Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Reagents
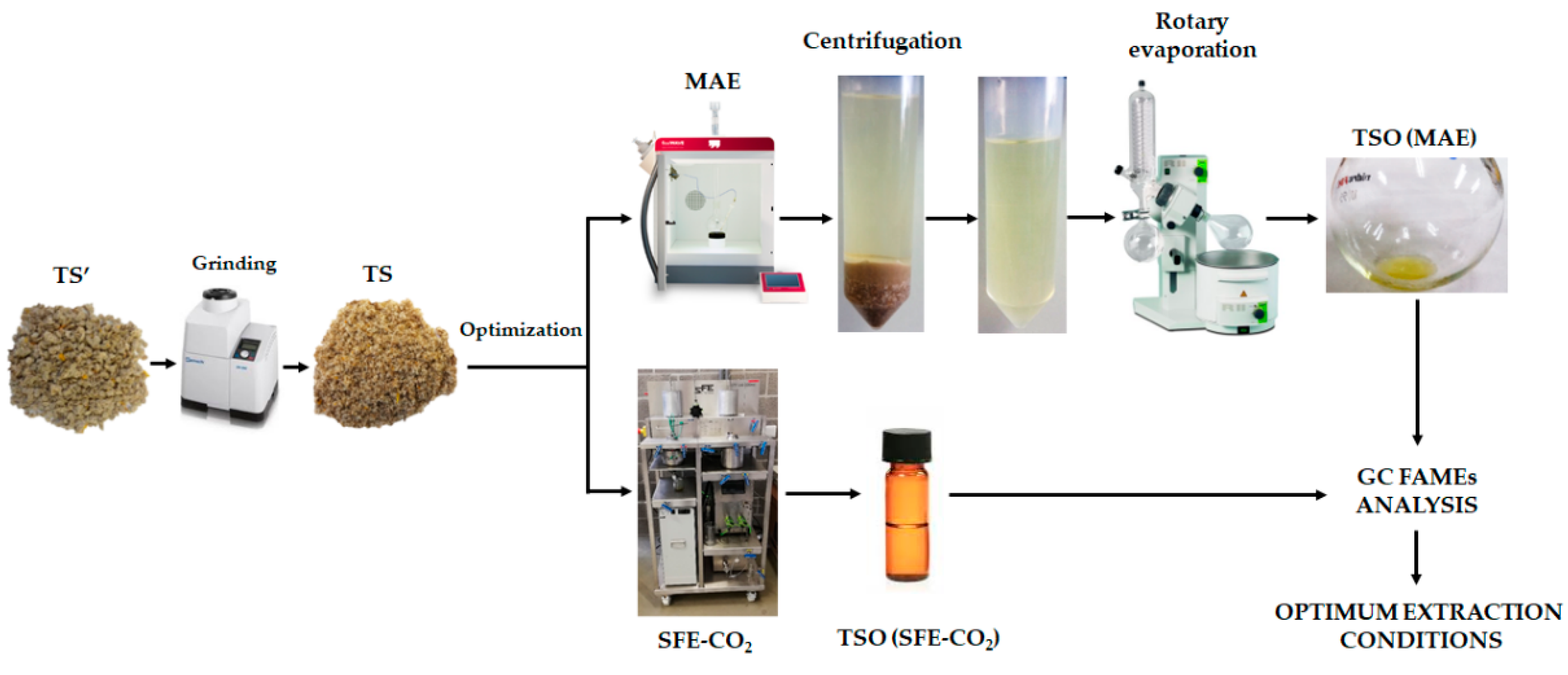
2.2. Microwave-Assisted Extraction (MAE)
2.3. Supercritical Fluid Extraction (SFE-CO2)
2.4. Box-Behnken Experimental Designs (BBD)
2.5. Tomato Seed Oil Characterization
2.5.1. Extraction Yield
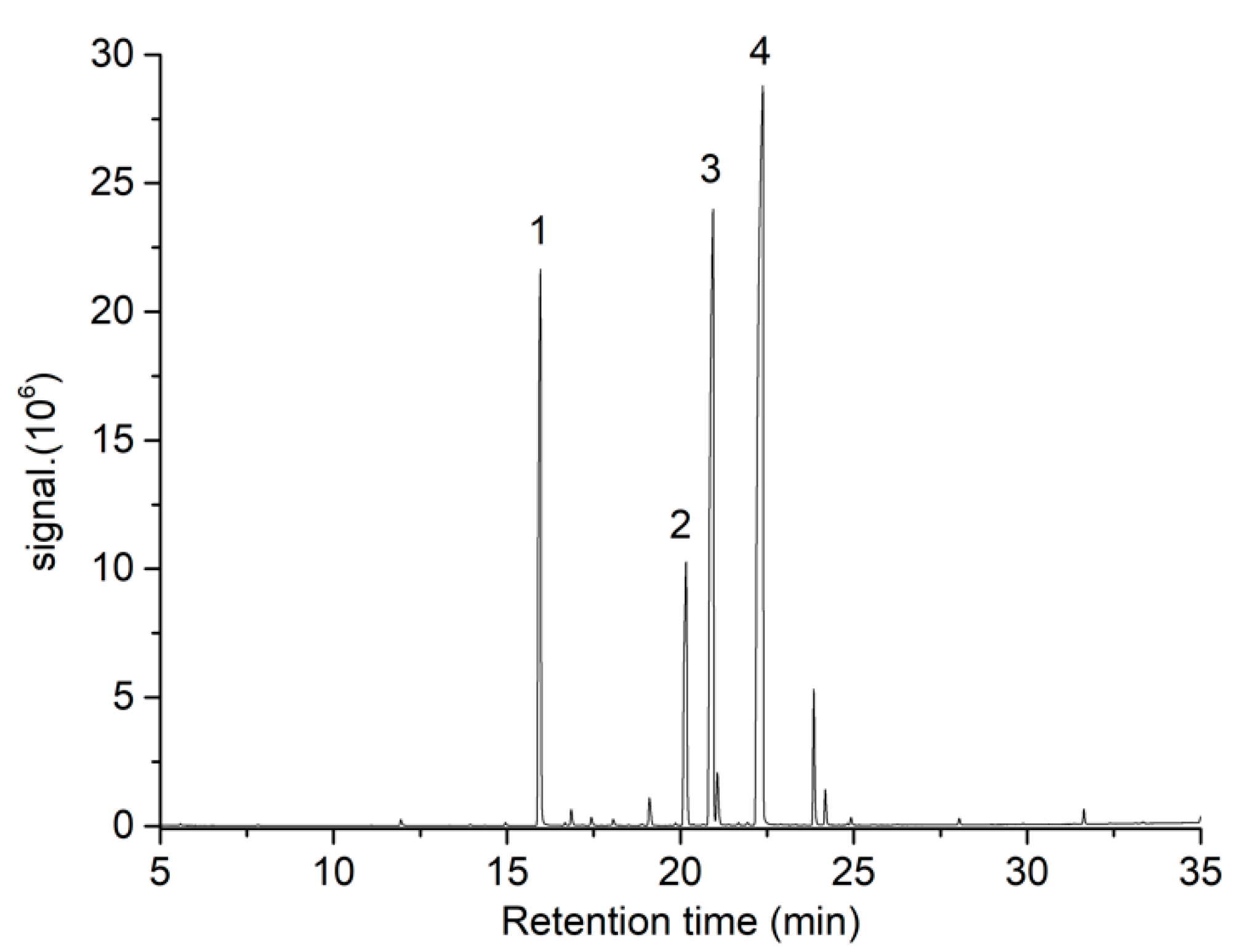
2.5.2. Fatty Acid Methyl Esters (FAMEs) Content by GC
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.4. Tocopherols Content by HPLC-DAD
2.5.5. Antioxidant Activity by DPPH• Radical Scavenging Method
2.6. Statistical Analysis
3. Results and Discussion
3.1. MAE Optimization
3.1.1. Model Fitting and Analysis
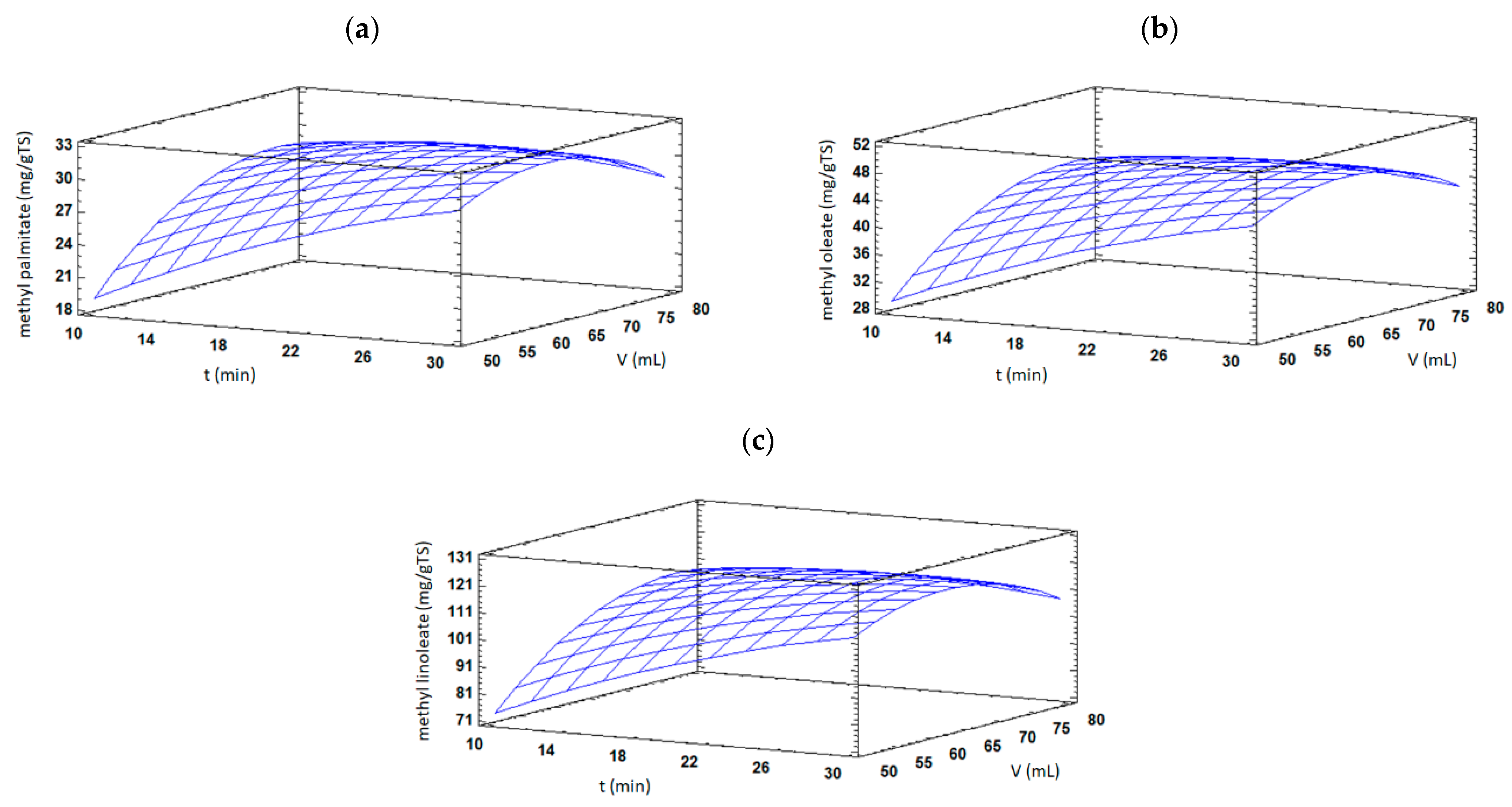
3.1.2. Effect of Extraction Variables on TSO Yield and FAMEs Content
3.1.3. Optimal Extraction Conditions and Verification Test
3.2. SFE-CO2 Optimization
3.2.1. Model Fitting and Analysis
3.2.2. Effect of Extraction Variables on TSO Extraction Yield and Optimal Extraction Conditions
3.3. Comparison of Extraction Techniques
| Source | C16:0 | C18:0 | C18:1 | C18:2 | Σ | Method | Ref. |
|---|---|---|---|---|---|---|---|
| TS | 14.83 | 5.87 | 22.59 | 56.71 | 100.0 | MAE | This study |
| TS | 16.50 | 5.70 | 20.30 | 50.20 | 92.7 | SC-CO2 | This study |
| TS | 12.97 | 5.74 | 25.71 | 51.90 | 96.3 | Soxhlet | [49] |
| TS | 14.42 | 3.95 | 17.88 | 61.73 | 98.0 | Stirring | [51] |
| TS | 17.08 | 5.97 | 23.64 | 49.70 | 96.4 | Stirring | [52] |
| TS | 13.81 | 5.53 | 23.50 | 52.99 | 95.8 | Soxhlet | [53] |
| TS | 18.47 | 0.51 | 20.89 | 56.81 | 96.7 | Semi-cont. Soxhlet | [54] |
| TS | 7.76 | 9.28 | 24.95 | 56.59 | 98.6 | CPE | [56] |
| TS | 7.98 | 6.86 | 25.29 | 57.77 | 97.9 | EAAE | [56] |
| TS | 18.80 | 7.40 | 23.10 | 44.80 | 94.1 | SC-CO2 | [46] |
| Pomace | 14.48 | 4.82 | 18.95 | 58.60 | 96.9 | SC-CO2 | [22] |
| TS | 12.26 | 5.15 | 22.1 | 56.12 | 95.6 | Soxhlet | [57] |
| TS | 16.81 | 7.34 | 27.16 | 48.69 | 100.0 | Vortex | [58] |
3.4. Characterization of TSO Obtained under Optimum MAE Conditions
3.4.1. ATR-FTIR Analysis
3.4.2. Tocopherols Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical Determination of Antioxidants in Tomato: Typical Components of the Mediterranean Diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Vázquez, J.A.; Ferreira, I.C.F.R. Optimization of Microwave-Assisted Extraction of Hydrophilic and Lipophilic Antioxidants from a Surplus Tomato Crop by Response Surface Methodology. Food Bioprod. Process. 2016, 98, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P.L.; Spigno, P.; Soressi, G.P.; Pietta, P.G. Polyphenol Pattern and Antioxidant Activity of Different Tomato Lines and Cultivars. Ann. Nutr. Metab. 