SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey and Plant Material
2.2. Extracts Preparation
2.3. Water Content
2.4. pH and Free Acidity
2.5. Conductivity
2.6. Color Analysis
2.7. A-Amylase Assay
2.8. Soluble Protein
2.9. Total Phenolic and Flavonoids Content
2.10. Antioxidant Capacity Assays
2.11. HPTLC Analyses
2.12. Protein Profiling by SDS-PAGE
2.13. Yeast Strain and Growth Conditions
2.14. Kinetics of the Yeast Growth Assay
2.15. Yeast Cell Viability
2.16. Bacteria Strain, Growth Condition, and Antibacterial Activity
2.17. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of Honey Quality
3.2. Protein Composition
3.3. HPTLC Analysis
3.4. Antioxidant Properties of Goldenrod Honey
3.5. Statistical Analysis
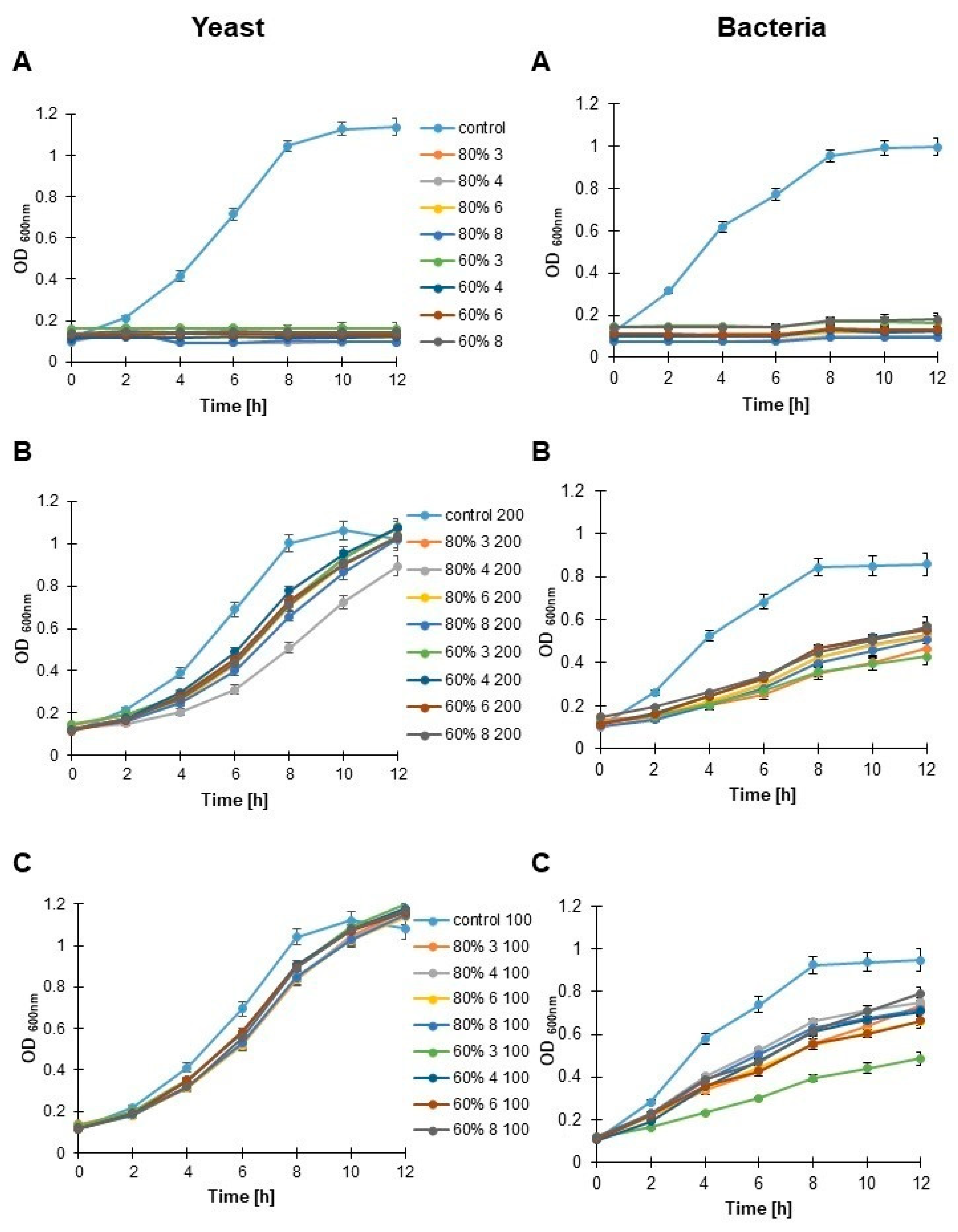
3.6. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Czigle, S.; Filep, R.; Balažová, E.; Szentgyörgyi, H.; Balázs, V.L.; Kocsis, M.; Purger, D.; Papp, N.; Farkas, Á. Antioxidant Capacity Determination of Hungarian-, Slovak-, and Polish-Origin Goldenrod Honeys. Plants 2022, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Popović, Z.; Mačukanović-Jocić, M.; Djurdjević, L.; Mijatović, M.; Karadžić, B.; Mitrović, M.; Pavlović, P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia). J. Ethnopharmacol. 