Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Whey Protein Solutions and Enzymatic Hydrolysis on Bench-Scale
2.3. Compositional Analyses
2.4. Degree of Hydrolysis of Whey Protein Hydrolysates
2.5. Inhibition of the Angiotensin-I-Converting Enzyme (ACE-I) of Whey Protein Hydrolysates
2.6. In Vitro Static Digestion of Whey Protein Hydrolysates
2.7. Peptide Analysis and Identification
2.8. Experimental Design and Statistical Analyses
3. Results
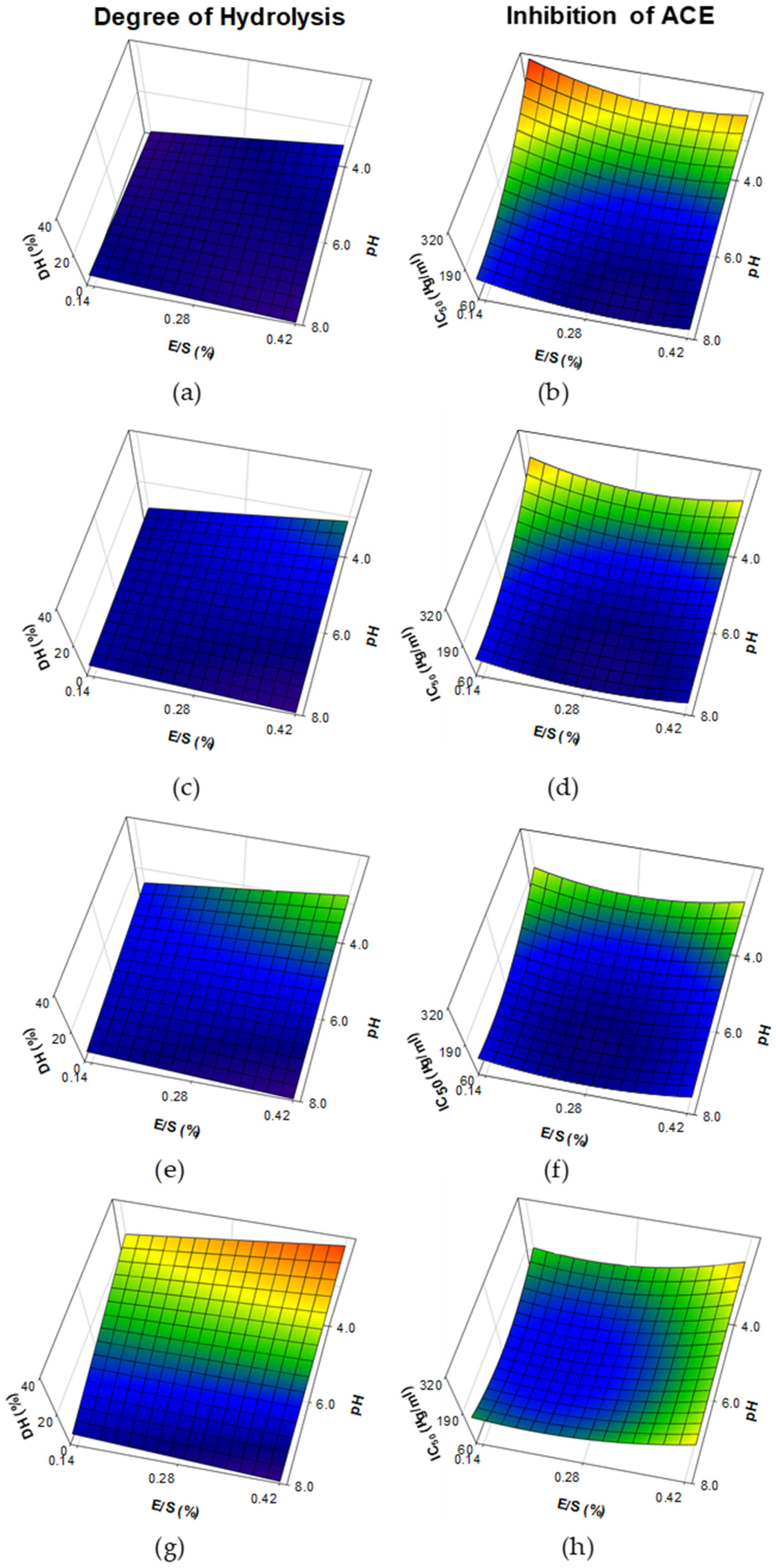
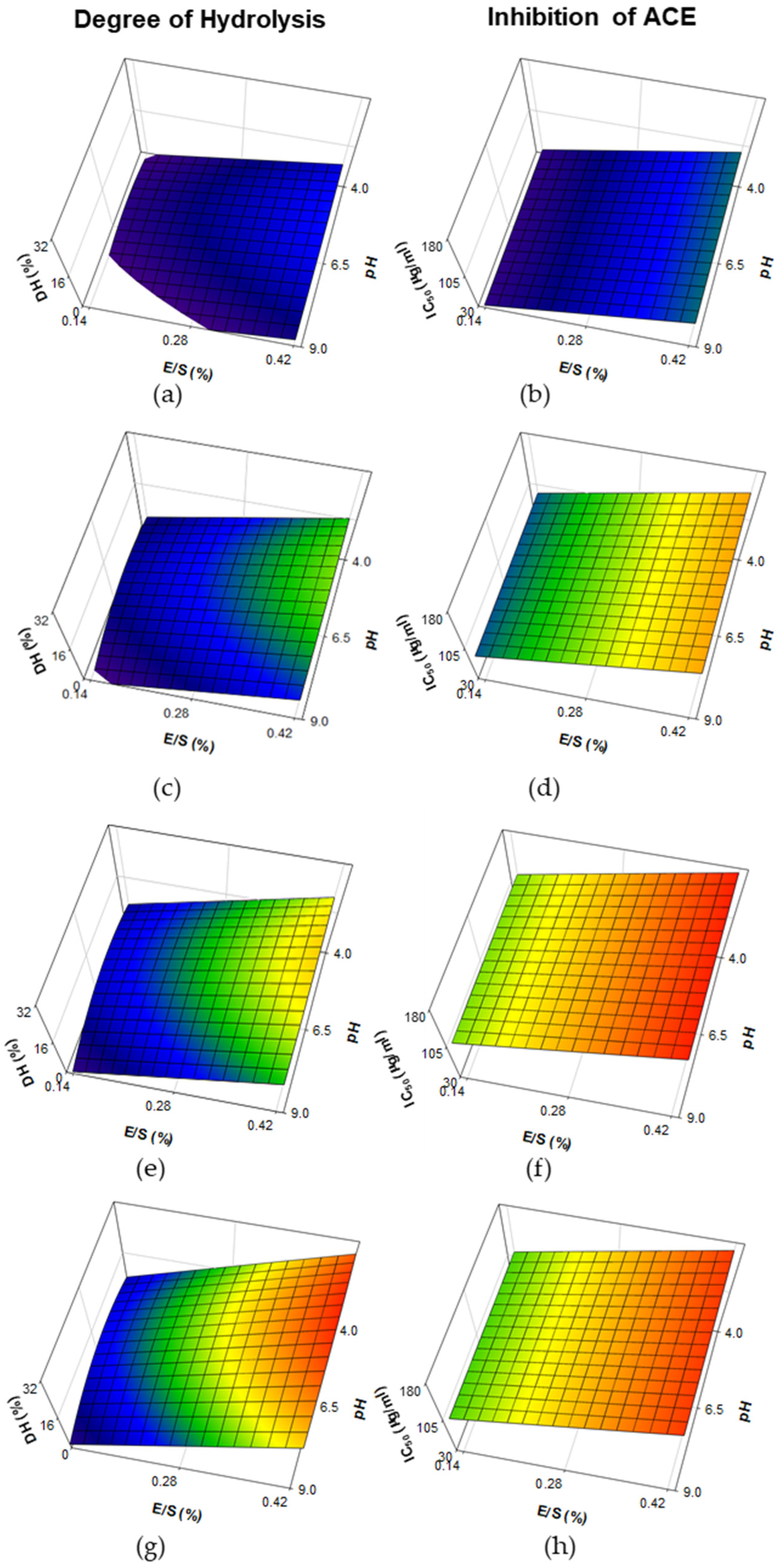
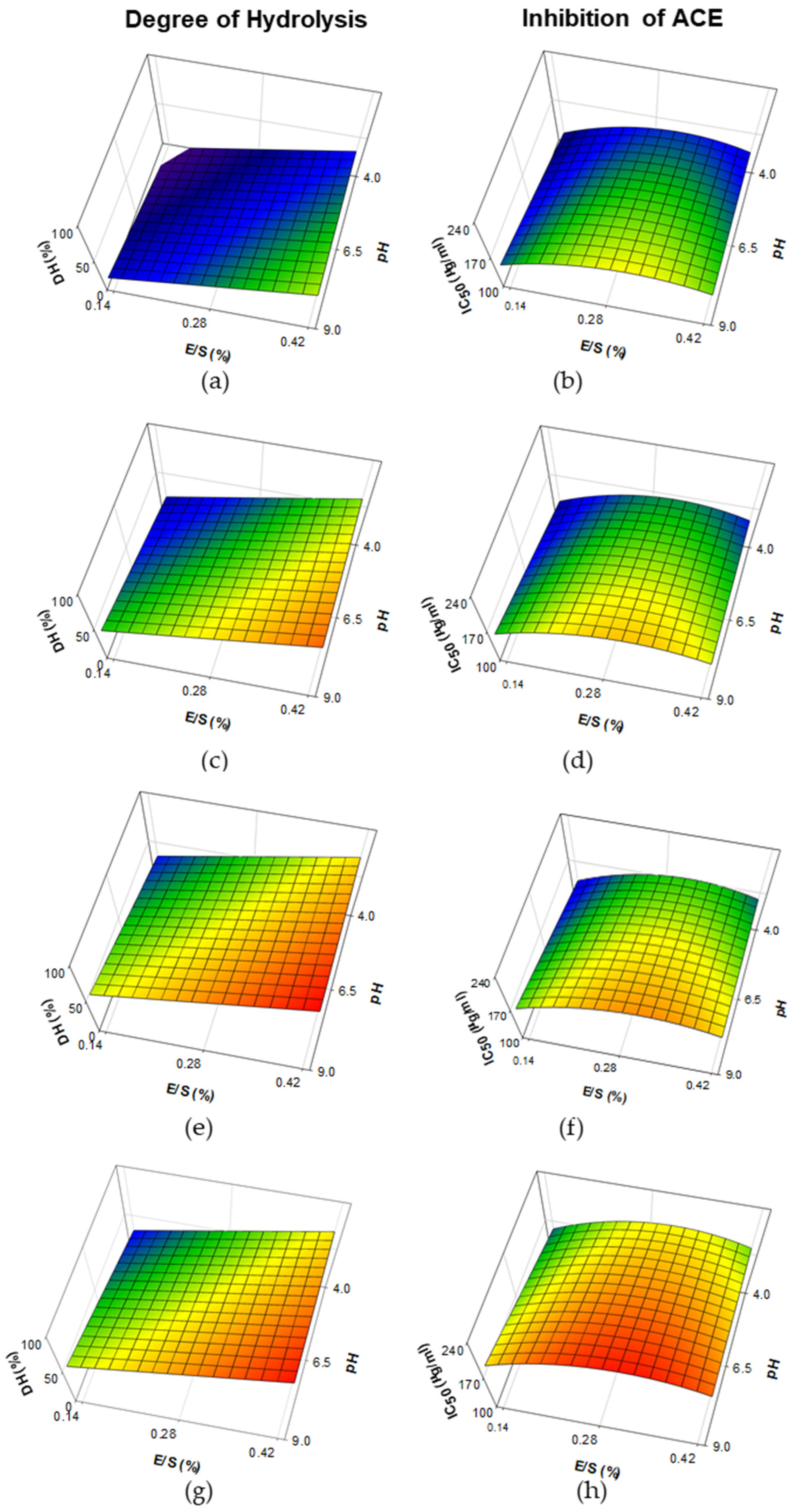
3.1. Degree of Hydrolysis and Inhibition of Angiotensin-I-Converting Enzyme from Whey Protein Hydrolysates Made with Papain, Bromelain and Ficin
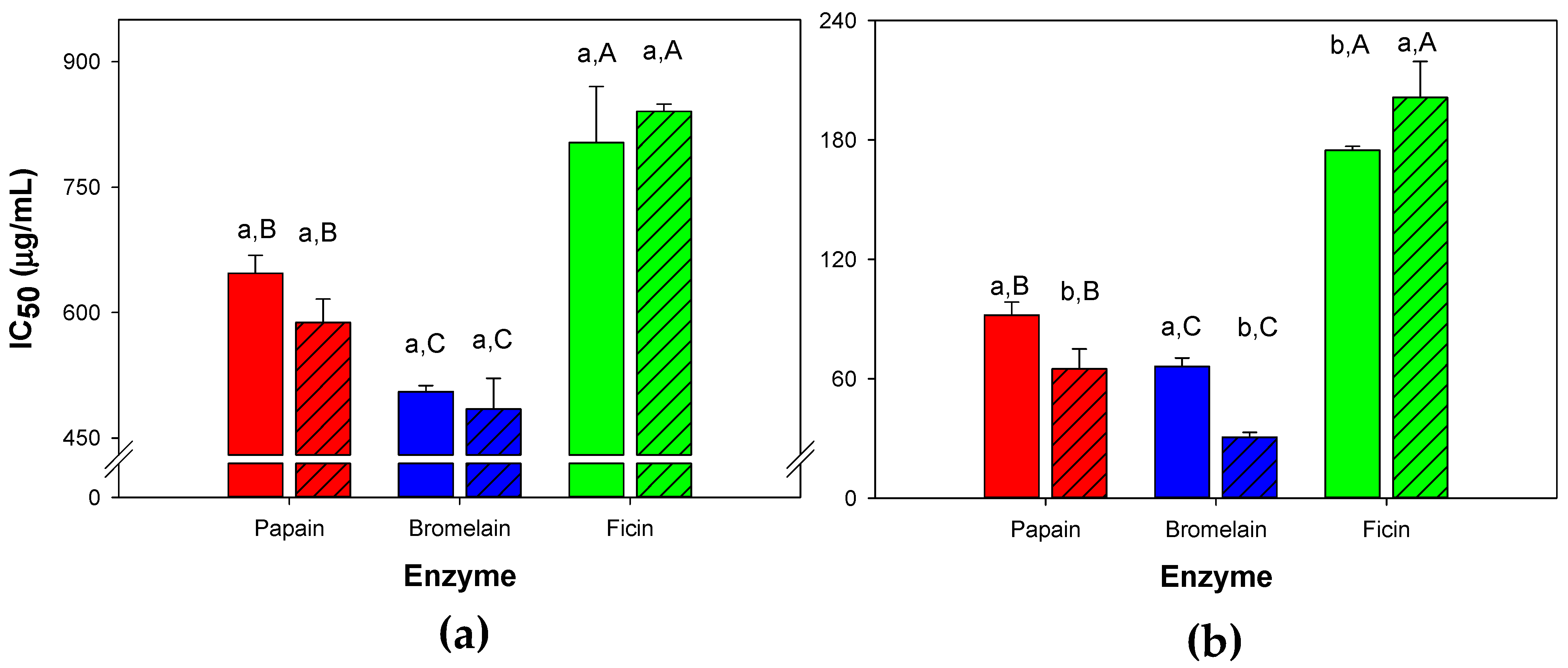
3.2. Predicted and Experimental In Vitro Antihypertensive Properties Obtained from Whey Protein Hydrolysates Made with Papain, Bromleain and Ficin under Selected Conditions
3.3. Influence of Simulated Gastrointestinal Digestion on the In Vitro Anihypertensive Properties of Selected Whey Protein Hydrolysates Made with Papain, Bromelain and Ficin
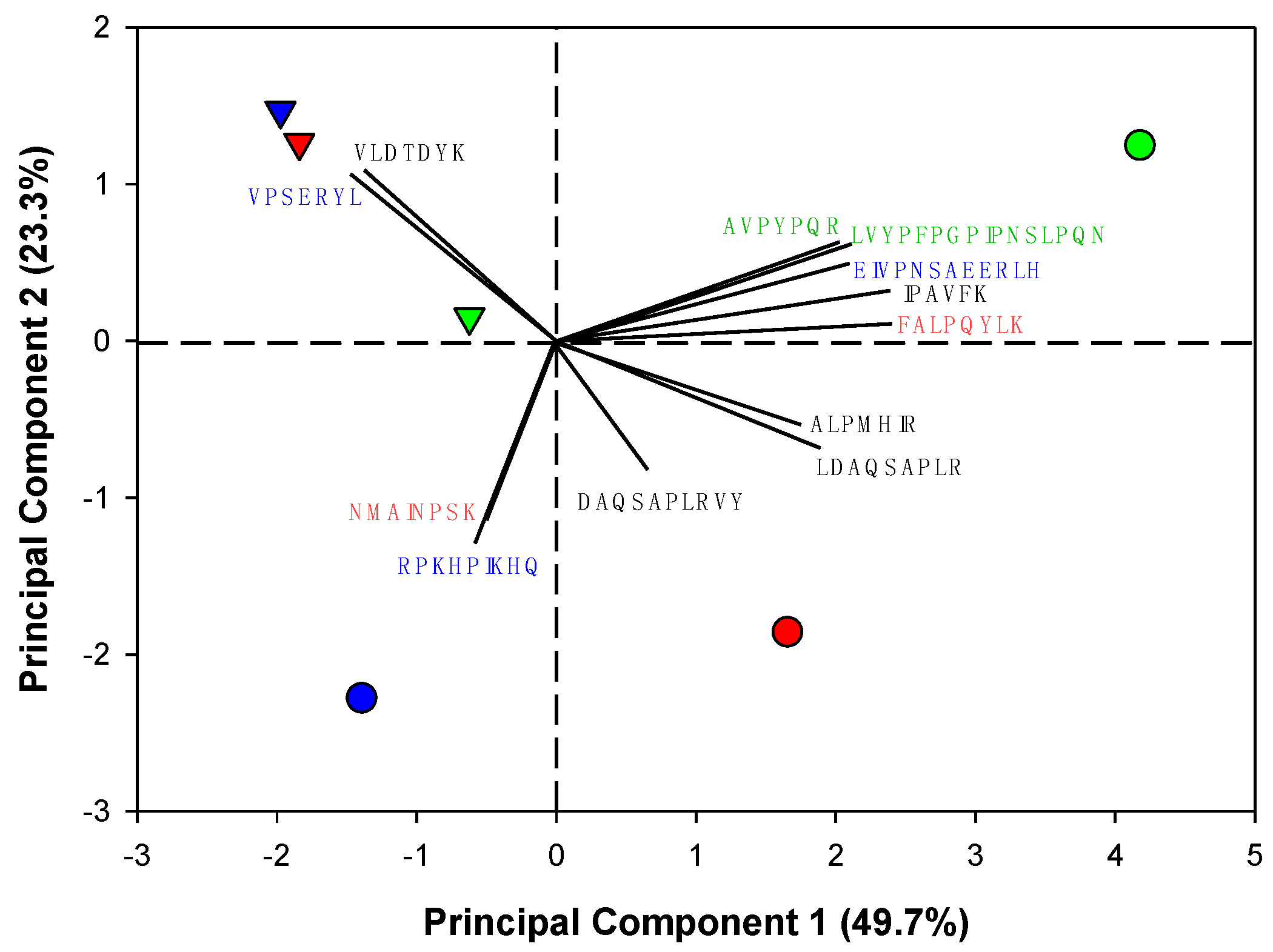
3.4. Identification of Antihypertensive Peptides in Selected Whey Protein Hydrolysates before and after Gastrointestinal Digestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez-Ledezma, B.; Garcia-Nebot, M.J.; Fernandez-Tome, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Pihlanto, A.; Korhonen, H. Advances in Fermented Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782420156. [Google Scholar]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- CDC. Centers for Desease Control and Prevention. Cardiovascular Diseases. Available online: https://www.cdc.gov/globalhealth/healthprotection/ncd/cardiovascular-diseases.html (accessed on 9 June 2022).
- Quirós, A.M.; Ramos, B.; Muguerza, B.; Delgado, M.A.; Miguel, M.; Aleixandre, A.; Recio, I. Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int. Dairy J. 2007, 17, 33–41. [Google Scholar] [CrossRef]