2003, 47, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable Valorisation of Tomato Pomace: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A.; Ferreira de Oliveira, J.M.P.; Simões, S.; Ascenso, A. Sustainable Valorization of Tomato by-products to Obtain Bioactive Compounds: Their Potential in Inflammation and Cancer Management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Benítez, J.J.; Castillo, P.M.; del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of Tomato Processing by-products: Fatty Acid Extraction and Production of Bio-Based Materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef] [Green Version]
- Casa, M.; Miccio, M.; De Feo, G.; Paulillo, A.; Chirone, R.; Paulillo, D.; Lettieri, P.; Chirone, R. A Brief Overview on Valorization of Industrial Tomato by-products Using the Biorefinery Cascade Approach. Detritus 2021, 15, 31–39. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.J.B.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Green Emerging Extraction Technologies to Obtain High-Quality Vegetable Oils from Nuts: A Review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Rani, H.; Sharma, S.; Bala, M. Technologies for Extraction of Oil from Oilseeds and Other Plant Sources in Retrospect and Prospects: A Review. J. Food Process Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martínez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: New York, NY, USA, 2013; pp. 15–52. [Google Scholar]
- Picot-allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of Carotenoids from Tomato Processing by-Products—A Review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Aniceto, J.P.S.; Rodrigues, V.H.; Portugal, I.; Silva, C.M. Valorization of Tomato Residues by Supercritical Fluid Extraction. Processes 2021, 10, 28. [Google Scholar] [CrossRef]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and Analysis of Tomato Seed Oil. J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Romano, R.; Aiello, A.; Pizzolongo, F.; Rispoli, A.; De Luca, L.; Masi, P. Characterisation of Oleoresins Extracted from Tomato Waste by Liquid and Supercritical Carbon Dioxide. Int. J. Food Sci. Technol. 2020, 55, 3334–3342. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Technical and Economic Evaluation of Supercritical CO2 Extraction of Oil from Sucupira Branca Seeds. J. Supercrit. Fluids 2022, 181, 105494. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of Process Parameters for Supercritical Carbon Dioxide Extraction of Passiflora Seed Oil by Response Surface Methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rigane, K.; Ferrari, G. Extraction of Citrullus Colocynthis L. Seed Oil by Supercritical Carbon Dioxide Process Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Approaches. Ind. Crops Prod. 2020, 158, 113002. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Md Norodin, N.S.; Sarkawi, N.S.; Hamzah, M.H.S.; Mohd Nasir, H.; Abang Zaidel, D.N.; Che Yunus, M.A.; Md Salleh, L. Valorisation of Plant Seed as Natural Bioactive Compounds by Various Extraction Methods: A Review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Bilgiç-Keleş, S.; Şahin-Yeşilçubuk, N.; Barla-Demirkoz, A.; Karakaş, M. Response Surface Optimization and Modelling for Supercritical Carbon Dioxide Extraction of Echium Vulgare Seed Oil. J. Supercrit. Fluids 2019, 143, 365–369. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M.; Assadpoor, E.; Nowrouzieh, S.; Alishah, O. Optimization of Microwave-Assisted Extraction of Cottonseed Oil and Evaluation of Its Oxidative Stability and Physicochemical Properties. Food Chem. 2014, 160, 90–97. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Yao, X.-H.; Luo, M.; Zhao, C.; Fu, Y.-J. Optimization of Negative Pressure Cavitation–Microwave Assisted Extraction of Yellow Horn Seed Oil and Its Application on the Biodiesel Production. Fuel 2016, 166, 67–72. [Google Scholar] [CrossRef]
- Dursun Capar, T.; Dedebas, T.; Yalcin, H.; Ekici, L. Extraction Method Affects Seed Oil Yield, Composition, and Antioxidant Properties of European Cranberrybush (Viburnum Opulus). Ind. Crops Prod. 2021, 168, 113632. [Google Scholar] [CrossRef]
- Ibrahim, A.P.; Omilakin, R.O.; Betiku, E. Optimization of Microwave-Assisted Solvent Extraction of Non-Edible Sandbox (Hura Crepitans) Seed Oil: A Potential Biodiesel Feedstock. Renew. Energy 2019, 141, 349–358. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Solaberrieta, I.; Jiménez, A.; Garrigós, M.C. Valorization of Aloe Vera Skin by-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Vallecilla-Yepez, L.; Ciftci, O.N. Increasing Cis-Lycopene Content of the Oleoresin from Tomato Processing Byproducts Using Supercritical Carbon Dioxide. LWT 2018, 95, 354–360. [Google Scholar] [CrossRef]
- Lisichkov, K.; Kuvendziev, S.; Lisichkov, B. Isolation of Tomato Seed Oil from Tomato Waste by Application of Supercritical Fluid CO2 Extraction. Qual. Life 2011, 2, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Gliszczyńska-Świgło, A.; Sikorska, E. Simple Reversed-Phase Liquid Chromatography Method for Determination of Tocopherols in Edible Plant Oils. J. Chromatogr. A 2004, 1048, 195–198. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Z.-G.; Gai, Q.-Y.; Li, X.-J.; Wei, F.-Y.; Fu, Y.-J.; Ma, W. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Pumpkin Seeds and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef]
- Quaisie, J.; Ma, H.; Golly, M.K.; Tuly, J.A.; Amaglo, N.K.; Jiaqi, Z. Effect of Ultrasound-Microwave Irradiation Hybrid Technique on Extraction, Physicochemical, Antioxidative, and Structural Properties of Stearic Acid-Rich Allanblackia Parviflora Seed Oil. Chem. Pap. 2021, 75, 4527–4541. [Google Scholar] [CrossRef]