2007, 111, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Michlewicz, M.; Dudek, M.; Tryjanowski, P. Invasive Canadian goldenrod (Solidago canadensis L.) as a preferred foraging habitat for spiders. Arthropod-Plant Interact. 2016, 10, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Bobul’ská, L.; Demková, L.; Čerevková, A.; Renčo, M. Invasive goldenrod (Solidago gigantea) influences soil microbial activities in forest and grassland ecosystems in central Europe. Diversity 2019, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Amtmann, M. The chemical relationship between the scent features of goldenrod (Solidago canadensis L.) flower and its unifloral honey. J. Food Compos. Anal. 2010, 23, 122–129. [Google Scholar] [CrossRef]
- Baroni, M.V.; Chiabrando, G.A.; Costa, C.; Wunderlin, D.A. Assessment of the floral origin of honey by SDS-page immunoblot techniques. J. Agric. Food Chem. 2002, 50, 1362–1367. [Google Scholar] [CrossRef]
- Nisbet, C.; Guler, A.; Ciftci, G.; Yarim, G.F. The Investigation of Protein Prophile of Different Botanic Origin Honey and Density Saccharose-Adulterated Honey by SDS-PAGE Method. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 443–446. [Google Scholar]
- Azevedo, M.S.; Valentim-Neto, P.A.; Seraglio, S.K.T.; da Luz, C.F.P.; Arisi, A.C.M.; Costa, A.C.O. Proteome comparison for discrimination between honeydew and floral honeys from botanical species Mimosa scabrella Bentham by principal component analysis. J. Sci. Food Agric. 2017, 97, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Borutinskaite, V.; Treigyte, G.; Čeksteryte, V.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J. Food Nutr. Res. 2018, 57, 57–69. [Google Scholar]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The comparison of physicochemical parameters, antioxidant activity and proteins for the raw local polish honeys and imported honey blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Matłok, N.; Kapusta, I.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Characterisation of some phytochemicals extracted from black elder (Sambucus nigra L.) flowers subjected to ozone treatment. Molecules 2021, 26, 5548. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2014/63/EU of the European Parliament and of the Council amending Council Directive2001/110/EC relating to honey. Off. J. Eur. Communities 2014, 57, L164/1. [Google Scholar]
- Rybak-Chmielewska, H.; Szczęsna, T.; Waś, E.; Jaśkiewicz, K.; Teper, D. Characteristics of Polish Unifloral Honeys IV. Honeydew Honey, Mainly Abies alba L. J. Apic. Sci. 2013, 57, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Bukowska, M.; Ligor, M.; Buszewski, B. Correlation study of honey regarding their physicochemical properties and sugars and cyclitols content. Molecules 2020, 25, 34. [Google Scholar] [CrossRef] [Green Version]