- Pihlanto-Leppala, A.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of bioactive peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods—Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Lu, Y.; Govindasamy-Lucey, S.; Lucey, J.A. Angiotensin-I-converting enzyme-inhibitory peptides in commercial Wisconsin Cheddar cheeses of different ages. J. Dairy Sci. 2016, 99, 41–52. [Google Scholar] [CrossRef]
- Pihlanto, A.; Virtanen, T.; Korhonen, H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010, 20, 3–10. [Google Scholar] [CrossRef]
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009, 112, 211–214. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Miracle, R.E.; Drake, M. Characterization of flavor of whey protein hydrolysates. J. Agric. Food Chem. 2010, 58, 6318–6327. [Google Scholar] [CrossRef]
- Godoy, J.; Peslerbes, M.; Vyhmeister, S.; Vargas-Bello-Pérez, E.; Fellenberg, M.A.; Ibáñez, R.A. Changes in the chemical and in-vitro antihypertensive properties of sweet whey obtained from miniature fresh, Chanco and Gouda-style model cheeses. J. Dairy Res. 2020, 87, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Zakynthinos, G.; Proestos, C. Effect of Food Processing, Quality, and Safety with Emphasis on Kosher, Halal, Vegetarian, and GM food. In Preparation and Processing of Religious and Cultural Foods; Woodhead Publishing: Cambridge, UK, 2018; pp. 193–214. [Google Scholar] [CrossRef]
- Tavares, T.; Contreras, M.D.M.; Amorim, M.; Pintado, M.; Recio, I.; Malcata, F.X. Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes. Peptides 2011, 32, 1013–1019. [Google Scholar] [CrossRef]
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Silva, M.R.; Silvestre, M.P.C.; Silva, V.D.M.; Souza, M.W.S.; Lopes Junior, C.O.; Afonso, W.O.; Lana, F.C.; Rodrigues, D.F. Production of ACE-inhibitory whey protein concentrate hydrolysates: Use of pancreatin and papain. Int. J. Food Prop. 2014, 17, 1002–1012. [Google Scholar] [CrossRef]
