A Comparative Study on Microbiological and Chemical Characteristics of Small Ruminant Carcasses from Abattoirs in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abattoirs and Carcasses Evaluated
2.2. Microbiological Assay
2.3. Physicochemical Characterization
2.4. Statistical Analysis
3. Results
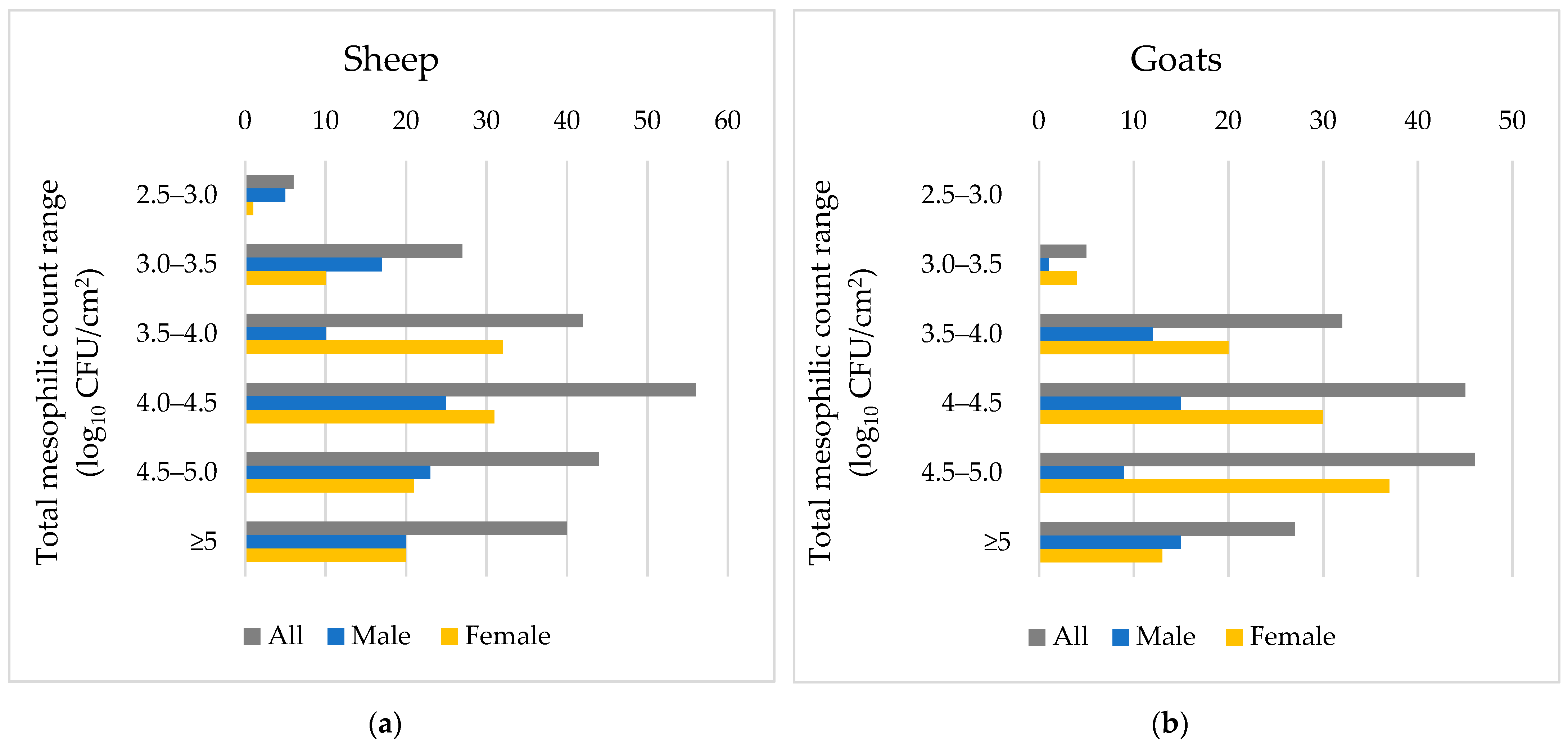
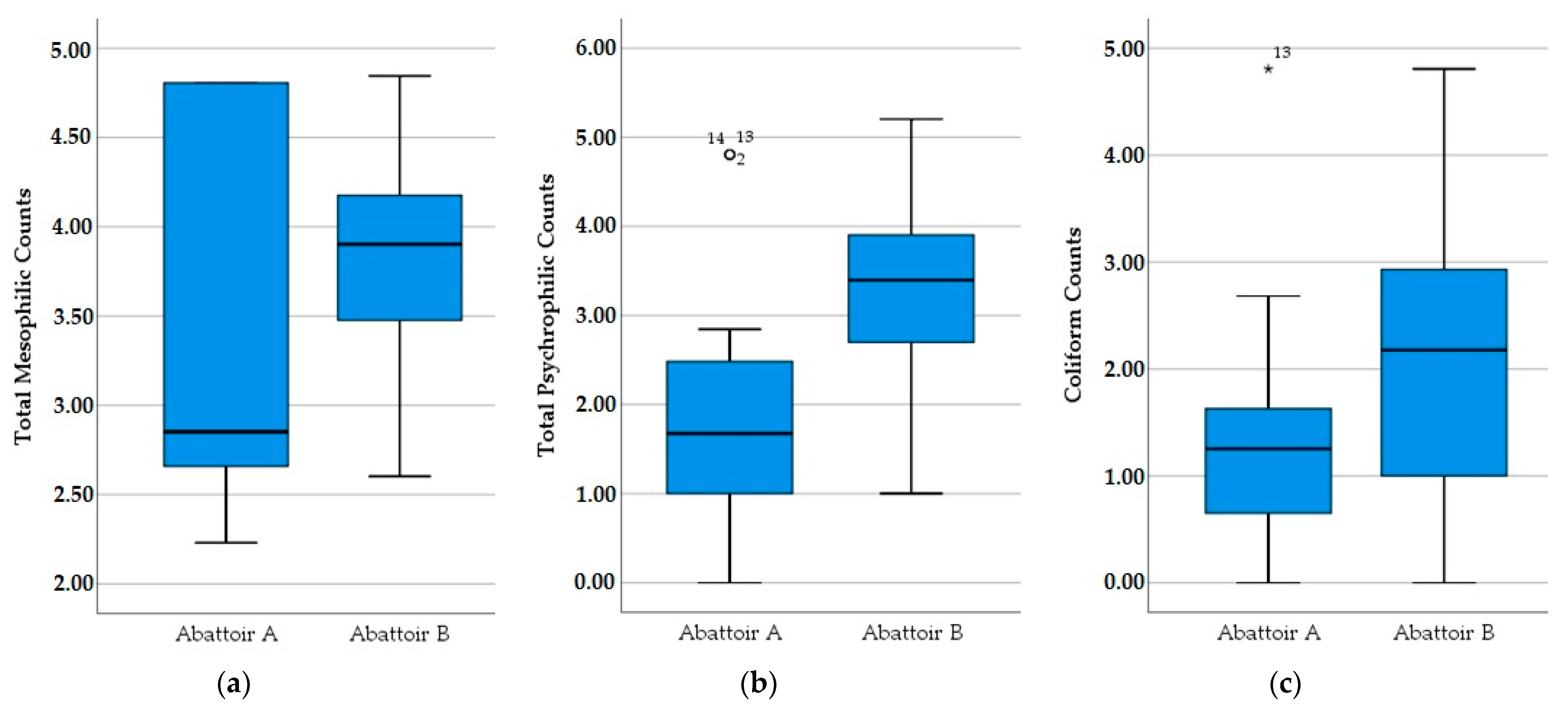
3.1. Microbiological Results
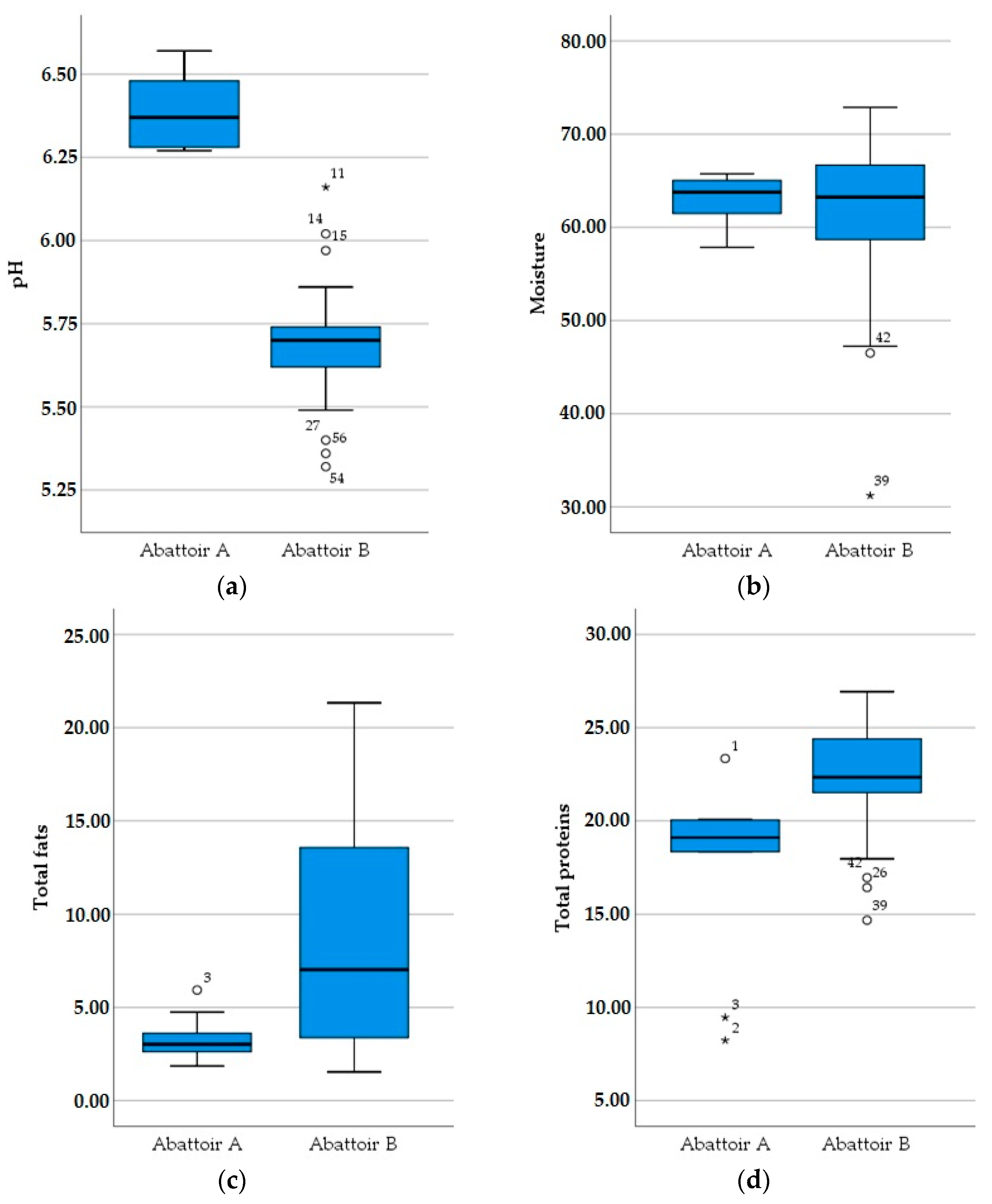
3.2. Physicochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, E.C.; Casey, N.H.; Simela, L. Goat meat quality. Small Rumin. Res. 2005, 60, 153–166. [Google Scholar] [CrossRef]
- Júnior, D.M.D.L.; De Carvalho, F.F.R.; Da Silva, F.J.S.; Rangel, A.H.N.; Novaes, L.P.; Difante, G.S. Intrinsic factors affecting sheep meat quality: A review. Rev. Colomb. Cienc. Pecu. 2016, 29, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Tsitsos, A.; Economou, V.; Arsenos, G.; Kalitsis, T.; Argyriadou, A.; Theodoridis, A. Greek and European consumer behaviour towards beef, lamb and mutton meat safety and quality: A review. Int. J. Agric. Resour. Gov. Ecol. 2021, 17, 414–431. [Google Scholar] [CrossRef]
- Elmasry, G.; Barbin, D.F.; Sun, D.-W.; Allen, P. Meat quality evaluation by hyperspectral imaging technique: An overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Skapetas, B.; Sinapis, E.; Hatziminaouglou, J.; Karalazos, A.; Katanos, J. Effect of age at slaughter on carcass characteristics and carcass composition in lambs of mountain Greek breeds. Czech. J. Anim. Sci. 2006, 51, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Arsenos, G.; Banos, G.; Fortomaris, P.; Katsaounis, N.; Stamataris, C.; Tsaras, L.; Zygoyiannis, D. Eating quality of lamb meat: Effects of breed, sex, degree of maturity and nutritional management. Meat Sci. 2002, 60, 379–387. [Google Scholar] [CrossRef]
- Mandolesi, S.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. Motivations and barriers for sheep and goat meat consumption in Europe: A means-end chain study. Animals 2020, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, A.; Vouraki, S.; Morin, E.; Rupérez, L.R.; Davis, C.; Arsenos, G. Efficiency Analysis as a Tool for Revealing Best Practices and Innovations: The Case of the Sheep Meat Sector in Europe. Animals 2021, 11, 3242. