An Acidic Polysaccharide with Anti-Inflammatory Effects from Blackened Jujube: Conformation and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Morphology and Conformational Analysis of BIP-4
2.2.1. XRD Analysis
2.2.2. AFM Measurement
2.2.3. CD Analysis
2.2.4. Congo Red Experiment
2.3. Rheological Measurements
2.3.1. Steady Shear Analysis
Effect of Concentration on Viscosity Characteristics of BJP-4
Viscosity Properties of BJP-4 at Different pH
2.3.2. Dynamic Oscillatory Analysis
2.4. Anti-Inflammatory Effect of BJP-4 In Vitro
2.4.1. Cell Culture and Viability
2.4.2. Measurement of NO and Cytokines
2.4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4.4. Western Blot
2.5. Statistical Analysis
3. Results and Discussion
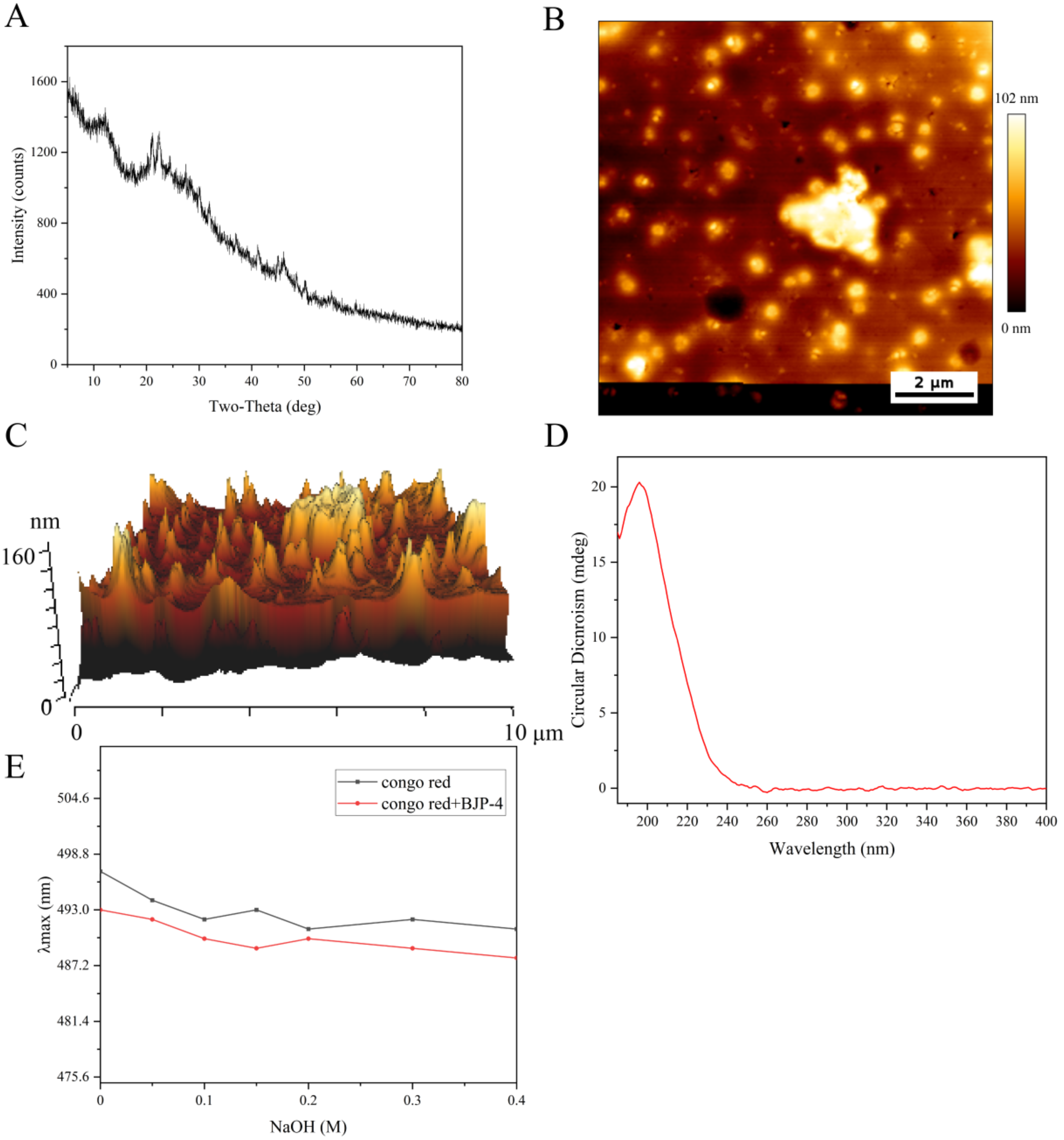
3.1. Morphological Properties of BJP-4
3.2. Advanced Structure of the Polysaccharide BJP-4
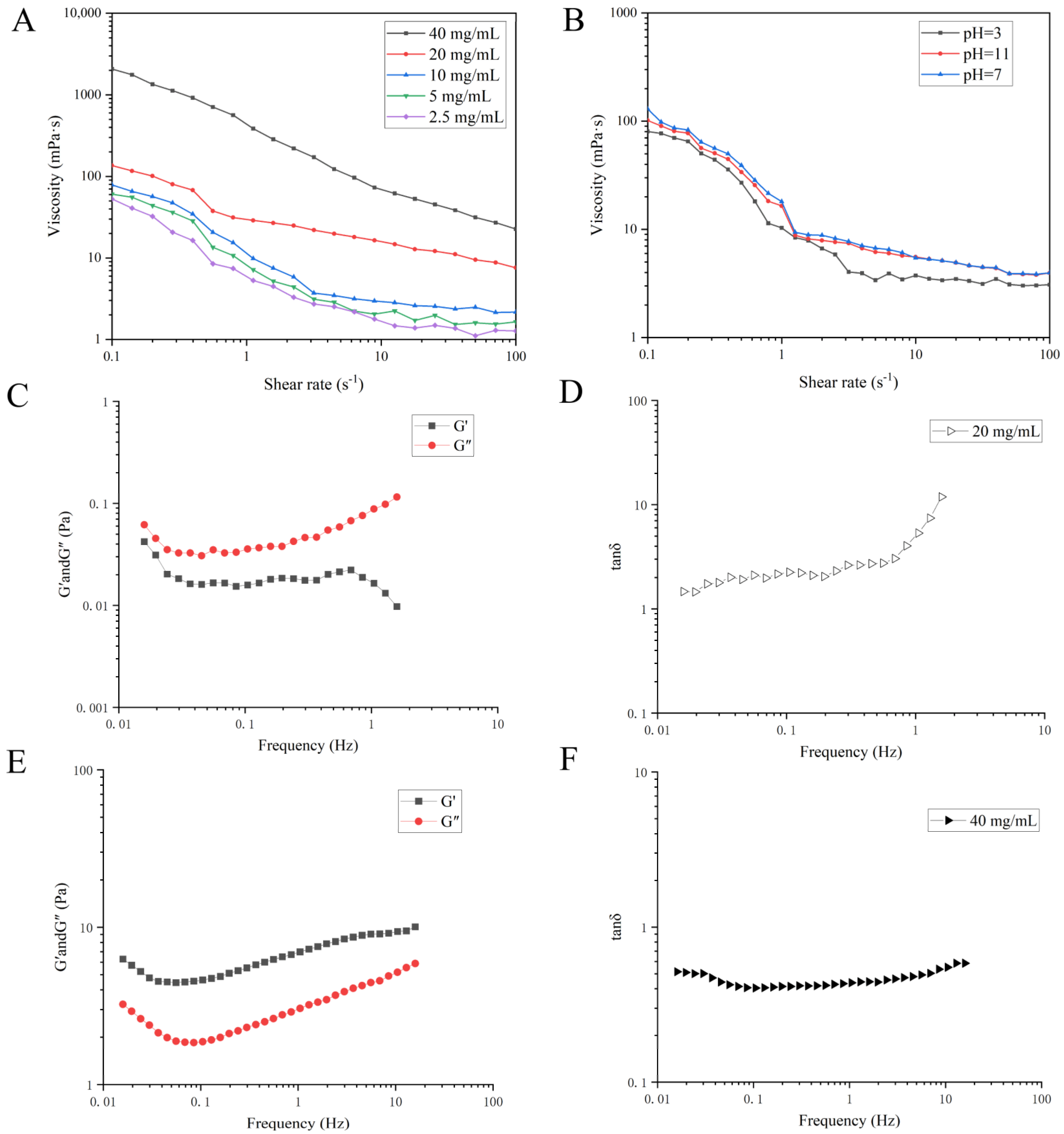
3.3. Rheological Measurements
3.3.1. Steady Shear Flow Properties
3.3.2. Dynamic Oscillation Shear Test
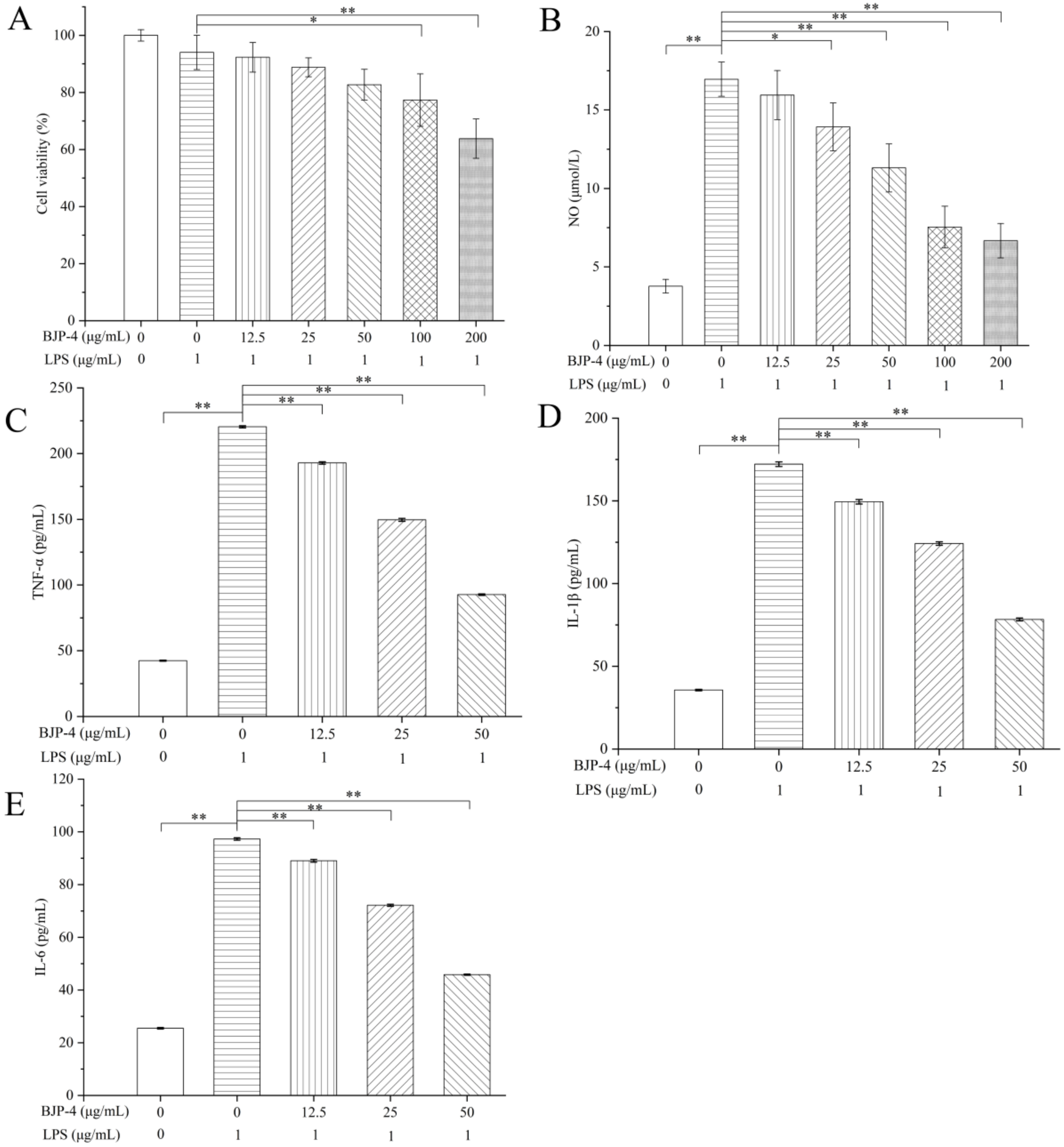
3.4. Anti-Inflammatory Effects of BJP-4 In Vitro
3.4.1. Evaluation of BJP-4 on Cell Viability and NO Production
3.4.2. Effects of BJP-4 on the Mediation of Pro-Inflammatory Cytokines
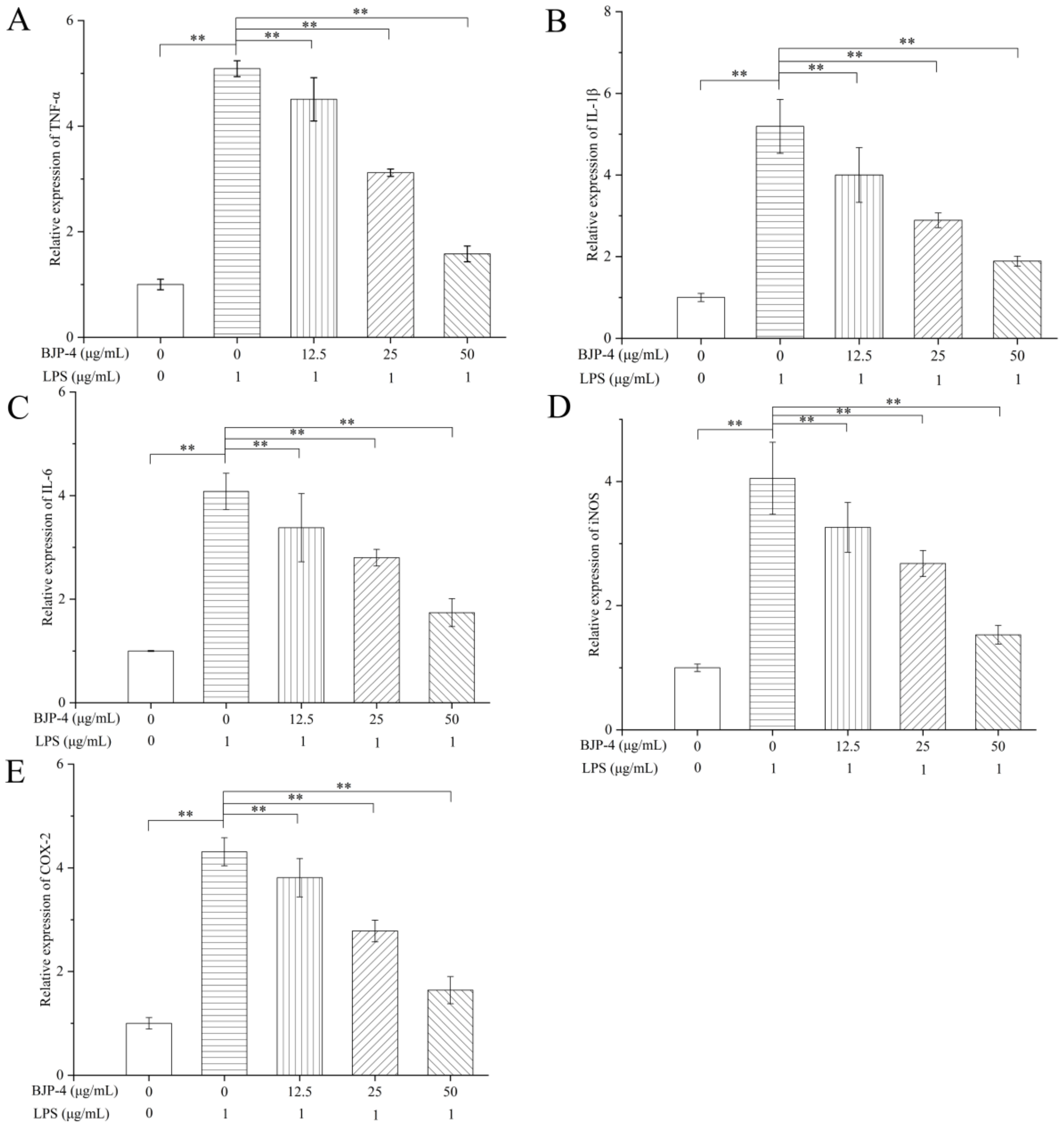
3.4.3. Regulation of BJP-4 on mRNA Expression of Multiple Cytokines
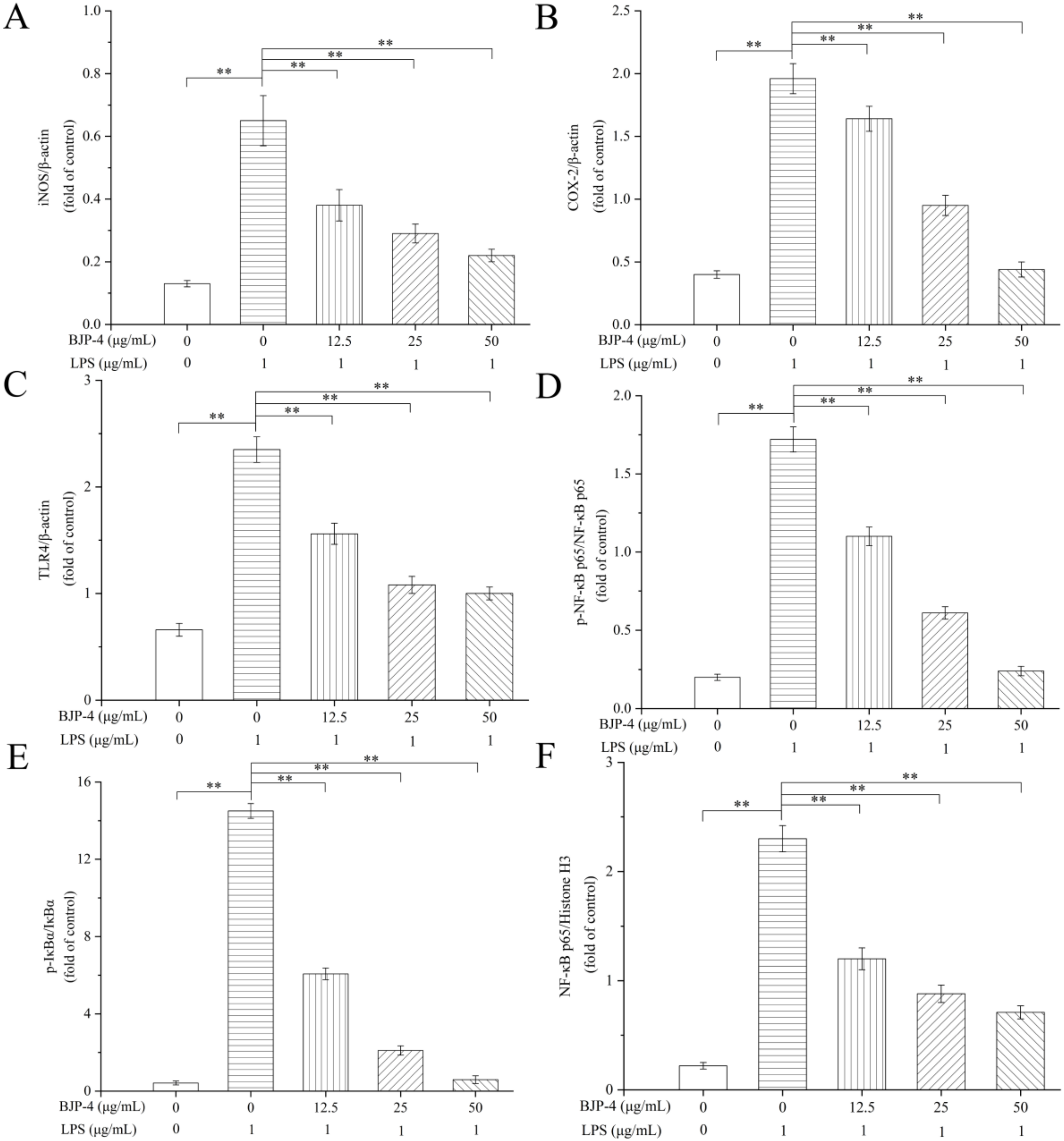