- Soroush, D.R.; Solaimanimehr, S.; Azizkhani, M.; Kenari, R.E.; Dehghan, B.; Mohammadi, G.; Sadeghi, E. Optimization of Microwave-Assisted Solvent Extraction of Hemp (Cannabis sativa L.) Seed Oil Using RSM: Evaluation of Oil Quality. J. Food Meas. Charact. 2021, 15, 5191–5202. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-Assisted Extraction of Hempseed Oil: Studying and Comparing of Fatty Acid Composition, Antioxidant Activity, Physiochemical and Thermal Properties with Soxhlet Extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Naghdi, F.G.; Thomas-Hall, S.R.; Durairatnam, R.; Pratt, S.; Schenk, P.M. Comparative Effects of Biomass Pre-Treatments for Direct and Indirect Transesterification to Enhance Microalgal Lipid Recovery. Front. Energy Res. 2014, 2, 57. [Google Scholar] [CrossRef]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.A.; Wali, A.; Aadil, R.M. Advances in Green Processing of Seed Oils Using Ultrasound-Assisted Extraction: A Review. J. Food Process. Preserv. 2020, 44, e14740. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; Hao, Q.; Gao, Y. Supercritical CO2 Extraction Optimization of Pomegranate (Punica granatum L.) Seed Oil Using Response Surface Methodology. LWT-Food Sci. Technol. 2009, 42, 1491–1495. [Google Scholar] [CrossRef]
- Lukic, I.; Milovanovic, S.; Pantic, M.; Srbljak, I.; Djuric, A.; Tadic, V.; Tyśkiewicz, K. Separation of High-Value Extracts from Silybum Marianum Seeds: Influence of Extraction Technique and Storage on Composition and Bioactivity. LWT 2022, 160, 113319. [Google Scholar] [CrossRef]
- Pavlić, B.; Pezo, L.; Marić, B.; Tukuljac, L.P.; Zeković, Z.; Solarov, M.B.; Teslić, N. Supercritical Fluid Extraction of Raspberry Seed Oil: Experiments and Modelling. J. Supercrit. Fluids 2020, 157, 104687. [Google Scholar] [CrossRef]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of Pomegranate, Tomato and Grapes: An Underestimated Source of Natural Bioactive Molecules and Antioxidants from Agri-Food by-Products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical Fluid Extraction of Lycopene from Tomato Processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef]
- Teslić, N.; Bojanić, N.; Čolović, D.; Fišteš, A.; Rakić, D.; Solarov, M.B.; Zeković, Z.; Pavlić, B. Conventional versus Novel Extraction Techniques for Wheat Germ Oil Recovery: Multi-Response Optimization of Supercritical Fluid Extraction. Sep. Sci. Technol. 2020, 56, 1546–1561. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical Composition of Tomato Seed Oil for an Edible Use: The Effect of Cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C. Tomato Seed Oil for Edible Use: Cold Break, Hot Break, and Harvest Year Effects. J. Food Process. Preserv. 2017, 41, e13309. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef]
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards Sustainable Management of Tomato Pomace through the Recovery of Valuable Compounds and Sequential Production of Low-Cost Biosorbent. Environ. Sci. Pollut. Res. 2020, 27, 39402–39412. [Google Scholar] [CrossRef] [PubMed]