- Lafarga, T.; Aluko, R.E.; Rai, D.K.; O’Connor, P.; Hayes, M. Identification of bioactive peptides from a papain hydrolysate of bovine serum albumin and assessment of an antihypertensive effect in spontaneously hypertensive rats. Food Res. Int. 2016, 81, 91–99. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Mora-Cortes, W.G.; Leandro-Roldan, M.M.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Ramírez-Suarez, J.C.; González-Córdova, A.F.; Vallejo-Córdoba, B. Production of whey protein hydrolysates with angiotensin-converting enzyme-inhibitory activity using three new sources of plant proteases. Biocatal. Agric. Biotechnol. 2020, 28, 101724. [Google Scholar] [CrossRef]
- Bertucci, J.I.; Liggieri, C.S.; Colombo, M.L.; Vairo Cavalli, S.E.; Bruno, M.A. Application of peptidases from Maclura pomifera fruit for the production of active biopeptides from whey protein. LWT Food Sci. Technol. 2015, 64, 157–163. [Google Scholar] [CrossRef]
- Tavares, T.G.; Contreras, M.M.; Amorim, M.; Martín-Álvarez, P.J.; Pintado, M.E.; Recio, I.; Malcata, F.X. Optimisation, by response surface methodology, of degree of hydrolysis and antioxidant and ACE-inhibitory activities of whey protein hydrolysates obtained with cardoon extract. Int. Dairy J. 2011, 21, 926–933. [Google Scholar] [CrossRef]
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; Fitzgerald, R.J. Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chem. 2016, 199, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, S.Z.; González, J.G.; Sforza, S.; Tedeschi, T. Bioactivity and peptide profile of whey protein hydrolysates obtained from Colombian double-cream cheese production and their products after gastrointestinal digestion. LWT 2021, 145, 111334. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, M.; Nebbia, S.; Briard-Bion, V.; Jardin, J.; Peila, C.; Coscia, A.; Dupont, D.; Cavallarin, L.; Deglaire, A. Simulated dynamic digestion reveals different peptide releases from human milk processed by means of holder or high temperature-short time pasteurization. Food Chem. 2022, 369, 130998. [Google Scholar] [CrossRef]
