99 a | 30.54 ± 2.45 a | 36.12 ± 4.69 a | 34.13 ± 5.03 a | |
| genistein | 3 d | 55.85 ± 3.46 b | 61.49 ± 4.42 b | 65.41 ± 2.13 ab | 63.88 ± 5.00 ab | 60.78 ± 2.29 b | 77.16 ± 6.18 a |
| 6 d | 56.68 ± 0.26 b | 69.25 ± 0.66 ab | 64.73 ± 1.45 b | 66.24 ± 6.61 b | 67.92 ± 7.95 ab | 84.70 ± 4.35 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhao, Y.; Gu, Q.; Chen, W.; Guo, X. Effects of Naringin on Postharvest Storage Quality of Bean Sprouts. Foods 2022, 11, 2294. https://doi.org/10.3390/foods11152294
Yang X, Zhao Y, Gu Q, Chen W, Guo X. Effects of Naringin on Postharvest Storage Quality of Bean Sprouts. Foods. 2022; 11(15):2294. https://doi.org/10.3390/foods11152294
Chicago/Turabian StyleYang, Xufeng, Yihan Zhao, Qiuming Gu, Weiling Chen, and Xinbo Guo. 2022. "Effects of Naringin on Postharvest Storage Quality of Bean Sprouts" Foods 11, no. 15: 2294. https://doi.org/10.3390/foods11152294
APA StyleYang, X., Zhao, Y., Gu, Q., Chen, W., & Guo, X. (2022). Effects of Naringin on Postharvest Storage Quality of Bean Sprouts. Foods, 11(15), 2294. https://doi.org/10.3390/foods11152294