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science+Business Media: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Garcia-Lopez, M.L.; Prieto, M.; Otero, A. The Physiological Attributes of Gram-Negative Bacteria Associated with Spoilage of Meat and Meat Products. In Microbiology of Meat and Poultry; Davies, A.R., Board, R.J., Board, R.G., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; pp. 1–34. [Google Scholar]
- Ayaz, N.D.; Onaran, B.; Cufaoglu, G.; Goncuoglu, M.; Ormanci, F.S.; Erol, I. Prevalence and characterization of Listeria monocytogenes isolated from beef and sheep carcasses in Turkey with characterization of locally isolated listeriophages as a control measure. J. Food Prot. 2018, 81, 2045–2053. [Google Scholar] [CrossRef]
- Bhoomika; Shakya, S.; Patyal, A.; Gade, N.E. Occurrence and characteristics of extended-spectrum β-lactamases producing Escherichia coli in foods of animal origin and human clinical samples in Chhattisgarh, India. Vet. World 2016, 9, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.W.; Agga, G.E.; Bosilevac, J.M.; Brichta-Harhay, D.M.; Shackelford, S.D.; Wang, R.; Wheeler, T.L.; Arthur, T.M. Occurrence of antimicrobial-resistant Escherichia coli and Salmonella enterica in the beef cattle production and processing continuum. Appl. Environ. Microbiol. 2015, 81, 713–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, L.M.; Gilmour, A. Incidence of Listeria spp. and Listeria monocytogenes in a poultry processing environment and in poultry products and their rapid confirmation by multiplex PCR. Appl. Environ. Microbiol. 1994, 60, 4600–4604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011, 9, 2322. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2021. [Google Scholar]
- Bhandare, S.G.; Sherikar, A.T.; Paturkar, A.M.; Waskar, V.S.; Zende, R.J. A comparison of microbial contamination on sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food Control 2007, 18, 854–858. [Google Scholar] [CrossRef]
- Tanganyika, J.; Mfitilodze, W.M.; Mtimuni, J.P.; Phoya, R.R.K.D. Microbial quality of goat carcasses in Lilongwe, Malawi. Chem. Biol. Technol. Agric. 2017, 4, 27. [Google Scholar] [CrossRef]