- Langella, O.; Valot, B.; Balliau, T.; Blein-Nicolas, M.; Bonhomme, L.; Zivy, M. X!TandemPipeline: A Tool to Manage Sequence Redundancy for Protein Inference and Phosphosite Identification. J. Proteome Res. 2017, 16, 494–503. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Valot, B.; Langella, O.; Nano, E.; Zivy, M. MassChroQ: A versatile tool for mass spectrometry quantification. Proteomics 2011, 11, 3572–3577. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Piraino, P.; Parente, E.; Mcsweeney, P.L.H. Processing of chromatographic data for chemometric analysis of peptide profiles from cheese extracts: A novel approach. J. Agric. Food Chem. 2004, 52, 6904. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Fernández, A.; Norris, R.; Riera, F.A.; FitzGerald, R.J. Selective enrichment of bioactive properties during ultrafiltration of a tryptic digest of β-lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Rokka, T.; Korhonen, H. Angiotensin I converting enzyme inhibitory peptides derived from bovine milk proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef]
- Ruiz, J.Á.G.; Ramos, M.; Recio, I. Angiotensin converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int. Dairy J. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Villegas, J.M.; Picariello, G.; Mamone, G.; Turbay, M.B.E.; de Giori, G.S.; Hebert, E.M. Milk-derived angiotensin-I-concerting enzyme-inhibitory peptides generated by Lactobacillus delbrueckii subsp. lactis CRL 581. Peptidomics 2014, 1, 22–29. [Google Scholar] [CrossRef]
- Tauzin, J.; Miclo, L.; Gaillard, J.L. Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine as2-casein. FEBS Lett. 2002, 531, 369–374. [Google Scholar] [CrossRef]
