Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review
Abstract
:1. Introduction
2. Mechanism of the Anti-Inflammatory Effects of Plant-Derived Bioactive Peptides
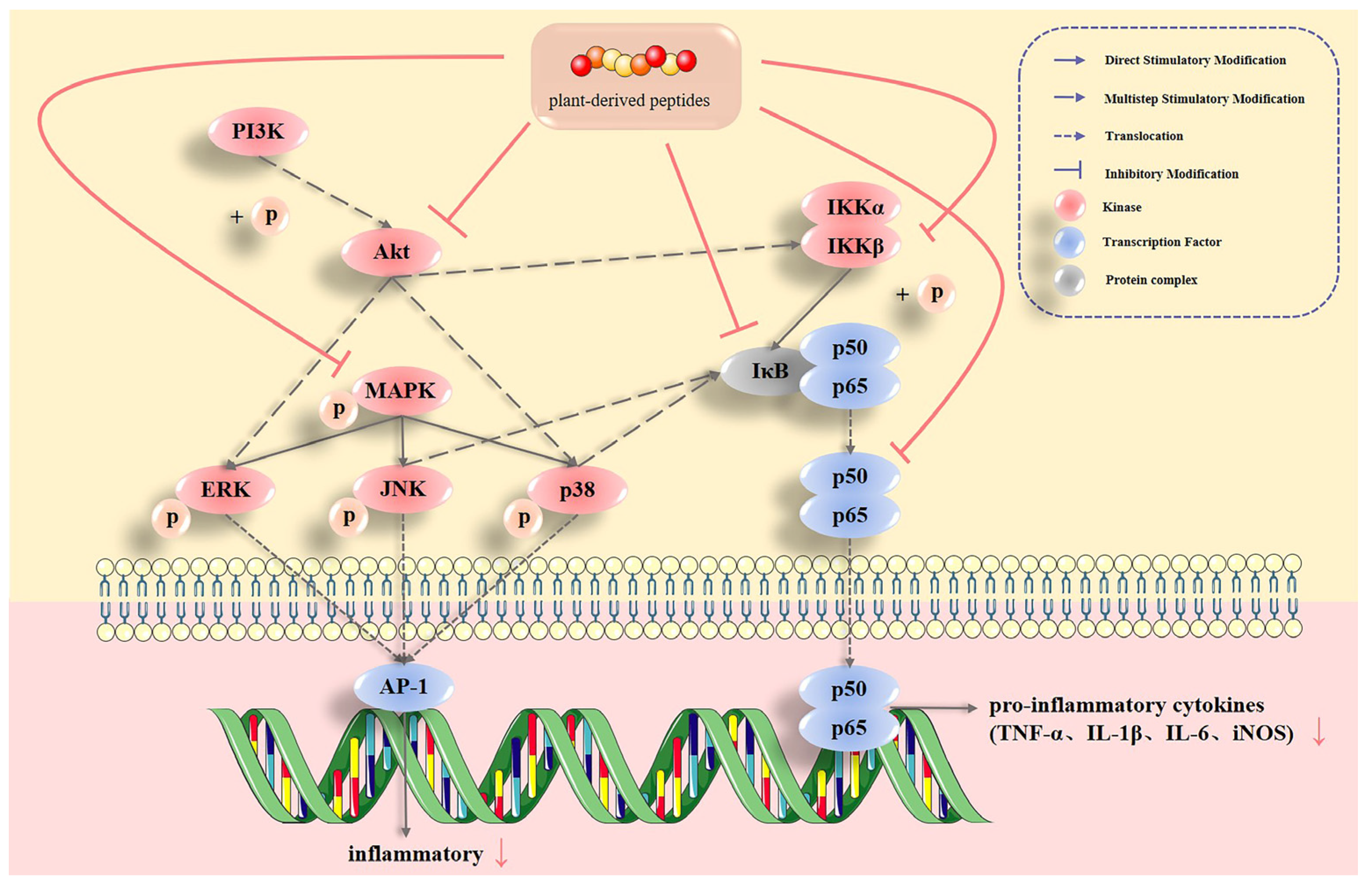
2.1. Plant-Derived Bioactive Peptides Regulate Single Inflammatory Signaling Pathways and Their Inflammatory Factors
2.1.1. MAPK Pathways
2.1.2. NF-κB Pathway
2.2. Plant-Derived Bioactive Peptides Regulate Multiple Inflammatory Signaling Pathways and Their Inflammatory Factors
3. Factors Affecting the Anti-Inflammatory Effects of Plant-Derived Bioactive Peptides
3.1. Effect of Molecular Weight on the Anti-Inflammatory Properties of Plant-Derived Bioactive Peptides
3.2. Effect of Amino Acid Composition on the Anti-Inflammatory Properties of Low-Molecular-Weight Plant-Derived Bioactive Peptides
3.2.1. Hydrophobic Amino Acids
3.2.2. Positively Charged Amino Acids
3.2.3. Specific Amino Acids
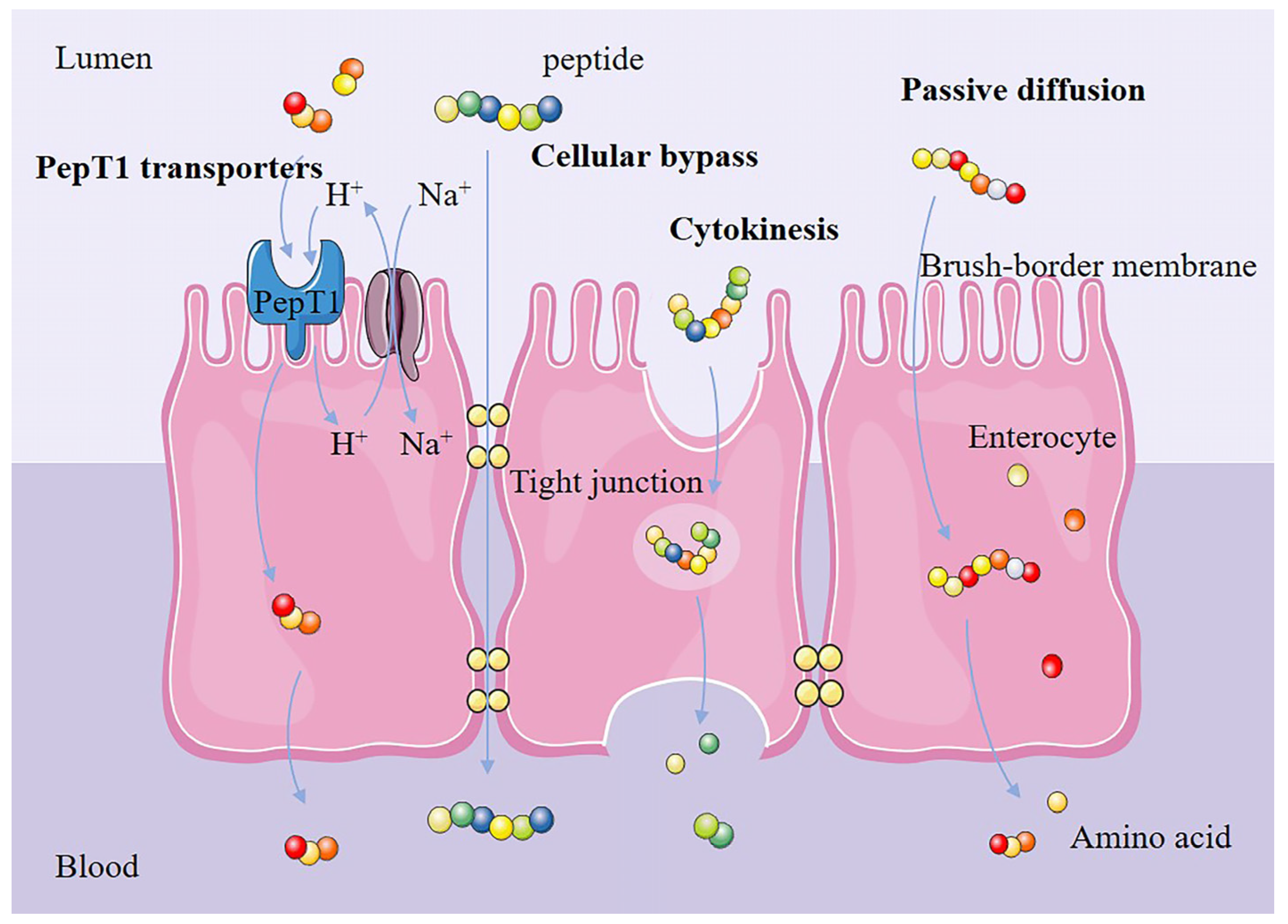
3.3. Effects of Amino Acid Positions in Plant-Derived Bioactive Peptides on Their Anti-Inflammatory Properties
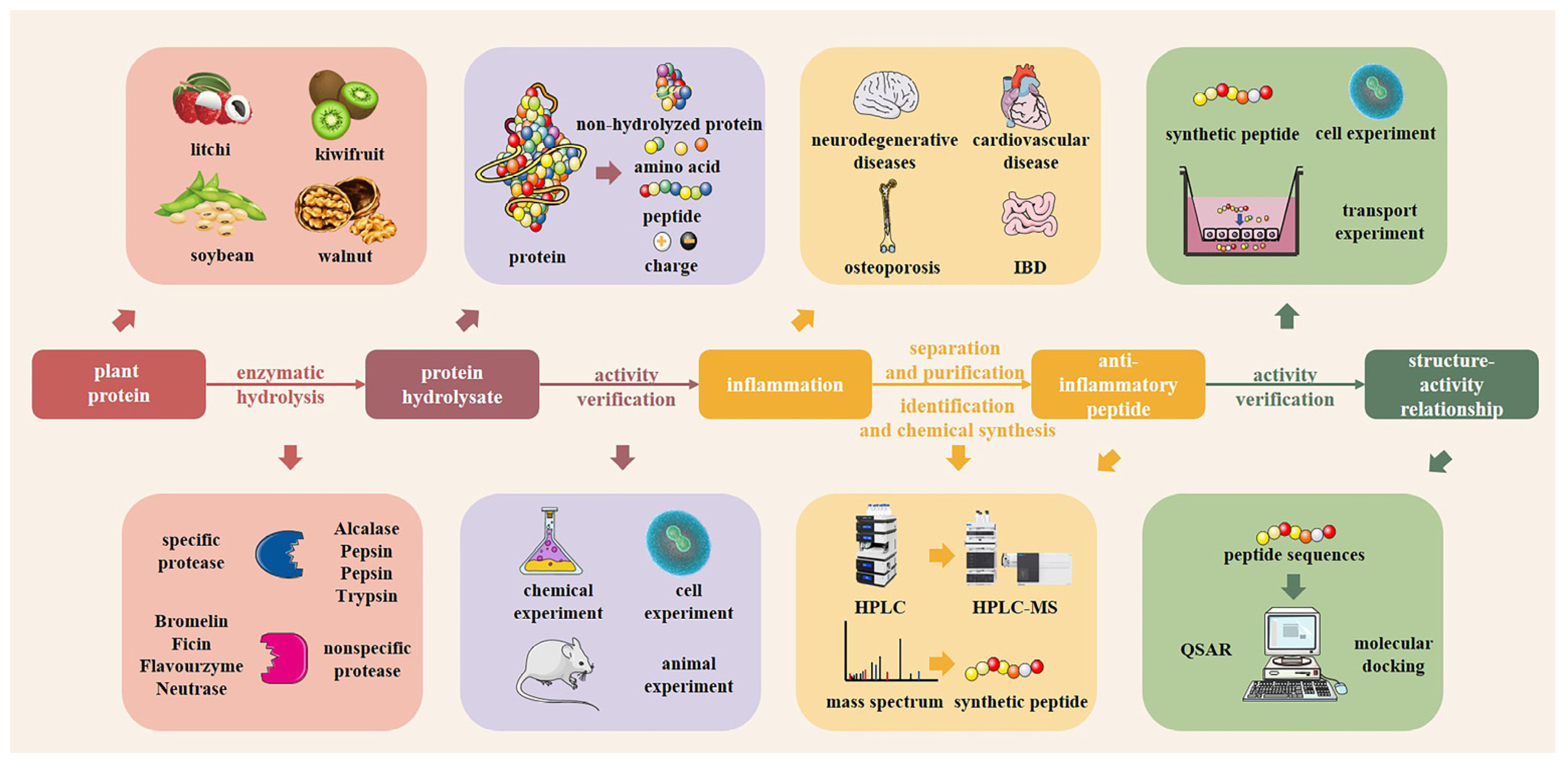
4. Preparation of Plant-Derived Anti-Inflammatory Peptides
4.1. Preparation of Plant-Derived Anti-Inflammatory Peptides
4.1.1. Chemical Hydrolysis
4.1.2. Microbial Fermentation
4.1.3. Enzymatic Hydrolysis
4.2. Influence of Enzyme Type in Enzymatic Processing on the Anti-Inflammatory Activity of Plant-Derived Anti-Inflammatory Peptides
4.2.1. Single Enzyme Digestion
4.2.2. Compound Enzyme Enzymatic Digestion
4.3. Isolation and Purification of Plant-Derived Protein Hydrolysates
4.4. Identification of Peptides from Plant-Derived Bioactive Peptides
5. Limitations of Plant-Derived Anti-Inflammatory Peptides
5.1. Difficulties in Determining the Enzymatic Solutions of Plant-Derived Anti-Inflammatory Peptides
5.2. Low Yield of Plant-Derived Anti-Inflammatory Peptide Processing
5.3. The Lack of Clinical Studies on Plant-Derived Anti-Inflammatory Peptides
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.J.; Gong, J.P.; Zhang, W. Transcriptional co-regulator RIP140: An important mediator of the inflammatory response and its associated diseases (review). Mol. Med. Rep. 2017, 16, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Padwad, Y. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci. Technol. 2020, 98, 41–52. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Food 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Bao, X.L.; Feng, G.X.; Zhang, M.; Ma, S. Effects of peptide-calcium complexes from sunflower seeds and peanuts on enhancing bone mineral density. Int. J. Food Sci. Technol. 2020, 55, 2942–2953. [Google Scholar] [CrossRef]