- Yalçin, S.; Nizamlioglu, M.; Gürbüz, Ü. Microbiological conditions of sheep carcasses during the slaughtering process. J. Food Saf. 2004, 24, 87–93. [Google Scholar] [CrossRef]
- Zweifel, C.; Stephan, R. Microbiological monitoring of sheep carcass contamination in three Swiss abattoirs. J. Food Prot. 2003, 66, 946–952. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Arthur, T.M.; Bosilevac, J.M.; Guerini, M.; Shackelford, S.D.; Wheeler, T.L. Post-harvest interventions to reduce/eliminate pathogens in beef. Meat Sci. 2005, 71, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Aynewa, D.; Gizaw, Z.; Haile, A.F. Assessment of bacteriological quality of sheep carcasses, effect level of 2.5% citric acid spray on bacterial contamination of meat, and hygiene practices of workers in a selected abattoir in Debrezeit town, central Ethiopia. Environ. Health Insights 2021, 15, 11786302211037555. [Google Scholar] [CrossRef]
- Sierra, M.L.; Sheridan, J.J.; McGuire, L. Microbial quality of lamb carcasses during processing and the acridine orange direct count technique (a modified DEFT) for rapid enumeration of total viable counts. Int. J. Food Microbiol. 1997, 36, 61–67. [Google Scholar] [CrossRef]
- Milios, K.; Mataragas, M.; Pantouvakis, A.; Drosinos, E.H.; Zoiopoulos, P.E. Evaluation of control over the microbiological contamination of carcasses in a lamb carcass dressing process operated with or without pasteurizing treatment. Int. J. Food Microbiol. 2011, 146, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Røssvoll, E.; Røtterud, O.J.; Hauge, S.J.; Alvseike, O. A comparison of two evisceration methods on hygienic quality in the pelvic area of sheep carcasses. Meat Sci. 2018, 137, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Salmela, S.P.; Fredriksson-Ahomaa, M.; Hatakka, M.; Nevas, M. Microbiological contamination of sheep carcasses in Finland by excision and swabbing sampling. Food Control 2013, 31, 372–378. [Google Scholar] [CrossRef]
- Kim, C.; Stein, R.A.; Pao, S. Comparison of the microbial quality of lamb and goat meat acquired from internet and local retail markets. J. Food Prot. 2015, 78, 1980–1987. [Google Scholar] [CrossRef]
- Ahmad, M.U.D.; Sarwar, A.; Najeeb, M.I.; Nawaz, M.; Anjum, A.A.; Ali, M.A.; Mansur, N. Assessment of microbial load of raw meat at abattoirs and retail outlets. J. Anim. Plant Sci. 2013, 23, 745–748. [Google Scholar]