3.4.4. BJP-4 Inhibited the TLR4/NF-κB Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herold, K.; Mrowka, R. Inflammation-Dysregulated inflammatory response and strategies for treatment. Acta Physiol. 2019, 226, e13284. [Google Scholar] [CrossRef]
- Byun, E.-B.; Sung, N.-Y.; Yang, M.-S.; Lee, B.-S.; Song, D.-S.; Park, J.-N.; Kim, J.-H.; Jang, B.-S.; Choi, D.-S.; Park, S.-H. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem. Toxicol. 2014, 74, 255–264. [Google Scholar] [CrossRef]
- Divate, R.D.; Chung, Y.-C. In vitro and in vivo assessment of anti-inflammatory and immunomodulatory activities of Xylaria nigripes mycelium. J. Funct. Foods 2017, 35, 81–89. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Roth, P.; Wick, W.; Weller, M. Steroids in neurooncology: Actions, indications, side-effects. Curr. Opin. Neurol. 2010, 23, 597–602. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M.; Saklani, A. Anti-inflammatory effect of Ajuga bracteosa Wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J. Ethnopharmacol. 2011, 133, 928–930. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Huang, J.; Qian, N.; Shen, G.; Chen, L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264. 7 macrophages. Int. J. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, X.; Zhu, Y.; Zhang, J.; Liu, H.; Jia, L. Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 2018, 111, 121–128. [Google Scholar] [CrossRef]