- Daghero, H.; Masso, J.R.F.; Astrada, S.; Vallespi, M.G.; Bollati-Fogolin, M. The anticancer peptide CIGB-552 exerts anti-inflammatory and anti-angiogenic effects through COMMD1. Molecules 2020, 26, 152. [Google Scholar] [CrossRef]
- Wang, G.L.; Yang, X.Y.; Wang, J.; Zhong, D.Y.; Zhang, R.G.; Zhang, Y.N.; Feng, L.L.; Zhang, Y.L. Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease and colonic tissue damage in high-fat diet fed rats. Int. J. Biol. Macromol. 2021, 182, 879–898. [Google Scholar] [CrossRef]
- Wang, S.G.; Sun-Waterhouse, D.X.; Waterhouse, G.I.N.; Zheng, L.; Su, G.W.; Zhao, M.M. Effects of food-derived bioactive peptides on cognitive deficits and memory decline in neurodegenerative diseases: A review. Trends Food Sci. Technol. 2021, 116, 712–732. [Google Scholar] [CrossRef]
- Brennan, R.; Wazaify, M.; Shawabkeh, H.; Boardley, I.; McVeigh, J.; Van Hout, M.C. A scoping review of non-medical and extra-medical use of non-steroidal anti-inflammatory drugs (NSAIDs). Drug Saf. 2021, 44, 917–928. [Google Scholar] [CrossRef]
- Zhang, W.T.; Wang, M.R.; Hua, G.D.; Li, Q.Y.; Wang, X.J.; Lang, R.; Weng, W.L.; Xue, C.M.; Zhu, B.C. Inhibition of aspirin-induced gastrointestinal injury: Systematic review and network meta-analysis. Front. Pharmacol. 2021, 12, 730681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, X.H.; Li, W.H.; Li, H.; Ying, Z.W.; Liu, X.Q.; Yin, L.D. Enhancement of nutritional soy protein and peptide supplementation on skin repair in rats. J. Funct. Food 2020, 75, 104231. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhang, J.; Li, H.; Liu, W.L.; Xi, Y.; Liu, X.Q. A comprehensive comparison of different selenium supplements: Mitigation of heat stress and exercise fatigue-induced liver injury. Front. Nutr. 2022, 9, 917349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.H.; Ying, Z.W.; Zhao, D.; Yi, G.F.; Li, H.; Liu, X.Q. Soybean protein-derived peptide nutriment increases negative nitrogen balance in burn injury-induced inflammatory stress response in aged rats through the modulation of white blood cells and immune factors. Food Nutr. Res. 2020, 64, 3677. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Food 2020, 64, 103680. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A.H. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Mondanelli, G.; Albini, E.; Orecchini, E.; Pallotta, M.T.; Belladonna, M.L.; Ricci, G.; Grohmann, U.; Orabona, C. Pathogenetic interplay between IL-6 and tryptophan metabolism in an experimental model of obesity. Front. Immunol. 