- Clauss, M.; Lechner-Doll, M. Differences in selective reticulo-ruminal particle retention as a key factor in ruminant diversification. Oecologia 2001, 129, 321–327. [Google Scholar] [CrossRef]
- Ivanovic, S.; Baltic, M.Z.; Nesic, K.; Zujovic, M.; Ivanovic, J.; Vukovic, S. Superficial bacterial contamination of goats carcasses. J. Pure Appl. Microbiol. 2014, 8, 101–108. [Google Scholar]
- Okraszewska-Lasica, W.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. Airborne Salmonella and Listeria associated with Irish commercial beef, sheep and pig plants. Meat Sci. 2014, 97, 255–261. [Google Scholar] [CrossRef]
- Lotfollahi, L.; Chaharbalesh, A.; Rezaee, M.A.; Hasani, A. Prevalence, antimicrobial susceptibility and multiplex PCR-serotyping of Listeria monocytogenes isolated from humans, foods and livestock in Iran. Microb. Pathog. 2017, 107, 425–429. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlaw, N.A.; Keelara, S.; Correa, M.; Foster, D.; Gebreyes, W.; Aidara-Kane, A.; Harden, L.; Thakur, S.; Fedorka-Cray, P.J. Evidence of sheep and abattoir environment as important reservoirs of multidrug resistant Salmonella and extended-spectrum beta-lactamase Escherichia coli. Int. J. Food Microbiol. 2022, 363, 109516. [Google Scholar] [CrossRef] [PubMed]
- Gozi, K.S.; Deus Ajude, L.P.T.; Barroso, M.D.V.; Silva, C.R.D.; Peiró, J.R.; Mendes, L.C.N.; Nogueira, M.C.L.; Casella, T. Potentially pathogenic multidrug-resistant Escherichia coli in lamb meat. Microb. Drug Resist. 2021, 27, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Shija, D.S.; Mtenga, L.A.; Kimambo, A.E.; Laswai, G.H.; Mushi, D.E.; Mgheni, D.M.; Mwilawa, A.J.; Shirima, E.J.; Safari, J.G. Chemical composition and meat quality attributes of indigenous sheep and goats from traditional production system in Tanzania. Asian-Australas J. Anim. Sci. 2013, 26, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsaounis, N.; Zygogiannis, D.; Giannakopoulos, A.; Kalogianni, E.; Stamataris, K.; Arsenos, G.; Sossidou, E.; Tsiaras, L. Production of “Heavy” Carcass Lambs from Indigenous Greek Dairy Breeds. Aristotle University Publishing Services: Thessaloniki, Greece, 1996. (In Greek) [Google Scholar]