- Zhai, X.-T.; Zhang, Z.-Y.; Jiang, C.-H.; Chen, J.-Q.; Ye, J.-Q.; Jia, X.-B.; Yang, Y.; Ni, Q.; Wang, S.-X.; Song, J. Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway. J. Ethnopharmacol. 2016, 183, 159–165. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; Zhu, J.; Huang, C.; Bi, S.; Song, L.; Hu, X.; Yu, R. Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Ma, X.; Liu, X.; Yang, M.; Fan, W.; Ren, H.; Efehi, N.; Wang, X.; Zhu, X. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 2018, 118, 2138–2148. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Zhan, R.; Xia, L.; Shao, J.; Wang, C.; Chen, D. Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. J. Funct. Foods 2018, 40, 461–470. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Li, H.; Liu, F.; Wang, M. Chemical characterization and anti-inflammatory activity of polysaccharides from Zizyphus jujube cv. Muzao. Int. J. Food Eng. 2017, 13, 20160382. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, M.-A.; Kim, J.-S.; Park, D.-C.; Lee, S.-P. Enhancing the organoleptic and functional properties of jujube by a quick aging process. Prev. Nutr. Food Sci. 2013, 18, 50. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Vidyarthi, S.K.; Zhang, R. Physicochemical properties, and volatile compounds of blackened jujube vinegar as prepared by optimized fermentation process. Int. J. Food Prop. 2022, 25, 288–304. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yang, Y.; Liu, C.; Zhang, R. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 2022, 385, 132637. [Google Scholar] [CrossRef]
