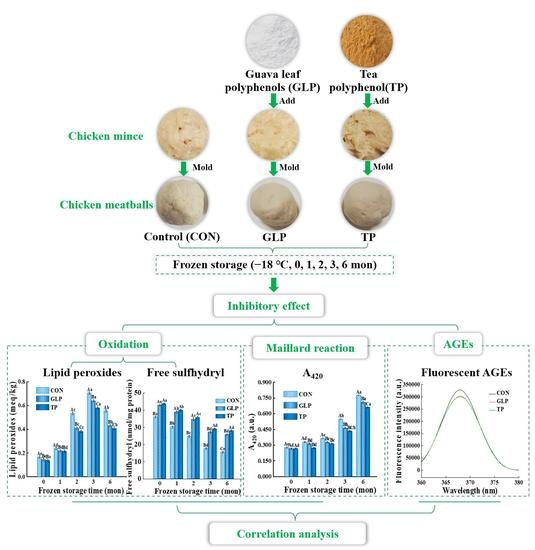
Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (−18 °C) and Its Mechanism Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chicken Meatballs
2.3. Oxidation Staility
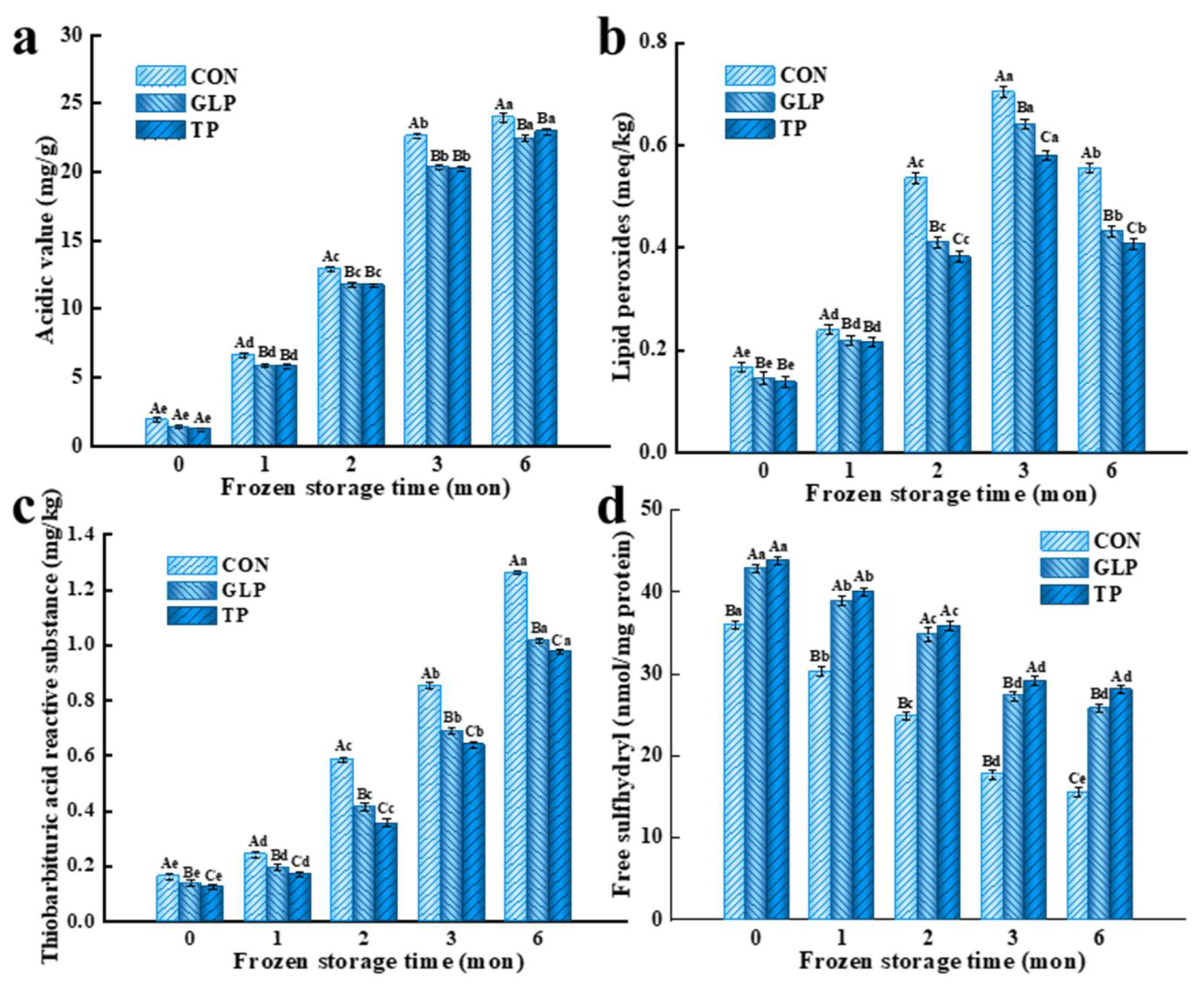
2.3.1. Acidic Value (AV)
2.3.2. Lipid Peroxides (POV)