- Arsenos, G.; Fortomaris, P.; Papadopoulos, E.; Sotiraki, S.; Stamataris, C.; Zygoyiannis, D. Growth and meat quality of kids of indigenous Greek goats (Capra prisca) as influenced by dietary protein and gastrointestinal nematode challenge. Meat Sci. 2009, 82, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kannan, G.; Eega, K.R.; Kouakou, B.; Getz, W.R. Nutritional and quality characteristics of meat from goats and lambs finished under identical dietary regime. Small Rumin. Res. 2008, 74, 255–259. [Google Scholar] [CrossRef]
- Okuskhanova, E.; Rebezov, M.; Yessimbekov, Z.; Suychinov, A.; Semenova, N.; Rebezov, Y.; Gorelik, O.; Zinina, O. Study of water binding capacity, pH, chemical composition and microstructure of livestock meat and poultry. Annu. Res. Rev. Biol. 2017, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Goetsch, A.L.; Merkel, R.C.; Gipson, T.A. Factors affecting goat meat production and quality. Small Rumin. Res. 2011, 101, 173–181. [Google Scholar] [CrossRef]
- Rudy, M. The analysis of correlations between the age and the level of bioaccumulation of heavy metals in tissues and the chemical composition of sheep meat from the region in SE Poland. Food Chem. Toxicol. 2009, 47, 1117–1122. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, M.; Rajput, I.R.; Faraz, S.; Rao, S.; Umer, M.; Devrajani, K. Effect of slaughtering age on chemical composition of goat meat. Pak. J. Nutr. 2010, 9, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Junkuszew, A.; Nazar, P.; Milerski, M.; Margetin, M.; Brodzki, P.; Bazewicz, K. Chemical composition and fatty acid content in lamb and adult sheep meat. Arch. Anim. Breed. 2020, 63, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.S.; De Araújo, W.O.; De Sousa, W.H.; Cézar, M.F.; Galvão, M.D.S.; Cunha, M.D.G.G. Effect of genotype and sex on chemical composition and fatty acid profile of sheep meat. [Efeito do genótipo e do sexo sobre a composição química e o perfil de ácidos graxos da carne de cordeiros]. Rev. Bras. Zootec. 2006, 35 (Suppl. S4), 1838–1844. [Google Scholar] [CrossRef] [Green Version]