2021, 12, 713989. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Liu, M.T.; Zou, Z.P.; Wang, M.J.; Wang, Z.G.; Ju, X.R.; Hao, G.F. Anti-inflammatory activity of peptides derived from millet bran in vitro and in vivo. Food Funct. 2022, 13, 1881–1889. [Google Scholar] [CrossRef]
- Xian, Y.; Yang, L.G.; Pan, D.; Liu, H.C.; Xia, H.; Wang, S.K.; Sun, G.J. Wheat peptide protects against ethanol-induced gastric mucosal damage through downregulation of TLR4 and MAPK. J. Funct. Food 2020, 75, 104271. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, Y.H.; Zhang, L.; Yu, H.X.; Xu, Z.; You, H.X.; Cheng, Y.H. Rice protein hydrolysates (RPHs) inhibit the LPS-stimulated inflammatory response and phagocytosis in RAW264.7 macrophages by regulating the NF-κB signaling pathway. RSC Adv. 2016, 6, 71295–71304. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.T.; Zhu, N.; Liu, X.R.; Kang, J.W.; Mao, R.X.; Hou, C.; Li, Y. The gastroprotective effect of small molecule oligopeptides isolated from walnut (Juglans regia L.) against ethanol-induced gastric mucosal injury in rats. Nutrients 2020, 12, 1138. [Google Scholar] [CrossRef]
- Ho, T.Y.; Li, C.C.; Lo, H.Y.; Chen, F.Y.; Hsiang, C.Y. Corn silk extract and its bioactive peptide ameliorated lipopolysaccharide-induced inflammation in mice via the nuclear factor-κB signaling pathway. J. Agric. Food Chem. 2017, 65, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.X.; Dun, B.Q.; Wei, Z.C.; Liu, C.Y.; Tian, J.; Ren, G.X.; Yao, Y. Peptides released from extruded adzuki bean protein through simulated gastrointestinal digestion exhibit anti-inflammatory activity. J. Agric. Food Chem. 2021, 69, 7028–7036. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Yang, Z.; Liu, M.; Zhang, C.; Zhao, Y.; Song, C. ω-3 DPA protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting NF-κB/MAPK p38 signaling and activating neuron-BDNF-PI3K/AKT pathways. Mar. Drugs 2021, 19, 587. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.N.; Ren, G.X.; Wu, C.E.; Qin, P.Y.; Yao, Y. Peptides from extruded lupin (Lupinus albus L.) regulate inflammatory activity via the p38 MAPK signal transduction pathway in RAW 264.7 Cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef]
- Feng, X.W.; Cheng, Q.L.; Fang, L.; Liu, W.Y.; Liu, L.W.; Sun, C.Q.; Lu, Z.H.; Li, G.M.; Gu, R.Z. Corn oligopeptides inhibit Akt/NF-κB signaling pathway and inflammatory factors to ameliorate CCl4 -induced hepatic fibrosis in mice. J. Food Biochem. 2022, 00, e14162. [Google Scholar] [CrossRef]