- Drenth, J.; Jansonious, J.N.; Koekoek, R.; Swen, H.M.; Wolthers, B.G. Structure of papain. Nature 1968, 218, 929–932. [Google Scholar] [CrossRef]
- Lieske, B.; Konrad, G. Interrelation between pH and availability of α-lactalbumin and β-lactoglobulin for proteolysis by papain. Int. Dairy J. 1996, 6, 359–370. [Google Scholar] [CrossRef]
- Lieske, B.; Konrad, G. Physico-chemical and functional properties of whey protein as affected by limited papain proteolysis and selective ultrafiltration. Int. Dairy J. 1996, 6, 13–31. [Google Scholar] [CrossRef]
- Ou, K.; Liu, Y.; Zhang, L.; Yang, X.; Huang, Z.; Nout, M.J.R.; Liang, J. Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 cells. J. Agric. Food Chem. 2010, 58, 4894–4900. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov. Food Sci. Emerg. Technol. 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Carullo, D.; Barbosa-Cánovas, G.V.; Ferrari, G. Changes of structural and techno-functional properties of high hydrostatic pressure (HHP) treated whey protein isolate over refrigerated storage. LWT 2021, 137, 110436. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Lin, X.; Wu, S.; Li, G.; Li, N.; Otter, D.; Zhu, F.; Hartinger, C.; Corke, H.; et al. In-flow SAXS investigation of whey protein isolate hydrolyzed by bromelain. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127662. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Wang, X.; Wang, H. Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases. LWT 2022, 159, 113257. [Google Scholar] [CrossRef]
- Aider, M. Potential applications of ficin in the production of traditional cheeses and protein hydrolysates. JDS Commun. 2021, 2, 233–237. [Google Scholar] [CrossRef]
- Kheroufi, A.; Brassesco, M.E.; Campos, D.A.; Boughellout, H.; Pintado, M.E. Functional properties of peptides obtained from whey proteins by ficin extract hydrolysis. Food Biosci. 2022, 47, 101707. [Google Scholar] [CrossRef]