2.3.3. Thiobarbituric Acid-Reactive Substances (TBARS)
2.3.4. Free Sulfhydryl Groups
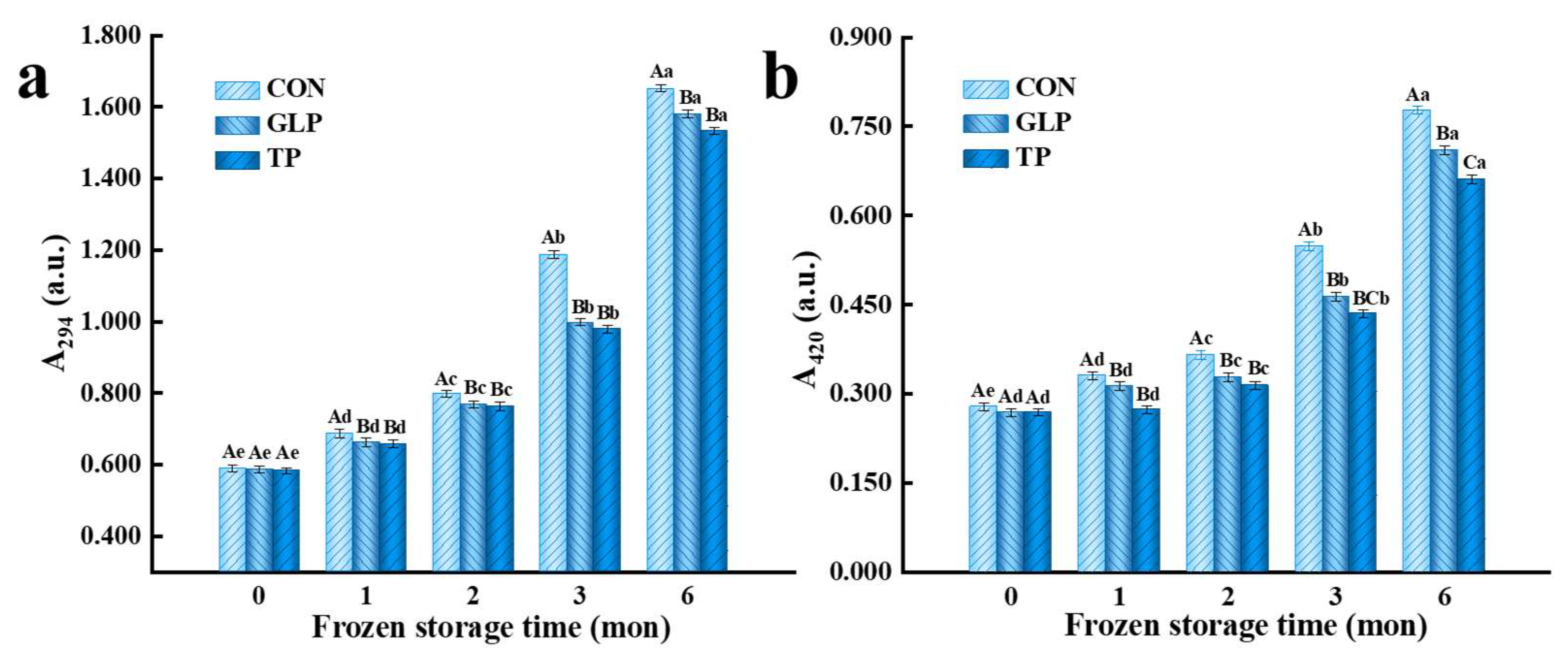
2.4. Degree of Maillard Reaction
2.5. Advanced Glycation end Products
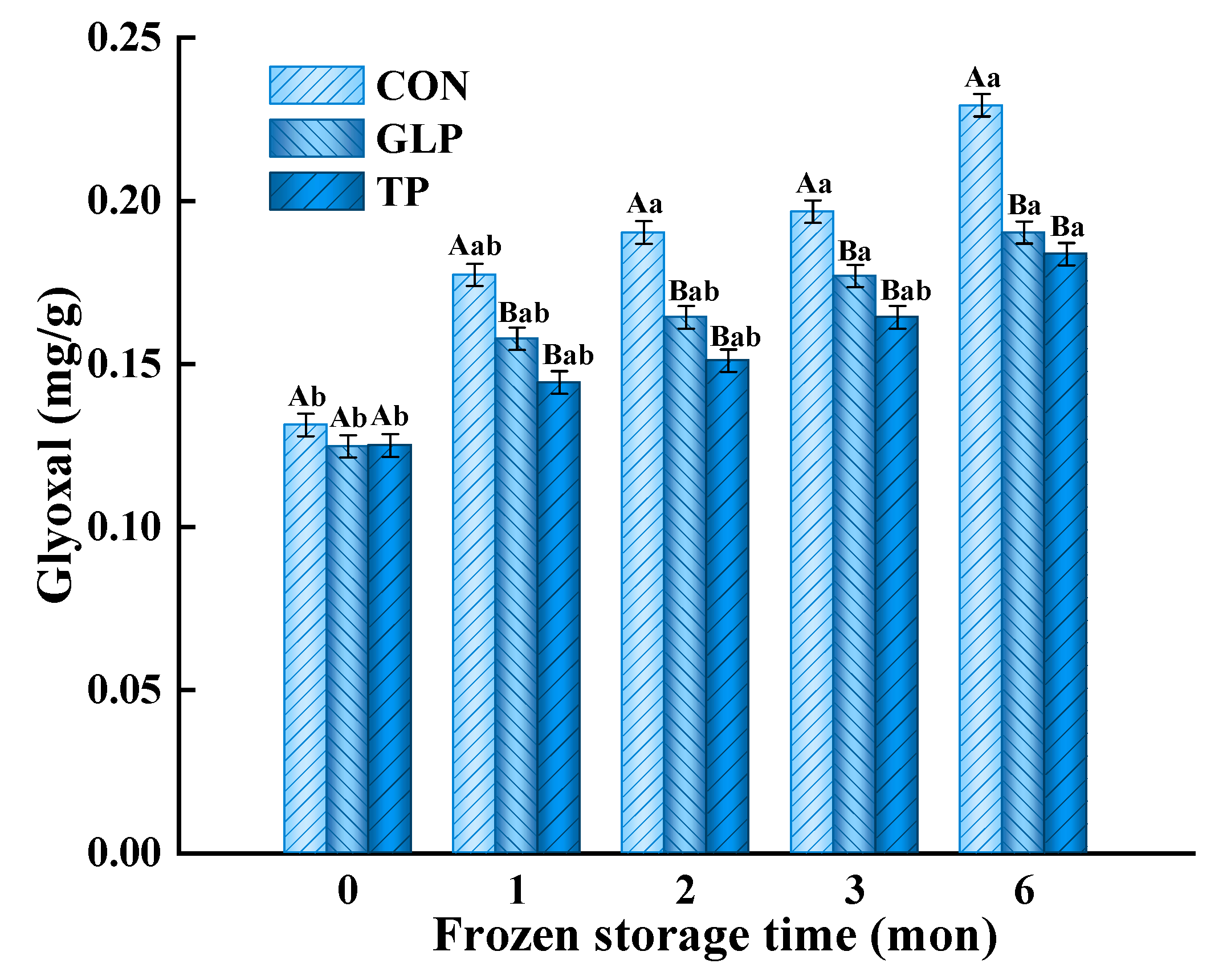
2.5.1. Glyoxal (GO)
2.5.2. Nε-Carboxymethyl-Lysine (CML)
2.5.3. Pentosidine