- Ji, Z.W.; Mao, J.Q.; Chen, S.G.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Gao, Y.W.; Qin, H.X.; Wu, D.; Liu, C.L.; Fang, L.; Wang, J.; Liu, X.T.; Min, W.H. Walnut peptide WEKPPVSH in alleviating oxidative stress and inflammation in lipopolysaccharide-activated BV-2 microglia via the Nrf2/HO-1 and NF-κB/p38 MAPK pathways. J. Biosci. Bioeng. 2021, 132, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Chanchao, C.; Reamtong, O.; Karnchanatat, A. Identification of novel anti-inflammatory peptides from bee pollen (Apis mellifera) hydrolysate in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Apic. Res. 2020, 60, 280–289. [Google Scholar] [CrossRef]
- Vo, T.; Ryu, B.M.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal spirulina maxima. J. Funct. Food 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Xue, H.Y.; Han, J.J.; He, B.Y.; Yi, M.X.; Liu, X.F.; Song, H.X.; Li, J.Y. Bioactive peptide release and the absorption tracking of casein in the gastrointestinal digestion of rats. Food Funct. 2021, 12, 5157–5170. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763. [Google Scholar] [CrossRef]
- Karaś, M. Influence of physiological and chemical factors on the absorption of bioactive peptides. Int. J. Food Sci. Technol. 2018, 54, 1486–1496. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Santos-Sanchez, G.; Bollati, C.; Bartolomei, M.; Li, J.Q.; Arnoldi, A.; Lammi, C. Hempseed (Cannabis sativa) peptides WVSPLAGRT and IGFLIIWV exert anti-inflammatory activity in the LPS-stimulated human hepatic cell line. J. Agric. Food Chem. 2022, 70, 577–583. [Google Scholar] [CrossRef]
- Feng, M.J.; Wang, X.Y.; Xiong, H.; Qiu, T.T.; Zhang, H.; Guo, F.H.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264.7 macrophages via NF-κB/MAPKs signaling pathways. J. Funct. Food 2021, 76, 104320. [Google Scholar] [CrossRef]
- Wang, Q.H.; Han, P.P.; Li, S.T.; Xia, J.X.; Chen, Z.; Wang, C.; Wu, Y.L.; Jia, Y.M.; Ma, A.J. Potential anti-inflammatory activity of walnut protein derived peptide leucine-proline-phenylalanine in lipopolysaccharides-irritated RAW264.7 cells. Food Agric. Immunol. 2021, 32, 663–678. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; de Mejia, E.G. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB Pathways in 3T3L-1 Adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Zheng, L.; Zhao, T.T.; Zhang, Q.; Liu, Y.; Sun, B.G.; Su, G.W.; Zhao, M.M. Inhibitory effects of walnut (Juglans regia) peptides on neuroinflammation and oxidative stress in lipopolysaccharide-induced cognitive impairment mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Sun, L.L.; Li, D.L.; Lai, X.F.; Wen, S.; Chen, R.H.; Zhang, Z.B.; Li, Q.H.; Sun, S.L. Green tea peptides ameliorate diabetic nephropathy by inhibiting the TGF-β/Smad signaling pathway in mice. Food Funct. 2022, 13, 3258–3270. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cui, X.J.; Lin, Q.L.; Cai, J.; Tang, L.H.; Liang, Y. Active peptide KF-8 from rice bran attenuates oxidative stress in a mouse model of aging induced by d-galactose. J. Agric. Food Chem. 2020, 68, 12271–12283. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.F.; Wang, W.Q.; Liao, J.M.; Liao, L.; Li, C.H.; Zhao, M.M. Walnut protein hydrolysates, rich with peptide fragments of WSREEQEREE and ADIYTEEAGR ameliorate UV-induced photoaging through inhibition of the NF-κB/MMP-1 signaling pathway in female rats. Food Funct. 2020, 11, 10601–10616. [Google Scholar] [CrossRef]
- Velliquette, R.A.; Fast, D.J.; Maly, E.R.; Alashi, A.M.; Aluko, R.E. Enzymatically derived sunflower protein hydrolysate and peptides inhibit NF-κB and promote monocyte differentiation to a dendritic cell phenotype. Food Chem. 2020, 319, 126563. [Google Scholar] [CrossRef]
- Sui, H.L.; Wang, F.; Weng, Z.B.; Song, H.Z.; Fang, Y.; Tang, X.Z.; Shen, X.C. A wheat germ-derived peptide YDWPGGRN facilitates skin wound-healing processes. Biochem. Biophys. Res. Commun. 2020, 524, 943–950. [Google Scholar] [CrossRef]
- Zhang, S.H.; Ren, M.; Zeng, X.F.; He, P.L.; Ma, X.; Qiao, S.Y. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar] [CrossRef]
- Bonvini, A.; Rogero, M.M.; Coqueiro, A.Y.; Raizel, R.; Bella, L.M.; Fock, R.A.; Borelli, P.; Tirapegui, J. Effects of different branched-chain amino acids supplementation protocols on the inflammatory response of LPS-stimulated RAW 264.7 macrophages. Amino Acids 2021, 53, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, A.; Schmidtchen, A.; Bhunia, A.; Malmsten, M. Tryptophan end-tagging for promoted lipopolysaccharide interactions and anti-inflammatory effects. Sci. Rep. 2017, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yin, J.; Wang, B.; Huang, X.G.; Yao, J.M.; Zheng, J.; Fan, W.J.; Li, T.J.; Yin, Y.L. Effects of dietary lysine restriction on inflammatory responses in piglets. Sci. Rep. 2018, 8, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.; Dou, X.J.; Li, J.W.; Yang, Y.; Xue, C.Y.; Wang, C.X.; Gao, N.; Shan, A. L-arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-κB and MAPK pathways and stimulating β-defensins expression in vivo and in vitro. J. Agric. Food Chem. 2020, 68, 2648–2663. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.Y.; Yu, Z.P.; Ma, S.T.; Du, Z.Y.; Zhang, T.; Liu, J.B. Importance of terminal amino acid residues to the transport of oligopeptides across the Caco-2 cell monolayer. J. Agric. Food Chem. 2017, 65, 7705–7712. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium binding to amino acids and small glycine peptides in aqueous solution: Toward peptide design for better calcium bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef]
- Li, X.G.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, J.; Chang, G.J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X.Z. Glutamine supplementation attenuates the inflammation caused by LPS-induced acute lung injury in mice by regulating the TLR4/MAPK signaling pathway. Inflammation 2021, 44, 2180–2192. [Google Scholar] [CrossRef]
- Jiang, H.F.; Chen, W.J.; Wang, J.; Zhang, R.S. Selective N-terminal modification of peptides and proteins: Recent progresses and applications. Chin. Chem. Lett. 2022, 33, 80–88. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.Z.; Lu, W.L.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. Trac-Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Sefora Rutella, G.; Taneyo Saa, D.L.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Ha, J.; Oh, H.; Oh, N.S.; Seo, Y.; Kang, J.; Park, M.H.; Kim, K.S.; Kang, S.H.; Yoon, Y. Anti-inflammatory effect of a peptide derived from the synbiotics, fermented cudrania tricuspidata with lactobacillus gasseri, on inflammatory bowel disease. Mediators Inflamm. 2020, 2020, 3572809. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Shang, W.T.; Zhou, Z.K. Functional peptides derived from rice bran proteins. Crit. Rev. Food Sci. Nutr. 2019, 59, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castano-Tostado, E.; Castillo-Herrera, G.A. Protein hydrolysis by subcritical water: A new perspective on obtaining bioactive peptides. Molecules 2021, 26, 6655. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.; Bowra, S.; Cooper, H.J. Subcritical water processing of proteins: An alternative to enzymatic digestion? Anal. Chem. 2016, 88, 6425–6432. [Google Scholar] [CrossRef] [Green Version]