- López, E.C.; Eberhardt, A.; Marino, F.; Mammarella, E.J.; Sihufe, G.A.; Manzo, R.M. Physicochemical characterisation of ACE-inhibitory and antioxidant peptides from Alcalase® whey protein hydrolysates using fractionation strategies. Int. J. Dairy Technol. 2022, 75, 538–552. [Google Scholar] [CrossRef]
- Tavares, T.G.; Amorim, M.; Gomes, D.; Pintado, M.E.; Pereira, C.D.; Malcata, F.X. Manufacture of bioactive peptide-rich concentrates from Whey: Characterization of pilot process. J. Food Eng. 2012, 110, 547–552. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Lestari, P.; Suyata. Antibacterial activity of hydrolysate protein from Etawa goat milk hydrolysed by crude extract bromelain. In IOP Conference Series: Materials Science and Engineering ; IOP Publishing: Bristol, UK, 2019; Volume 509, p. 012111. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Otte, J.; van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Halabi, A.; Croguennec, T.; Ménard, O.; Briard-Bion, V.; Jardin, J.; Le Gouar, Y.; Hennetier, M.; Bouhallab, S.; Dupont, D.; Deglaire, A. Protein structure in model infant milk formulas impacts their kinetics of hydrolysis under in vitro dynamic digestion. Food Hydrocoll. 2022, 126, 107368. [Google Scholar] [CrossRef]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The influence of peptidases in intestinal brush border membranes on the absorption of oligopeptides from whey protein hydrolysate: An ex vivo study using an Ussing chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
| Treatment Number | Coded Values | Actual Values | |||||
|---|---|---|---|---|---|---|---|
| pH | E/S | Time | pH Papain | pH Bromelain and Ficin | E/S (%) | Time (min) | |
| 1 | −1 | −1 | −1 | 4.78 | 5.00 | 0.194 | 103 |
| 2 | +1 | −1 | +1 | 7.22 | 8.00 | 0.194 | 397 |
| 3 | +1 | +1 | −1 | 7.22 | 8.00 | 0.366 | 103 |
| 4 | −1 | +1 | +1 | 4.78 | 5.00 | 0.366 | 397 |