- Ouatmani, T.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Mehidi-Terki, D.; Maouche, A.; Madani, K. A Sustainable Valorization of Industrial Tomato Seeds (Cv Rio Grande): Sequential Recovery of a Valuable Oil and Optimized Extraction of Antioxidants by Microwaves. J. Food Process. Preserv. 2022, 46, e16123. [Google Scholar] [CrossRef]
- Botineştean, C.; Gruia, A.T.; Jianu, I. Utilization of Seeds from Tomato Processing Wastes as Raw Material for Oil Production. J. Mater. Cycles Waste Manag. 2015, 17, 118–124. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Policosanol in Tomato (Solanum lycopersicum L.) Seed Oil: The Effect of Cultivar. J. Oleo Sci. 2015, 64, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozyurt, V.H.; Çakaloğlu, B.; Otles, S. Optimization of Cold Press and Enzymatic-Assisted Aqueous Oil Extraction from Tomato Seed by Response Surface Methodology: Effect on Quality Characteristics. J. Food Process. Preserv. 2021, 45, e15471. [Google Scholar] [CrossRef]
- Giannelos, P.N.; Sxizas, S.; Lois, E.; Zannikos, F.; Anastopoulos, G. Physical, Chemical and Fuel Related Properties of Tomato Seed Oil for Evaluating Its Direct Use in Diesel Engines. Ind. Crops Prod. 2005, 22, 193–199. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, F.; Wu, Y.; Zhang, Y.; Gao, B.; Yu, L. Triacylglycerols and Fatty Acid Compositions of Cucumber, Tomato, Pumpkin, and Carrot Seed Oils by Ultra-Performance Convergence Chromatography Combined with Quadrupole Time-of-Flight Mass Spectrometry. Foods 2020, 9, 970. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jorge, N. Bioactive Compounds of the Lipid Fractions of Agro-Industrial Waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Irnawati; Riyanto, S.; Martono, S.; Rohman, A. The Employment of FTIR Spectroscopy and Chemometrics for Authentication of Pumpkin Seed Oil from Sesame Oil. Food Res. 2019, 4, 42–48. [Google Scholar] [CrossRef]
- Riyanta, A.B.; Riyanto, S.; Lukitaningsih, E.; Rohman, A. The Employment of Fourier Transform Infrared Spectroscopy (FTIR) and Chemometrics for Analysis of Candlenut Oil in Binary Mixture with Grape Seed Oil. Food Res. 2019, 4, 184–190. [Google Scholar] [CrossRef]
- Riyanta, A.B.; Riyanto, S.; Lukitaningsih, E.; Rohman, A. Analysis of Sunflower Oil in Ternary Mixture with Grapeseed Oil and Candlenut Oil in the Ternary Mixture System Using FTIR Spectroscopy and Chemometrics. Food Res. 2020, 4, 1726–1731. [Google Scholar] [CrossRef]
- Uncu, O.; Napiórkowska, A.; Szajna, T.K.; Ozen, B. Evaluation of Three Spectroscopic Techniques in Determination of Adulteration of Cold Pressed Pomegranate Seed Oils. Microchem. J. 2020, 158, 105128. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Abu-sbeih, K.A.; Al-Trawneh, I.; Bourghli, L. Preparation and Characterization of Alkyd Resins of Jordan Valley Tomato Oil. J. Polym. Environ. 2014, 22, 553–558. [Google Scholar] [CrossRef]