- Brent Chesson, C.; Alvarado, R.E.; Rudra, J.S. Microwave-assisted synthesis and immunological evaluation of self-assembling peptide vaccines. Methods Mol. Biol. 2018, 1777, 249–259. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Deering, A.J.; Liceaga, A.M. New insights into the antimicrobial properties of hydrolysates and peptide fractions derived from chia seed (Salvia hispanica L.). Probiotics Antimicrob. Proteins 2020, 12, 1571–1581. [Google Scholar] [CrossRef]
- Falade, E.O.; Mu, T.-H.; Zhang, M. Improvement of ultrasound microwave-assisted enzymatic production and high hydrostatic pressure on emulsifying, rheological and interfacial characteristics of sweet potato protein hydrolysates. Food Hydrocoll. 2021, 117, 106684. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.J.; Chi, Z.P.; Guo, Z.W.; Zhang, L.P. Mung bean protein hydrolysate modulates the immune response through NF-κB pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Food Sci. 2019, 84, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Kang, H.A.; Cominguez, D.C.; Kim, S.H.; An, H.J. Papain ameliorates lipid accumulation and inflammation in high-fat diet-induced obesity mice and 3T3-L1 adipocytes via AMPK activation. Int. J. Mol. Sci. 2021, 22, 9885. [Google Scholar] [CrossRef]
- Qu, T.; He, S.; Ni, C.; Wu, Y.; Xu, Z.; Chen, M.L.; Li, H.; Cheng, Y.; Wen, L. In vitro anti-inflammatory activity of three peptides derived from the byproduct of rice processing. Plant Food Hum. Nutr. 2022, 77, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Lu, Y.L.; Han, C.H.; Hou, W.C.; Aluko, R.E. Flaxseed protein-derived peptide fractions: Antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem. 2009, 116, 277–284. [Google Scholar] [CrossRef]
- Millan-Linares, M.D.; Yust, M.D.; Alcaide-Hidalgo, J.M.; Millan, F.; Pedroche, J. Lupine protein hydrolysates inhibit enzymes involved in the inflammatory pathway. Food Chem. 2014, 151, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Toldra, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Zenezini Chiozzi, R.; Lagana, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milan-Noris, A.K.; Luna-Vital, D.A.; Milan-Carrillo, J.; Montoya-Rodriguez, A. Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef]
- Agyei, D.; Clarence, M.O.; Chan, Y.W.; Alan, S.C.; Michael, K.D. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and their applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffuler, P.; Bhullar, K.S.; Zani, S.C.D.; Wu, J.P. Bioactive peptides: From basic research to clinical trials and commercialization. J. Agric. Food Chem. 2022, 70, 3585–3595. [Google Scholar] [CrossRef] [PubMed]

| Amino Acid Composition Characteristics | Peptide Sequences | Source | Models | Signaling Pathways | Inhibition of Pro-Inflammatory Factors | References |