| 5 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| 6 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| 7 | +1 | −1 | −1 | 7.22 | 8.00 | 0.194 | 103 |
| 8 | −1 | −1 | +1 | 4.78 | 5.00 | 0.194 | 397 |
| 9 | −1 | +1 | −1 | 4.78 | 5.00 | 0.366 | 103 |
| 10 | 1 | +1 | +1 | 7.22 | 8.00 | 0.366 | 397 |
| 11 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| 12 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| 13 | −α 1 | 0 | 0 | 4.00 | 4.00 | 0.280 | 250 |
| 14 | +α | 0 | 0 | 8.00 | 9.00 | 0.280 | 250 |
| 15 | 0 | 0 | −α | 6.00 | 6.50 | 0.280 | 10 |
| 16 | 0 | 0 | +α | 6.00 | 6.50 | 0.280 | 490 |
| 17 | 0 | −α | 0 | 6.00 | 6.50 | 0.140 | 250 |
| 18 | 0 | +α | 0 | 6.00 | 6.50 | 0.420 | 250 |
| 19 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| 20 | 0 | 0 | 0 | 6.00 | 6.50 | 0.280 | 250 |
| Enzyme | Dependent Variable | Independent Variable | Coefficient | R2 (Adjusted) 1 | p-Value |
|---|---|---|---|---|---|
| Papain | Degree of hydrolysis (DH) | Constant pH *** Time *** pH × E/S ** pH × Time *** | 11.458 −6.645 7.028 −4.15 −6.86 | 0.9495 | <0.001 |
| IC50 of <3 kDa fraction | Constant pH *** pH2 ** E/S2 * Time2 + pH × Time * E/S × Time + | 111.36 −44.75 38.80 34.10 31.70 36.40 29.00 | 0.7890 | 0.001 | |
| Bromelain | Degree of hydrolysis (DH) | Constant E/S *** Time *** pH2 * Time2 ** E/S × Time + | 15.512 7.418 7.590 −2.86 −3.21 3.18 | 0.9065 | <0.001 |
| IC50 of <3 kDa fraction | Constant E/S* Time *** Time2 ** | 143.51 28.60 46.30 −49.50 | 0.6470 | <0.001 | |
| Ficin | Degree of hydrolysis (DH) | Constant pH ** E/S *** Time *** Time2 ** | 63.14 11.64 18.50 22.41 −23.21 | 0.8486 | <0.001 |
| IC50 of <3 kDa fraction | Constant pH * Time * E/S2 + | 187.20 16.71 22.64 −24.50 | 0.4525 | 0.013 |
| Enzyme | Treatments | Responses | |||
|---|---|---|---|---|---|
| IC50 (μg/mL) | |||||
| pH | E/S (%) | Time (min) | Predicted 1 | Experimental 2 | |
| Papain | 7.5 | 0.28 | 150 | 93.6 | 91.9 ± 6.6 |
| Bromelain | 6.5 | 0.28 | 30 | 65.1 | 66.2 ± 4.2 |