2.5.4. Fluorescent AGEs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Oxidation Stability
3.1.1. Lipid Oxidation Stability
3.1.2. Protein Oxidation Stability
3.2. Degree of Maillard Reaction
3.2.1. Intermediate Products of Maillard Reaction
3.2.2. Advanced Products of the Maillard Reaction
3.3. Advanced Glycation end Products Formation
3.3.1. Glyoxal (GO)
3.3.2. Nε-Carboxymethyl-Lysine (CML)
3.3.3. Pentosidine
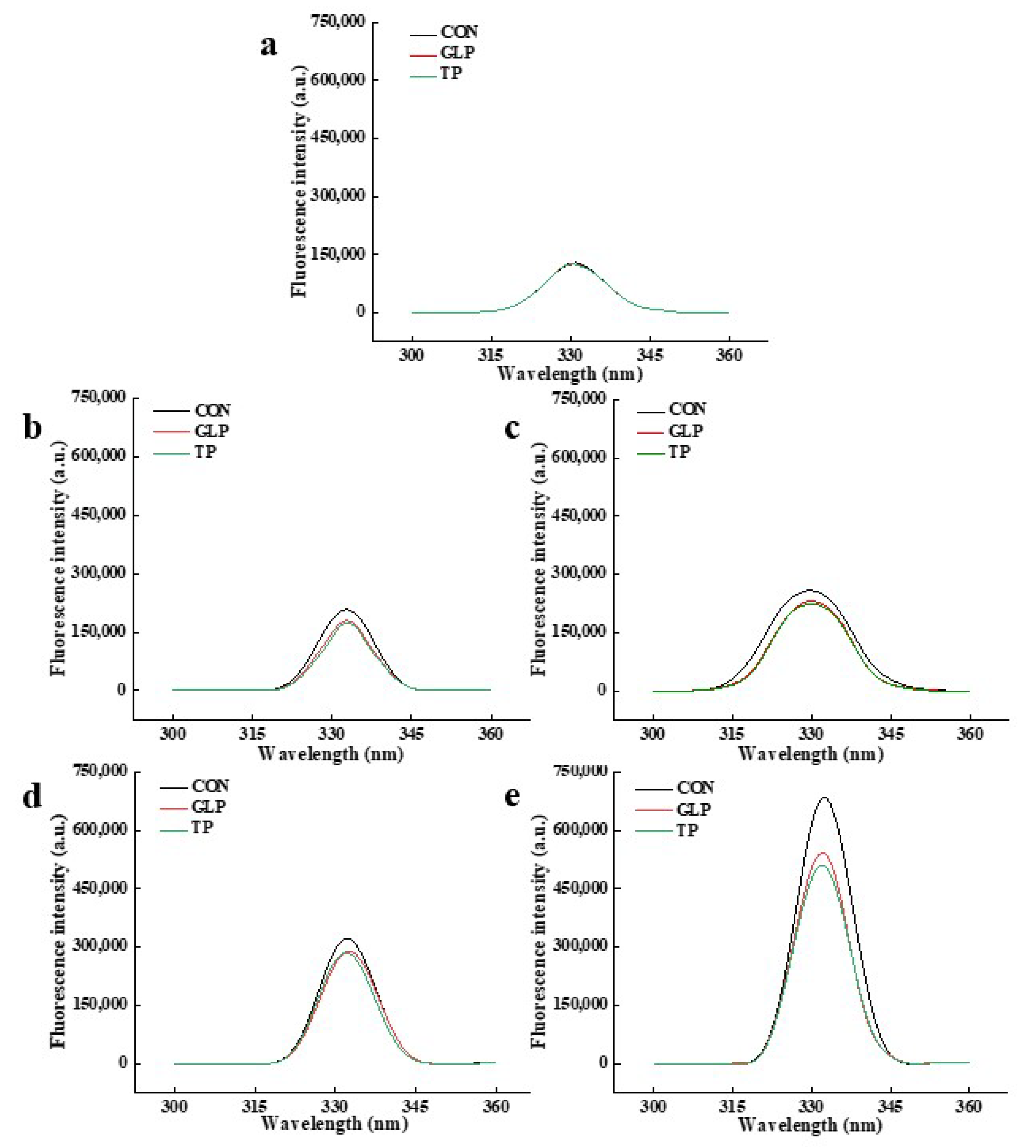
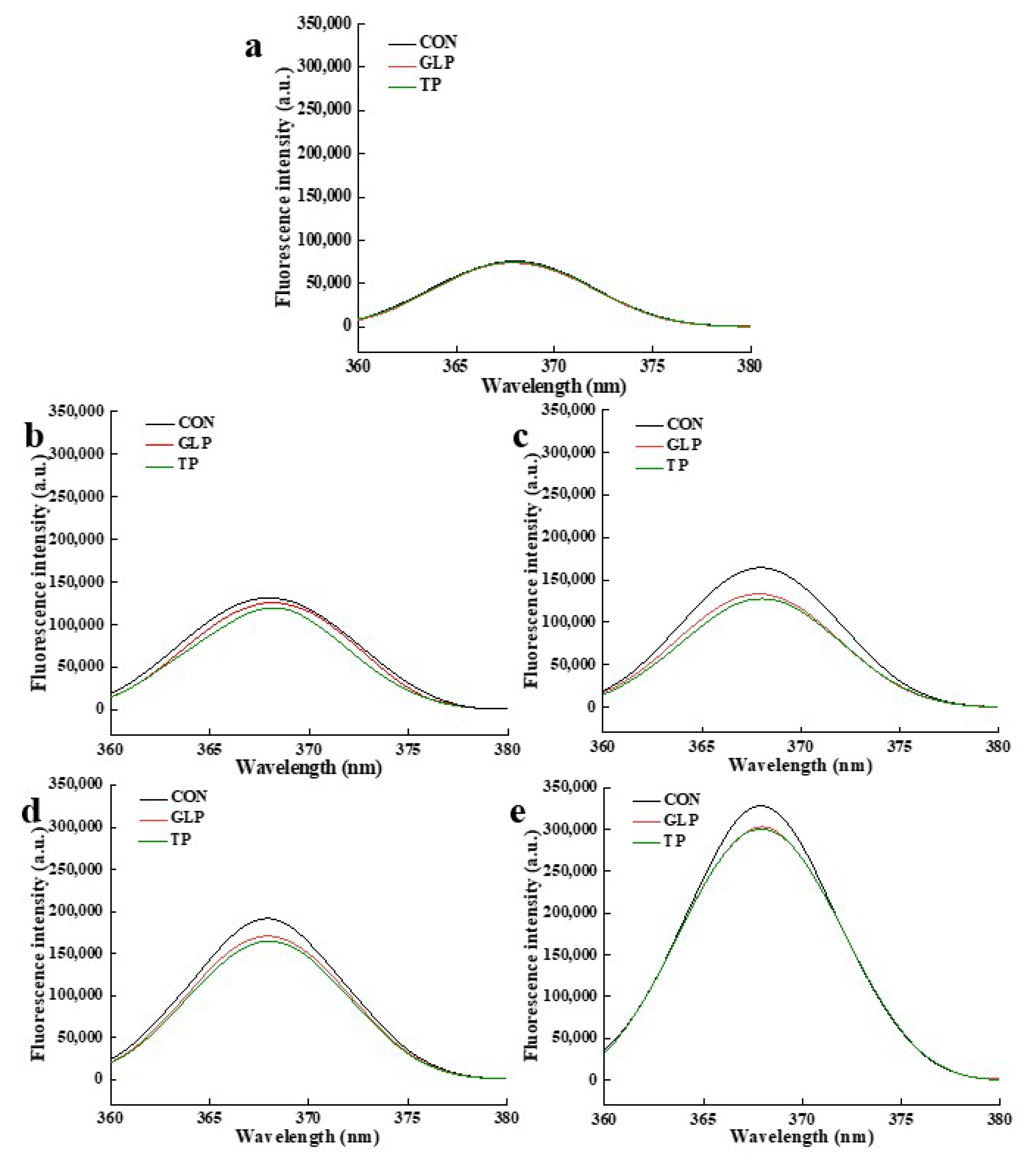
3.3.4. Fluorescent AGEs
3.4. Analysis of Correlation between Oxidation, Maillard Reaction, and AGEs Formation