|---|---|---|---|---|---|---|
| High hydrophobic amino acid content | WVSPLAGRT, IGFLIIWV | Hempseed | HepG2 cells | NF-κB | NO, iNOS | [40] |
| VLER, WVGK, VVRP, VLLF, VALVR, LFGK, FGPK | Millet bran | RAW264.7 cells | MAPK; NF-κB | TNF-α, IL-1β, PGE2 | [20] | |
| ALLLQAVQSQYEEK | Brown rice | RAW264.7 cells | MAPK; NF-κB | IL-6, IL-1β, TNF-α, iNOS, COX-2 | [41] | |
| LPF | Walnut | RAW264.7 cells | NF-κB | iNOS, COX-2, TNF-α, NO | [42] | |
| NSPGPHDVALDQ, RMVLPEYELLYE | Chia seed | RAW264.7 cells | NF-κB | iNOS, NO, PGE2, TNF-α | [43] | |
| LPF, GVYY, APTLW | Walnut | BV-2 cells | - | TNF-α, IL-1β, IL-6 | [44] | |
| PFLF, IALLIPF | Millet | RAW264.7 cells | MAPK; NF-κB | IL-6, TNF-α, NO, IL-β | [31] | |
| High positively charged amino acids content | LAEQAER, VECTIPK, DAYVGDEAQSK, MASLALK | Green tea | HK-2 cells | NF-κB | iNOS, TNF-α | [45] |
| WEKPPVSH | Walnut | BV-2 cells | MAPK; NF-κB | TNF-α, IL-6, IL-1β, iNOS, COX-2 | [32] | |
| KLRSRNLLHPT, TNGRHSAKKH | Bee pollen | RAW264.7 cells | - | COX-2, IL-6, iNOS, TNF-α | [34] | |
| KHNRGDEF | Rice bran | D-gal-treated mice | NF-κB | - | [46] | |
| WSREEQEREE, ADIYTEEAGR | Walnut | UV- induced mice | NF-κB | IL-1β, IL-6 | [47] | |
| High specific amino acids content | QLPY, EYPSIQ, LTDPAAS, LPVGPQ, LLPSSQ | Corn | CCl4- induced mice | PI3K/Akt; NF-κB | - | [30] |
| KQSESHFVDAQPEQQQR | Adzuki bean | RAW264.7 cells | NF-κB | IL-1, IL-6, TNF-α, MCP-1 | [26] | |
| IQDKEGIPPDQQR | Lupin | RAW264.7 cells | MAPK | TNF-α, IL-1, IL-6, MCP-1 | [29] | |
| YFVP, SGRDP, MVWGP, TGSYTEGWS | Sunflower | THP-1 cells | NF-κB | IL-1β | [48] | |
| YDWPGGRN | Wheat germ | RAW 264.7 cells | NF-κB | NO, IL-1β, IL-6, TNF-α | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Chen, X.; Li, H.; Zhang, J.; An, J.; Liu, X. Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods 2022, 11, 2361. https://doi.org/10.3390/foods11152361
Liu W, Chen X, Li H, Zhang J, An J, Liu X. Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods. 2022; 11(15):2361. https://doi.org/10.3390/foods11152361
Chicago/Turabian StyleLiu, Wanlu, Xinwei Chen, He Li, Jian Zhang, Jiulong An, and Xinqi Liu. 2022. "Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review" Foods 11, no. 15: 2361. https://doi.org/10.3390/foods11152361
APA StyleLiu, W., Chen, X., Li, H., Zhang, J., An, J., & Liu, X. (2022). Anti-Inflammatory Function of Plant-Derived Bioactive Peptides: A Review. Foods, 11(15), 2361. https://doi.org/10.3390/foods11152361