- Muresan, C.I.; Cornea-Cipcigan, M.; Suharoschi, R.; Erler, S.; Mărgăoan, R. Honey botanical origin and honey-specific protein pattern: Characterization of some European honeys. LWT 2021, 154, 112883. [Google Scholar] [CrossRef]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dzugan, M. Analysis of cytotoxicity of selected Asteraceae plant extracts in real time, their antioxidant properties and polyphenolic profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef] [PubMed]
- Avertseva, I.N.; Suleymanova, F.S.; Nesterova, O.V.; Reshetnyak, V.Y.; Matveenko, V.N.; Zhukov, P.A. Study of Polyphenolic Compounds in Extracts from Flowers and Leaves of Canadian Goldenrod and Dwarf Goldenrod (Solidago canadensis L. and Solidago nana Nitt.). Mosc. Univ. Chem. Bull. 2020, 75, 47–51. [Google Scholar] [CrossRef]
- Shelepova, O. Phytochemistry and Inflorescences Morphometry of Invasive Solidago L. (Goldenrods) Species-Valuable Late Autumn Mellifers. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 5, 209–214. [Google Scholar] [CrossRef]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and analyses of flavonoids and phenolic acids from canadian goldenrod and giant goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba-Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- Mietlińska, K.; Przybyt, M.; Kalemba, D. Polish plants as raw materials for cosmetic purposes. Biotechnol. Food Sci. 2019, 83, 95–106. [Google Scholar] [CrossRef]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant profiles of leaves and inflorescences of native, invasive and hybrid Solidago species. Ind. Crop. Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. Aktywność i stabilność termiczna diastazy występującej w Podkarpackich miodach odmianowych. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2017, 24, 103–112. [Google Scholar] [CrossRef]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; & Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2016, 24, 1255–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Efem, S.E.E.; Iwara, C.I. The antimicrobial spectrum of honey and its clinical significance. Infection 1992, 20, 227–229. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. 2019, 9, 18197. [Google Scholar] [CrossRef] [Green Version]
- Koc, A.N.; Silici, S.; Ercal, B.D.; Kasap, F.; Hörmet-Öz, H.T.; Mavus-Buldu, H. Antifungal Activity of Turkish Honey against Candida spp. and Trichosporon spp: An in vitro evaluation. Med. Mycol. 2009, 47, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Miłek, M.; Grabek-Lejko, D.; Stȩpień, K.; Sidor, E.; Mołoń, M.; Dżugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef]