- Chen, L.; Ge, M.-D.; Zhu, Y.-J.; Song, Y.; Cheung, P.C.; Zhang, B.-B.; Liu, L.-M. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.-J.; Sun, P.-L.; Zhang, F.-M.; Linhardt, R.J.; Zhang, A.-Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Guo, S.; Wen, L.; Yu, M.; Feng, L.; Jia, X. Structural Elucidation, Modification, and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 2022, 9, 908175. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Woźniak, Ł.; Zou, L.; Cao, H.; Xiao, J. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides frompotatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Meng, M.; Duan, S.Q.; Feng, C.C.; Wang, C.L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.K.; Hua, D.H.; Huang, D.; Zhang, Q.; Yan, C.Y. Characterization of a new heteropolysaccharide from green guava and its application as an α-glucosidase inhibitor for the treatment of type II diabetes. Food Funct. 2018, 9, 3997–4007. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Liu, X.; Xu, G.; Ye, M.; Bai, L.; Lin, R.; Sha, X.; Liang, L.; Huang, J.; Zhou, C.; et al. Identification of bioactive polysaccharide from Pseudostellaria heterophylla with its anti-inflammatory effects. J. Funct. Foods 2021, 78, 104353. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, S.; Yu, J.; Zhu, J.; Ji, D.; Hu, G.; Wu, C.; Li, Y. The aqueous extract of Phellinus igniarius (SH) ameliorates dextran sodium sulfate-induced colitis in C57BL/6 mice. PLoS ONE 2018, 13, e0205007. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Tan, H. Ultrasonic extraction optimization of L. macranthoides polysaccharides and its physicochemical properties. Int. J. Biol. Macromol. 2015, 74, 224–231. [Google Scholar] [CrossRef]
- Karimi, S.; Ghanbarzadeh, B.; Roufegarinejad, L.; Falcone, P.M. Polysaccharide extracted from Althaea officinalis L. root: New studies of structural, rheological and antioxidant properties. Carbohydr. Res. 2021, 510, 108438. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Zhang, J.; Wen, C.; Zhou, J.; Yao, H.; He, Y.; Ma, H.; Duan, Y. Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 246, 116595. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ji, X.; Wang, M.; Yin, S.; Peng, Q. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: Structural characterizations and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 607–615. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Zhang, B.; Chen, J.; Liu, R.; Song, D.; Li, W.; Lin, N.; Zou, X.; Wang, J. Purification, characterization, and in vitro antitumor activity of a novel glucan from the purple sweet potato Ipomoea Batatas (L.) Lam. Carbohydr. Polym. 2021, 257, 117605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; You, S.; Cao, L.; Zhou, R.; Wang, Q.; Cui, S.W. Chemical and rheological properties of polysaccharides from fruit body of Auricularia auricular-judae. Food Hydrocoll. 2016, 57, 30–37. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part III–Steady and dynamic shear rheology. Food Hydrocoll. 2017, 67, 243–250. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Bai, X.; Li, C.; Xiang, D. Rheological and physicochemical properties of polysaccharides extracted from stems of Dendrobium officinale. Food Hydrocoll. 2020, 103, 105706. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Liu, N.; Zhang, J.; Tan, G.; Kannan, B.; Liu, X.; Bell, A.E. Rheological properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 227, 64–72. [Google Scholar] [CrossRef]
- Nagy, L.; Szanto, A.; Szatmari, I.; Széles, L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol. Rev. 2012, 92, 739–789. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; OuYang, X.-K.; Qu, Y.-L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Wang, Y.; Zhang, L.; Wei, J.; Zhang, X.; He, F.; Zhang, L. Casein Kinase 2 Interacting Protein-1 regulates M1 and M2 inflammatory macrophage polarization. Cell. Signal. 2017, 33, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Liu, J.; Gou, Y.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Kan, J.; Qian, C. Structural characterization and anti-inflammatory activity of alkali-soluble polysaccharides from purple sweet potato. Int. J. Biol. Macromol. 2019, 131, 484–494. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Kan, J.; Wu, X.; Zhang, X.; Tang, S.; Sun, R.; Liu, J.; Qian, C.; Jin, C. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from Arctium lappa. Int. J. Biol. Macromol. 