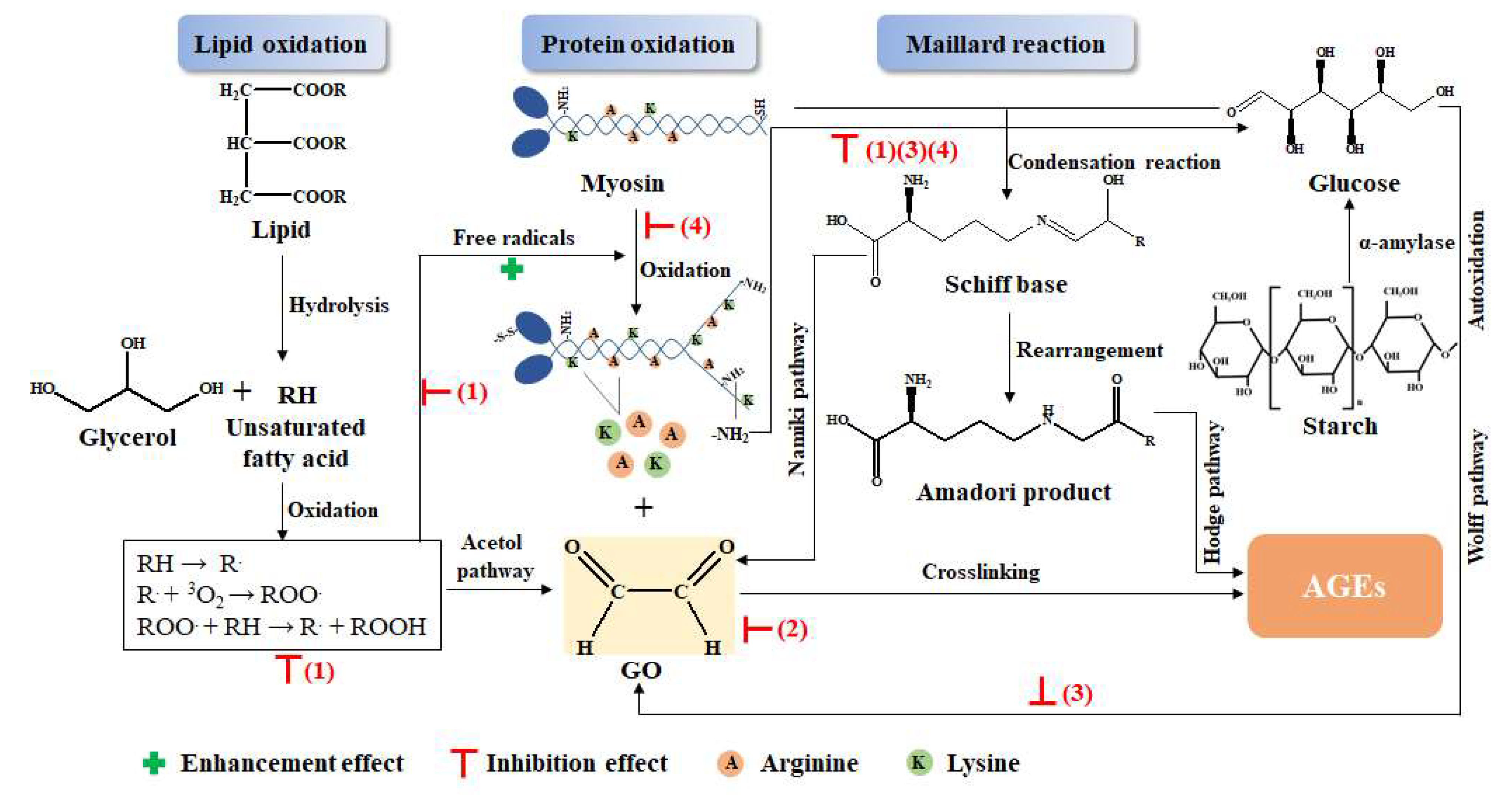
3.5. Possible Mechanism Analysis for the Inhibitory Effect of the GLP on the AGEs
- (1)
- GLP could scavenge free radicals produced by lipid oxidation, which stopped the chain reaction of lipid oxidation, thus preventing the formation of dicarbonyl compounds, the precursor of AGEs, and inhibiting the formation of AGEs [31]. Furthermore, protein oxidation was also prevented by scavenging free radicals; therefore, exposure of the amino group and free amino acid formation were inhibited, which reduced the substrate of the Maillard reaction and the precursor of AGEs [4].