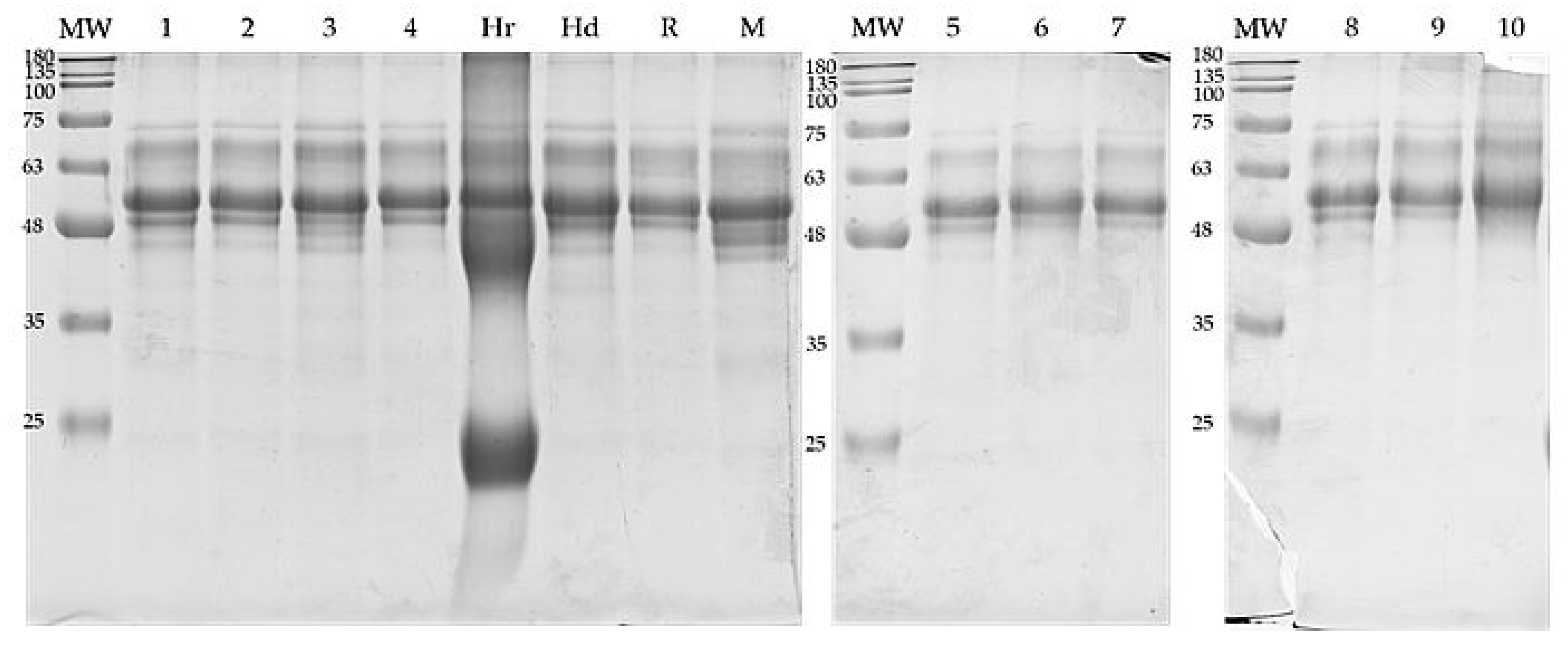

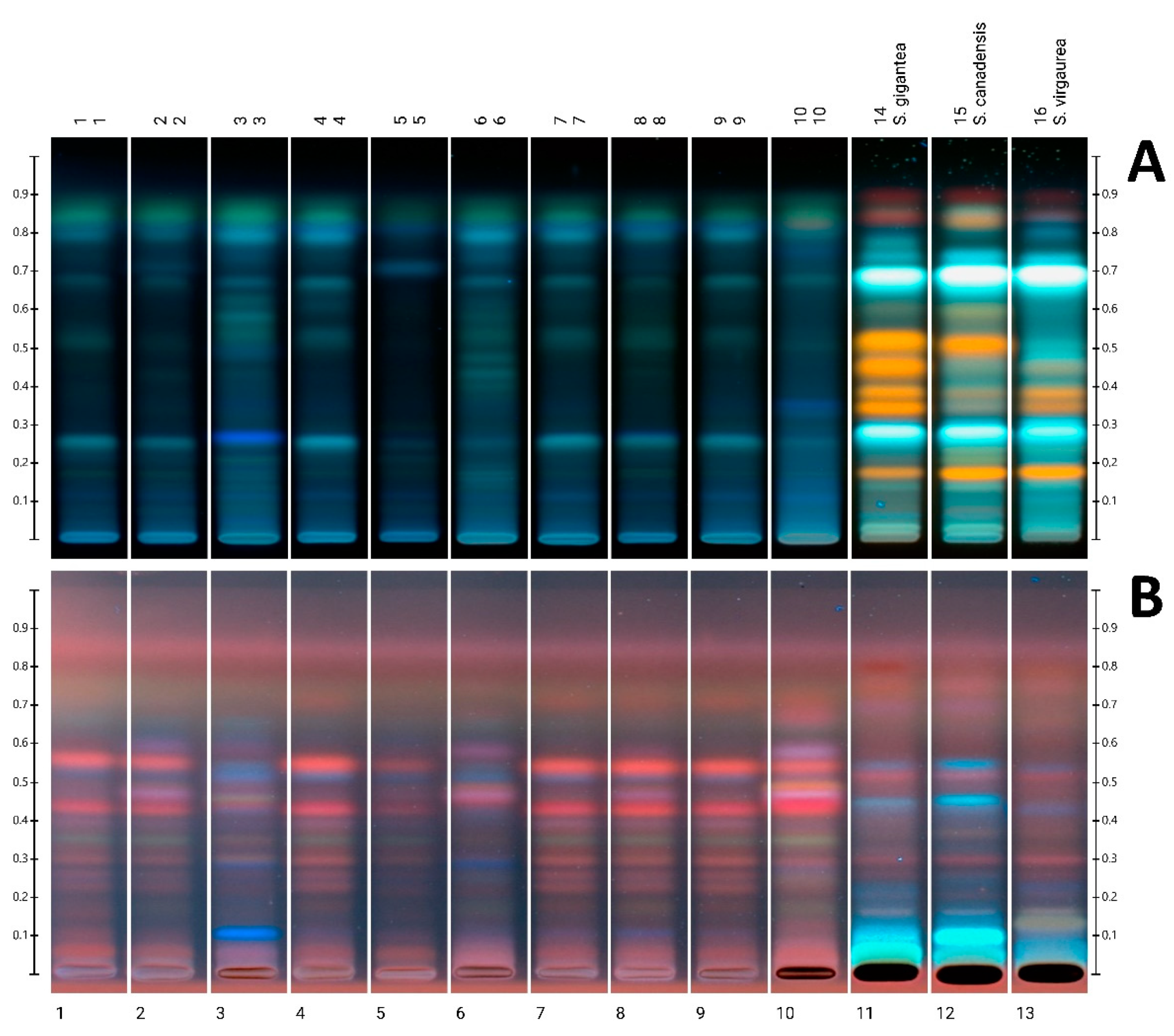
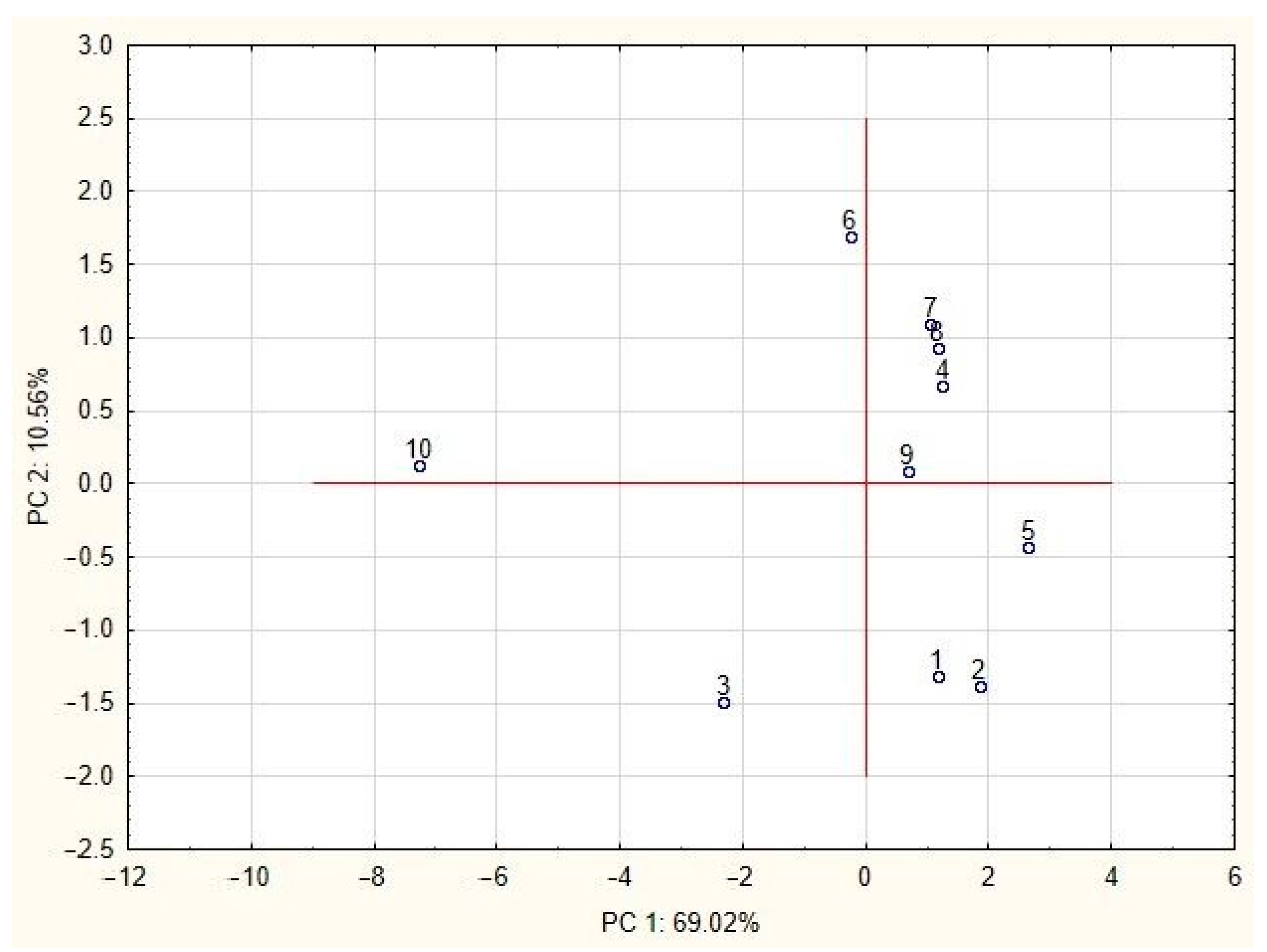
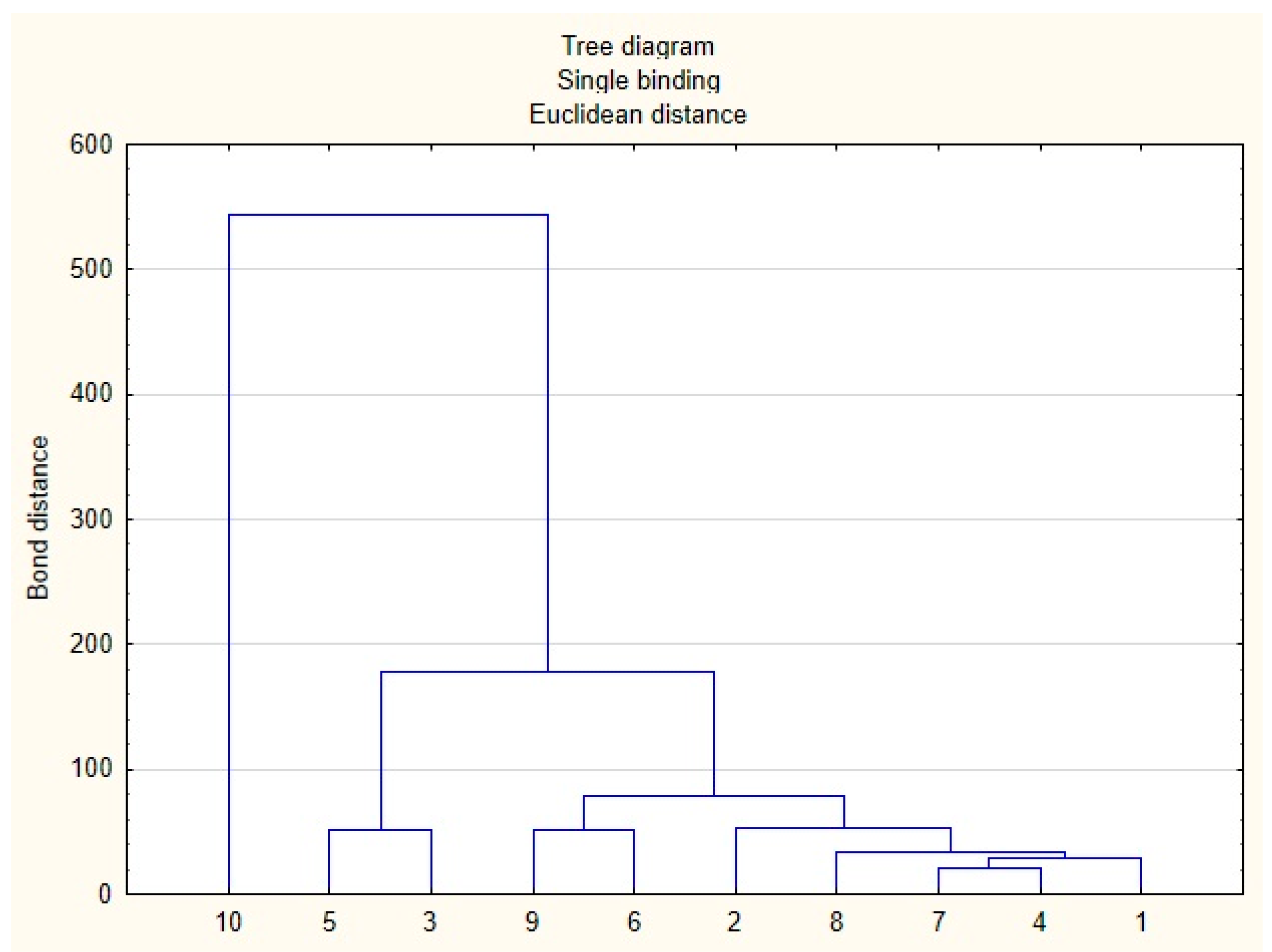

| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Soluble protein content [mg/100 g] | 38.11 ± 7.97 a | 22.68 ± 1.28 a | 38.91 ± 8.35 ab | 39.01 ± 8.98 ab | 35.39 ± 9.08 ac | 60.79 ± 11.55 bd | 51.72 ± 14.25 bc | 75.31 ± 8.98 d | 57.16 ± 6.42 bcd | 88.92 ± 10.27 d |
| Diastase number (DN) | 25.96 ± 0.42 a | 16.92 ± 1.15 b | 24.40 ± 0.68 a | 12.20 ± 0.85 cd | 17.68 ± 1.25 b | 10.68 ± 0.85 c | 11.41 ± 0.00 cd | 15.09 ± 0.42 be | 13.62 ± 0.42 de | 22.10 ± 0.85 f |
| Sample | TPC [mg GAE/100 g] | TFC [mg QE/100 g] | DPPH [μmol TE/100 g] | FRAP [μmol TE/100 g] | CUPRAC [μmol TE/100 g] |
|---|---|---|---|---|---|
| 1 | 17.36 ± 1.69 a | 0.31 ± 0.02 a | 22.81 ± 12.62 a | 19.74 ± 1.74 a | 302.47 ± 23.49 a |
| 2 | 18.06 ± 0.75 a | 0.26 ± 0.01 a | 24.66 ± 8.25 a | 21.93 ± 2.31 a | 379.37 ± 54.01 ac |
| 3 | 31.75 ± 0.91 b | 0.48 ± 0.02 b | 29.27 ± 16.94 a | 62.28 ± 2.74 b | 625.45 ± 46.99 b |
| 4 | 22.22 ± 1.89 c | 0.33 ± 0.01 ad | 25.58 ± 15.07 a | 33.11 ± 0.38 c | 333.23 ± 35.52 ac |
| 5 | 15.77 ± 1.95 a | 0.20 ± 0.03 c | 15.44 ± 12.45 a | 19.74 ± 3.29 a | 640.83 ± 38.71 b |