- Hundie, K.B. Optimization of Biodiesel Production Parameters from Cucurbita Maxima Waste Oil Using Microwave Assisted via Box-Behnken Design Approach. J. Chem. 2022, 2022, 8516163. [Google Scholar] [CrossRef]
- Westphal, A.; Bauerfeind, J.; Rohrer, C.; Ernawita; Böhm, V. Analytical Characterisation of the Seeds of Two Tomato Varieties as a Basis for Recycling of Waste Materials in the Food Industry. Eur. Food Res. Technol. 2014, 239, 613–620. [Google Scholar] [CrossRef]
- Müller, L.; Catalano, A.; Simone, R.; Cittadini, A.; Fröhlich, K.; Böhm, V.; Palozza, P. Antioxidant Capacity of Tomato Seed Oil in Solution and Its Redox Properties in Cultured Macrophages. J. Agric. Food Chem. 2013, 61, 346–354. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Enhancing the Bioaccessibility of Lycopene from Tomato Processing Byproducts via Supercritical Carbon Dioxide Extraction. Curr. Res. Food Sci. 2022, 5, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical Carbon Dioxide Extraction of Carotenoids, Tocopherols and Sitosterols from Industrial Tomato by-Products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Kiralan, M.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Physicochemical Properties and Stability of Black Cumin (Nigella Sativa) Seed Oil as Affected by Different Extraction Methods. Ind. Crops Prod. 2014, 57, 52–58. [Google Scholar] [CrossRef]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Li, X. Study of Optimal Extraction Conditions for Achieving High Yield and Antioxidant Activity of Tomato Seed Oil. J. Food Sci. 2012, 77, E202–E208. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]

| Experimental Design | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | t (min) | T (°C) | V (mL) | Yield (wt%) | C16:0 (mg gTS−1) * | C18:0 (mg gTS−1) * | C18:1 (mg gTS−1) * | C18:2 (mg gTS−1) * |
| 1 | 30 | 40 | 65 | 25.2 | 26.5 ± 1.0 | 10.3 ± 0.5 | 39.9 ± 1.6 | 101.1 ± 3.9 |
| 2 | 30 | 55 | 50 | 24.3 | 27.8 ± 1.6 | 11.0 ± 0.7 | 42.1 ± 2.7 | 106.1 ± 6.3 |
| 3 | 10 | 55 | 50 | 23.9 | 19.1 ± 0.1 | 7.6 ± 0.1 | 29.2 ± 0.1 | 74.1 ± 0.2 |
| 4 | 30 | 55 | 80 | 25.4 | 28.1 ± 0.6 | 11.1 ± 0.4 | 42.7 ± 1.3 | 107.4 ± 3.2 |
| 5 | 30 | 70 | 65 | 26.2 | 30.7 ± 1.2 | 12.2 ± 0.4 | 46.5 ± 1.7 | 117.4 ± 4.2 |
| 6 | 20 | 40 | 80 | 25.4 | 24.4 ± 0.2 | 9.5 ± 0.2 | 37.0 ± 0.7 | 93.9 ± 1.6 |
| 7 | 20 | 55 | 65 | 24.6 | 32.1 ± 1.2 | 12.7 ± 0.5 | 48.0 ± 1.8 | 121.0 ± 4.5 |
| 8 | 20 | 70 | 80 | 26.4 | 23.9 ± 1.2 | 9.5 ± 0.5 | 36.6 ± 1.9 | 92.1 ± 4.6 |
| 9 | 10 | 70 | 65 | 25.4 | 21.2 ± 0.1 | 8.4 ± 0.1 | 32.3 ± 0.1 | 82.4 ± 0.2 |
| 10 | 20 | 55 | 65 | 24.8 | 29.8 ± 1.2 | 11.8 ± 0.5 | 44.8 ± 1.7 | 112.9 ± 4.1 |