- Montossi, F.; Font-i-Furnols, M.; del Campo, M.; San Juliána, R.; Britoa, G.; Sañudo, C. Sustainable sheep production and consumer preference trends: Compatibilities, contradictions, and unresolved dilemmas. Meat Sci. 2013, 95, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Ådnøy, T.; Haug, A.; Sørheim, O.; Thomassen, M.S.; Varszegi, Z.; Eik, L.O. Grazing on mountain pastures-does it affect meat quality in lambs? Livest. Prod. Sci. 2005, 94, 25–31. [Google Scholar] [CrossRef]
- Theriez, M.; Touraine, B.; Vigneron, P.; Prud’hon, M. Effects of indoor or outdoor rearing on the chemical composition of lambs. Anim. Sci. 1992, 54, 389–393. [Google Scholar] [CrossRef]
- Parrini, S.; Sirtori, F.; Acciaioli, A.; Becciolini, V.; Crovetti, A.; Bonelli, A.; Franci, O.; Bozzi, R. Effect of farming system on meat traits of native Massese suckling lamb. Ital. J. Anim. Sci. 2021, 20, 71–83. [Google Scholar] [CrossRef]
| Species | Gender | Age | Total | |||
|---|---|---|---|---|---|---|
| Male | Female | <35% | 35–70% | >70% | ||
| Sheep | 100 | 115 | 86 | 36 | 93 | 215 |
| Goat | 51 | 104 | 51 | 21 | 83 | 155 |
| Total | 151 | 219 | 137 | 57 | 176 | 370 |
| Gene | Primers | Product (bp) | Target |
|---|---|---|---|
| prs | For: GCTGAAGAGATTGCGAAAGAAG | 370 | All Listeria species |
| Rev: CAAAGAAACCTTGGATTTGCG | |||
| ORF2819 | For: AGCAAAATGCCAAAACTCGT | 471 | Serovars 1/2b, 3b, 4b, 4d and 4e |
| Rev: CATCACTAAAGCCTCCCATTG | |||
| ORF2110 | For: AGTGGACAATTGATTGGTGAA | 597 | Serovars 4b, 4d and 4e |
| Rev: CATCCATCCCTTACTTTGGAC | |||
| lmo0737 | For: AGGGCTTCAAGGACTTACCC | 691 | Serovars 1/2a, 1/2c, 3a and 3c |
| Rev: ACGATTTCTGCTTGCCATTC | |||
| lmo1118 | For: AGGGGTCTTAAATCCTGGAA | 906 | Serovars 1/2c and 3c |
| Rev: CGGCTTGTTCGGCATACTTA |
| TMVC | TPVC | Coliforms | |||
|---|---|---|---|---|---|
| Sheep | Gender | Male | 3.75 (0.76) | 2.92 (1.24) | 2.08 (1.27) |
| Female | 3.77 (0.63) | 3.01 (0.78) | 1.56 (1.27) | ||
| Age | <35% | 3.75 (0.82) | 2.66 (1.13) | 1.89 (1.22) | |
| 35–70% | 3.63 (0.63) | 3.04 (0.96) | 2.04 (1.11) | ||
| >70% | 3.81 (0.58) | 3.22 (0.85) | 1.64 (1.42) | ||
| Total | 3.76 (0.69) | 2.97 (1.02) | 1.80 (1.30) | ||
| Goat | Gender | Male | 3.97 (0.60) | 3.12 (1.05) | 2.66 (1.44) |
| Female | 3.90 (0.52) | 3.42 (0.86) | 1.90 (1.20) | ||
| Age | <35% | 4.17 (0.52) | 3.63 (0.92) | 3.17 (1.21) | |
| 35–70% | 4.13 (0.33) | 3.72 (0.48) | 2.43 (0.77) | ||