2019, 135, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yin, H.; Li, R.; Shi, W.; Mou, J.; Yang, J. The activation effects of fucoidan from sea cucumber Stichopus chloronotus on RAW264. 7 cells via TLR2/4-NF-κB pathway and its structure-activity relationship. Carbohydr. Polym. 2021, 270, 118353. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chen, S.; Wang, X.; Yuan, G.; Jiang, F.; Chen, X.; Wang, L. Characterization of Moringa oleifera roots polysaccharide MRP-1 with anti-inflammatory effect. Int. J. Biol. Macromol. 2019, 132, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, Z.; Zhou, R.; Cao, S. A polysaccharide from Umbilicaria yunnana: Structural characterization and anti-inflammation effects. Int. J. Biol. Macromol. 2020, 151, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Hu, Y.; Li, J.; Cai, J.; Qiu, Y.; Dong, C. Anti-inflammatory Effect of a Novel Pectin Polysaccharide from Rubus chingii Hu on Colitis Mice. Front. Nutr. 2022, 9, 868657. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Wassie, T.; Wang, H.-h.; Li, T.; Xie, C.; Wu, X. Enteromorpha prolifera polysaccharide–zinc complex modulates the immune response and alleviates LPS-induced intestinal inflammation via inhibiting the TLR4/NF-κB signaling pathway. Food Funct. 2022, 13, 52–63. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.; Sang, R.; Ge, B.; Wang, M.; Zhou, H.; Zhang, X. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2020, 146, 832–840. [Google Scholar] [CrossRef]