| 11 | 10 | 40 | 65 | 23.5 | 21.7 ± 1.5 | 8.8 ± 0.6 | 33.4 ± 2.3 | 83.2 ± 5.4 |
| 12 | 10 | 55 | 80 | 25.0 | 30.4 ± 2.1 | 11.9 ± 0.8 | 45.1 ± 3.0 | 114.5 ± 8.3 |
| 13 | 20 | 70 | 50 | 24.8 | 18.6 ± 0.9 | 7.3 ± 0.4 | 28.2 ± 1.6 | 71.8 ± 3.7 |
| 14 | 20 | 55 | 65 | 25.8 | 30.2 ± 1.3 | 11.9 ± 0.6 | 45.2 ± 2.0 | 114.4 ± 5.1 |
| 15 | 20 | 40 | 50 | 23.8 | 25.3 ± 1.4 | 10.0 ± 0.6 | 37.9 ± 2.1 | 96.0 ± 5.3 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Yield | |||||
| A | 1.28 | 1 | 1.28 | 3.05 | 0.2230 |
| B | 2.97 | 1 | 2.97 | 7.07 | 0.1171 |
| C | 3.56 | 1 | 3.56 | 8.48 | 0.1005 |
| AA | 0.18 | 1 | 0.18 | 0.42 | 0.5841 |
| AB | 0.23 | 1 | 0.23 | 0.56 | 0.5331 |
| AC | 0.00 | 1 | 0.00 | 0.01 | 0.9445 |
| BB | 0.22 | 1 | 0.22 | 0.52 | 0.5468 |
| BC | 0.00 | 1 | 0.00 | 0.00 | 0.9907 |
| CC | 0.12 | 1 | 0.12 | 0.29 | 0.6453 |
| Lack-of-fit | 0.65 | 3 | 0.22 | 0.51 | 0.7128 |
| Pure error | 0.84 | 2 | 0.42 | ||
| Total (corr.) | 10.09 | 14 | |||
| R2 | 0.8525 | ||||
| Adj R2 | 0.5870 | ||||
| Methyl palmitate (C16:0) | |||||
| A | 53.42 | 1 | 53.42 | 36.76 | 0.0261 * |
| B | 1.62 | 1 | 1.62 | 1.11 | 0.4021 |
| C | 31.43 | 1 | 31.43 | 21.63 | 0.0433 * |
| AA | 5.42 | 1 | 5.42 | 3.73 | 0.1931 |
| AB | 5.72 | 1 | 5.72 | 3.94 | 0.1857 |
| AC | 30.51 | 1 | 30.51 | 21.00 | 0.0445 * |
| BB | 74.52 | 1 | 74.52 | 51.27 | 0.0190 * |
| BC | 9.45 | 1 | 9.45 | 6.50 | 0.1255 |
| CC | 36.77 | 1 | 36.77 | 25.30 | 0.0373 * |
| Lack-of-fit | 29.14 | 3 | 9.71 | 6.68 | 0.1329 |
| Pure error | 2.91 | 2 | 1.45 | ||
| Total (corr.) | 269.70 | 14 | |||
| R2 | 0.8812 | ||||
| Adj R2 | 0.6673 | ||||
| Methyl stearate (C18:0) | |||||
| A | 7.85 | 1 | 7.85 | 31.91 | 0.0299 * |
| B | 0.21 | 1 | 0.21 | 0.85 | 0.4529 |
| C | 4.89 | 1 | 4.89 | 19.87 | 0.0468 * |
| AA | 0.72 | 1 | 0.72 | 2.92 | 0.2295 |
| AB | 1.20 | 1 | 1.20 | 4.89 | 0.1575 |
| AC | 4.45 | 1 | 4.45 | 18.07 | 0.0511 |
| BB | 11.58 | 1 | 11.58 | 47.04 | 0.0206 * |
| BC | 1.70 | 1 | 1.70 | 6.91 | 0.1194 |
| CC | 6.25 | 1 | 6.25 | 25.39 | 0.0372 * |
| Lack-of-fit | 4.09 | 3 | 1.36 | 5.54 | 0.1566 |
| Pure error | 0.49 | 2 | 0.25 | ||
| Total (corr.) | 41.70 | 14 | |||
| R2 | 0.8900 | ||||
| Adj R2 | 0.6920 | ||||
| Methyl oleate (C18:1) | |||||
| A | 121.12 | 1 | 121.12 | 40.52 | 0.0238 * |
| B | 2.67 | 1 | 2.67 | 0.90 | 0.4438 |
| C | 71.66 | 1 | 71.66 | 23.97 | 0.0393 * |
| AA | 9.07 | 1 | 9.07 | 3.04 | 0.2236 |
| AB | 14.77 | 1 | 14.77 | 4.94 | 0.1563 |
| AC | 58.70 | 1 | 58.70 | 19.64 | 0.0473 * |
| BB | 152.24 | 1 | 152.24 | 50.93 | 0.0191 * |
| BC | 21.53 | 1 | 21.53 | 7.20 | 0.1153 |
| CC | 79.93 | 1 | 79.93 | 26.74 | 0.0354 * |
| Lack-of-fit | 54.35 | 3 | 18.12 | 6.06 | 0.1449 |
| Pure error | 5.98 | 2 | 2.99 | ||
| Total (corr.) | 569.52 | 14 | |||
| R2 | 0.8941 | ||||
| Adj R2 | 0.7034 | ||||
| Methyl linoleate (C18:2) | |||||