| >70% | 3.72 (0.53) | 3.03 (0.94) | 1.45 (1.45) | ||
| Total | 3.92 (0.55) | 3.32 (0.94) | 2.15 (1.33) |
| pH | Moisture | Total Fat | Total Proteins | |||
|---|---|---|---|---|---|---|
| Sheep | Gender | Male | 5.89 (0.38) | 63.27% (4.78) | 5.98% (4.5) | 20.73% (4.39) |
| Female | 5.77 (0.24) | 60.19% (9.40) | 8.50% (6.46) | 21.92% (3.32) | ||
| Age | <35% | 5.86 (0.33) | 62.75% (4.98) | 6.41% (4.93) | 20.22% (4.53) | |
| 35–70% | 5.95 (0.41) | 60.85% (3.96) | 8.10% (6.05) | 21.25% (3.16) | ||
| >70% | 5.74 (0.25) | 61.15% (10.56) | 7.63% (6.32) | 22.48% (3.32) | ||
| Total | 5.83 (0.32) | 61.76% (7.48) | 7.21% (5.63) | 21.31% (3.90) | ||
| Goat | Gender | Male | 5.71 (0.10) | 63.27% (3.59) | 5.59% (4.12) | 24.29% (2.72) |
| Female | 5.68 (0.15) | 64.60% (2.41) | 5.83% (2.17) | 23.82% (1.73) | ||
| Age | <35% | 5.67 (0.12) | 64.49% (3.34) | 5.36% (4.11) | 24.68% (2.47) | |
| 35–70% | 5.70 (0.12) | 63.75% (3.10) | 5.68% (3.22) | 24.05% (2.26) | ||
| >70% | 5.73 (0.10) | 62.77% (2.78) | 6.17% (2.11) | 23.22% (1.13) | ||
| Total | 5.70 (0.11) | 63.80% (3.14) | 5.96% (3.38) | 24.10% (2.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsos, A.; Economou, V.; Chouliara, E.; Ambrosiadis, I.; Arsenos, G. A Comparative Study on Microbiological and Chemical Characteristics of Small Ruminant Carcasses from Abattoirs in Greece. Foods 2022, 11, 2370. https://doi.org/10.3390/foods11152370
Tsitsos A, Economou V, Chouliara E, Ambrosiadis I, Arsenos G. A Comparative Study on Microbiological and Chemical Characteristics of Small Ruminant Carcasses from Abattoirs in Greece. Foods. 2022; 11(15):2370. https://doi.org/10.3390/foods11152370
Chicago/Turabian StyleTsitsos, Anestis, Vangelis Economou, Eirini Chouliara, Ioannis Ambrosiadis, and Georgios Arsenos. 2022. "A Comparative Study on Microbiological and Chemical Characteristics of Small Ruminant Carcasses from Abattoirs in Greece" Foods 11, no. 15: 2370. https://doi.org/10.3390/foods11152370
APA StyleTsitsos, A., Economou, V., Chouliara, E., Ambrosiadis, I., & Arsenos, G. (2022). A Comparative Study on Microbiological and Chemical Characteristics of Small Ruminant Carcasses from Abattoirs in Greece. Foods, 11(15), 2370. https://doi.org/10.3390/foods11152370