| Gene | Product Size (bp) | ||
|---|---|---|---|
| TNF-α | F | TCTCATTCCTGCTTGTGG | 195 |
| R | CTTGGTGGTTTGCTACG | ||
| IL-6 | F | TAACAGATAAGCTGGAGTC | 115 |
| R | TAGGTTTGCCGAGTAGA | ||
| IL-1β | F | AGCATCCAGCTTCAAATC | 252 |
| R | ATCTCGGAGCCTGTAGTG | ||
| iNOS | F | CCTGAGGGCTTTACTACAC | 136 |
| R | GGTCTTTGCTGGCTGAT | ||
| COX-2 | F | GGGGTGATGAGCAACTA | 199 |
| R | GGGTGCCAGTGATAGAG | ||
| β-actin | F | CTGTGCCCATCTACGAGGGCTAT | 155 |
| R | TTTGATGTCACGCACGATTTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, F.; Zhang, R. An Acidic Polysaccharide with Anti-Inflammatory Effects from Blackened Jujube: Conformation and Rheological Properties. Foods 2022, 11, 2488. https://doi.org/10.3390/foods11162488
Liu C, Wang F, Zhang R. An Acidic Polysaccharide with Anti-Inflammatory Effects from Blackened Jujube: Conformation and Rheological Properties. Foods. 2022; 11(16):2488. https://doi.org/10.3390/foods11162488
Chicago/Turabian StyleLiu, Chuang, Fangzhou Wang, and Rentang Zhang. 2022. "An Acidic Polysaccharide with Anti-Inflammatory Effects from Blackened Jujube: Conformation and Rheological Properties" Foods 11, no. 16: 2488. https://doi.org/10.3390/foods11162488
APA StyleLiu, C., Wang, F., & Zhang, R. (2022). An Acidic Polysaccharide with Anti-Inflammatory Effects from Blackened Jujube: Conformation and Rheological Properties. Foods, 11(16), 2488. https://doi.org/10.3390/foods11162488