| A | 758.10 | 1 | 758.10 | 41.26 | 0.0234 * |
| B | 13.74 | 1 | 13.74 | 0.75 | 0.4783 |
| C | 446.88 | 1 | 446.88 | 24.32 | 0.0387 * |
| AA | 59.07 | 1 | 59.07 | 3.22 | 0.2148 |
| AB | 73.18 | 1 | 73.18 | 3.98 | 0.1841 |
| AC | 383.57 | 1 | 383.57 | 20.88 | 0.0447 * |
| BB | 953.97 | 1 | 953.97 | 51.92 | 0.0187 * |
| BC | 125.42 | 1 | 125.42 | 6.83 | 0.1206 |
| CC | 496.28 | 1 | 496.28 | 27.01 | 0.0351 * |
| Lack-of-fit | 382.33 | 3 | 127.44 | 6.94 | 0.1286 |
| Pure error | 36.74 | 2 | 18.37 | ||
| Total (corr.) | 3587.66 | 14 | |||
| R2 | 0.8832 | ||||
| Adj R2 | 0.6729 |
| Response | t (min) | T (°C) | V (mL) | Predicted Value |
|---|---|---|---|---|
| Yield a | 23.0 | 70.0 | 80.0 | 26.4 |
| C16:0 b | 30.0 | 56.0 | 63.4 | 32.2 |
| C18:0 b | 30.0 | 56.4 | 63.8 | 12.7 |
| C18:1 b | 30.0 | 56.4 | 64.0 | 48.4 |
| C18:2 b | 30.0 | 56.2 | 63.8 | 122.1 |
| Experimental Design | Response Variable | |||
|---|---|---|---|---|
| Run | P (bar) | T (°C) | F (g min−1) | Yield (wt%) |
| 1 | 400 | 60 | 75 | 16.8 |
| 2 | 400 | 60 | 25 | 13.8 |
| 3 | 250 | 40 | 25 | 4.1 |
| 4 | 100 | 60 | 25 | 2.1 |
| 5 | 100 | 80 | 50 | 4.3 |
| 6 | 250 | 60 | 50 | 8.5 |
| 7 | 250 | 40 | 75 | 3.0 |
| 8 | 400 | 80 | 50 | 10.4 |
| 9 | 250 | 60 | 50 | 9.8 |
| 10 | 100 | 60 | 75 | 1.8 |
| 11 | 250 | 80 | 25 | 1.7 |
| 12 | 400 | 40 | 50 | 13.9 |
| 13 | 250 | 80 | 75 | 8.7 |
| 14 | 250 | 60 | 50 | 7.5 |
| 15 | 100 | 40 | 50 | 2.5 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Yield | |||||
| A | 244.43 | 1 | 244.43 | 187.39 | 0.0053 * |
| B | 0.30 | 1 | 0.30 | 0.23 | 0.6768 |
| C | 9.37 | 1 | 9.37 | 7.19 | 0.1155 |
| AA | 11.02 | 1 | 11.02 | 8.45 | 0.1008 |
| AB | 6.97 | 1 | 6.97 | 5.34 | 0.1470 |
| AC | 2.82 | 1 | 2.82 | 2.16 | 0.2791 |
| BB | 23.40 | 1 | 23.40 | 17.94 | 0.0515 |
| BC | 16.24 | 1 | 16.24 | 12.45 | 0.0718 |
| CC | 10.27 | 1 | 10.27 | 7.87 | 0.1070 |
| Lack-of-fit | 15.02 | 3 | 5.01 | 3.84 | 0.2136 |
| Pure error | 2.61 | 2 | 1.30 | ||
| Total (corr.) | 344.56 | 14 | |||
| R2 | 0.9489 | ||||
| Adj R2 | 0.8568 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solaberrieta, I.; Mellinas, A.C.; Espagnol, J.; Hamzaoui, M.; Jiménez, A.; Garrigós, M.C. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods 2022, 11, 2408. https://doi.org/10.3390/foods11162408
Solaberrieta I, Mellinas AC, Espagnol J, Hamzaoui M, Jiménez A, Garrigós MC. Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques. Foods. 2022; 11(16):2408. https://doi.org/10.3390/foods11162408
Chicago/Turabian StyleSolaberrieta, Ignacio, Ana Cristina Mellinas, Jérémy Espagnol, Mahmoud Hamzaoui, Alfonso Jiménez, and María Carmen Garrigós. 2022. "Valorization of Tomato Seed By-Products as a Source of Fatty Acids and Bioactive Compounds by Using Advanced Extraction Techniques" Foods 11, no. 16: 2408. https://doi.org/10.3390/foods11162408