| 6 | 25.50 ± 1.20 cd | 0.36 ± 0.01 d | 31.34 ± 14.21 a | 46.93 ± 1.00 d | 435.76 ± 44.40 c |
| 7 | 21.83 ± 0.69 c | 0.34 ± 0.02 ad | 21.43 ± 2.39 a | 33.55 ± 0.66 c | 317.85 ± 8.88 ac |
| 8 | 18.65 ± 1.64 ac | 0.30 ± 0.01 a | 20.28 ± 4.17 a | 23.46 ± 1.00 a | 297.34 ± 49.44 a |
| 9 | 27.78 ± 0.45 d | 0.38 ± 0.02 d | 20.74 ± 2.07 a | 43.20 ± 1.00 d | 451.14 ± 72.68 c |
| 10 | 50.69 ± 0.69 e | 0.77 ± 0.01 e | 39.18 ± 2.88 a | 96.93 ± 3.38 e | 1168. 87 ± 30.76 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżugan, M.; Miłek, M.; Kielar, P.; Stępień, K.; Sidor, E.; Bocian, A. SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey. Foods 2022, 11, 2390. https://doi.org/10.3390/foods11162390
Dżugan M, Miłek M, Kielar P, Stępień K, Sidor E, Bocian A. SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey. Foods. 2022; 11(16):2390. https://doi.org/10.3390/foods11162390
Chicago/Turabian StyleDżugan, Małgorzata, Michał Miłek, Patrycja Kielar, Karolina Stępień, Ewelina Sidor, and Aleksandra Bocian. 2022. "SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey" Foods 11, no. 16: 2390. https://doi.org/10.3390/foods11162390
APA StyleDżugan, M., Miłek, M., Kielar, P., Stępień, K., Sidor, E., & Bocian, A. (2022). SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey. Foods, 11(16), 2390. https://doi.org/10.3390/foods11162390