| Ficin | 4.0 | 0.28 | 150 | 162.0 | 174.8 ± 2.0 |
| Peptide Sequence | ACE-Inhibitory Peptide | Papain | Bromelain | Ficin | Reported IC50 (mM) | |||
|---|---|---|---|---|---|---|---|---|
| Before 3 | After 4 | Before 3 | After 4 | Before 3 | After 4 | |||
| LDAQSAPLR | β-lg f(32–40) | + 5 | + | + | + | + | + | 635.0 [9] |
| DAQSAPLRVY | β-lg f(33–42) | + | + | − | + | − | + | 12.2 [17] |
| IPAVFK | β-lg f(79–83) | + | − | − | − | + | − | 144.8 [39] |
| VLDTDYK | β-lg f(94–100) | − | + | − | + | + | − | 946.0 [9] |
| ALPMHIR | β-lg f(142–148) | + | + | + | + | + | + | 42.6 [40] |
| RPKHPIKHQ | αs1-CN f(1–9) | + | − | + | − | − | − | 13.4 [41] |
| VPSERYL | αs1-CN f(86–92) | − | + | − | + | + | − | 232.8 [42] |
| EIVPNSAEERLH | αs1-CN f(110–121) | + | − | − | − | + | + | NA 6 [43] |
| NMAINPSK | αs2-CN f(25–32) | + | + | + | + | + | − | 60.0 [44] |
| FALPQYLK | αs2-CN f(174–181) | + | − | − | + | + | − | 4.3 [44] |
| LVYPFPGPIPNSLPQN | β-CN f(11–26) | + | − | + | + | + | + | 71.0 [12] |
| AVPYPQR | β-CN f(177–183) | + | − | − | + | + | − | 274.0 [40] |
| Number of total ACE-inhibitory peptides | 10 | 6 | 5 | 9 | 10 | 5 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peslerbes, M.; Fellenberg, A.; Jardin, J.; Deglaire, A.; Ibáñez, R.A. Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity. Foods 2022, 11, 2429. https://doi.org/10.3390/foods11162429
Peslerbes M, Fellenberg A, Jardin J, Deglaire A, Ibáñez RA. Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity. Foods. 2022; 11(16):2429. https://doi.org/10.3390/foods11162429
Chicago/Turabian StylePeslerbes, Marie, Angélica Fellenberg, Julien Jardin, Amélie Deglaire, and Rodrigo A. Ibáñez. 2022. "Manufacture of Whey Protein Hydrolysates Using Plant Enzymes: Effect of Processing Conditions and Simulated Gastrointestinal Digestion on Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity" Foods 11, no. 16: 2429. https://doi.org/10.3390/foods11162429