- (2)
- GLP directly captured dicarbonyl compounds that were the precursors of AGEs to inhibit the formation of AGEs. The benzene ring and phenolic hydroxyl group in GLP electronically conjugated to produce phenoxy anions, which could combine with dicarbonyl compounds, thus reducing the precursor of AGEs [52,53].
- (3)
- GLP reduced the production of glucose that was the substrate of the Maillard reaction. Polyphenol in guava leaves inhibited the activity of α-amylase, which reduced the decomposition of polysaccharides into glucose, thereby reducing the substrate for the Maillard reaction [46]. In addition, GLP also reduced the content of the dicarbonyl compounds generated by glucose oxidation [45].
- (4)
- Polyphenols in GLP combined with protein to form polyphenol–protein compounds, which reduced the Maillard reaction between proteins and sugars, resulting in decreased AGEs [11].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, N.; Wan, W.; Kong, B.; Liu, Q.; Lv, H.; Xia, X.; Li, F. Mechanisms of Change in Emulsifying Capacity Induced by Protein Denaturation and Aggregation in Quick-Frozen Pork Patties with Different Fat Levels and Freeze-Thaw Cycles. Foods 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.; Xu, Y.; Zhao, X.; Zhao, L.; Yang, H. Preservative effect of slightly acid electrolysed water ice generated by the developed sanitising unit on shrimp (Penaeus vannamei). Food Control 2022, 136, 108876. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; He, Z.; Wang, Z.; Qin, F.; Zeng, M. Effect of Freeze-Thaw Cycles on the Oxidation of Protein and Fat and Its Relationship with the Formation of Heterocyclic Aromatic Amines and Advanced Glycation End Products in Raw Meat. Molecules 2021, 26, 1264. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Xia, X.; Kong, B.; Diao, X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019, 54, 311–320. [Google Scholar] [CrossRef]
- Li, T.; Niu, L.; Li, X.; Wang, F.; Huang, Y.; Liu, Y. Formation of advanced glycation end-products in silver carp (Hypophthalmichthys molitrix) surimi products during heat treatment as affected by freezing-thawing cycles. Food Chem. 2022, 395, 133612. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Khan, I.A.; Huang, M.; Zhang, X. Inhibitory mechanism of catechins against advanced glycation end products of glycated myofibrillar protein through anti-aggregation and anti-oxidation. LWT Food Sci. Technol. 2021, 147, 111550. [Google Scholar] [CrossRef]
- Zhao, W.; Cai, P.; Zhang, N.; Wu, T.; Sun, A.; Jia, G. Inhibitory effects of polyphenols from black chokeberry on advanced glycation end-products (AGEs) formation. Food Chem. 2022, 392, 133295. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese bayberry (Myrica rubra) phenolics mitigated protein glycoxidation and formation of advanced glycation end-products: A mechanistic investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef]
- Szkudlarz, S.M.; Siger, A.; Szwengiel, A.; Bajerska, J. Natural compounds from grape by-products enhance nutritive value and reduce formation of CML in model muffins. Food Chem. 2015, 172, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium guajava L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Foods 2021, 10, 752. [Google Scholar] [CrossRef]
- Kumar, M.; Kapoor, S.; Dhumal, S.; Tkaczewska, J.; Changan, S.; Saurabh, V.; Mekhemar, M.; Radha; Rais, N.; Satankar, V.; et al. Guava (Psidium guajava L.) seed: A low-volume, high-value byproduct for human health and the food industry. Food Chem. 2022, 386, 132694. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Asi, M.R.; Hameed, A.; Bourquin, L.D. Effect of Postharvest Application of Aloe Vera Gel on Shelf Life, Activities of Anti-Oxidative Enzymes, and Quality of ‘Gola’ Guava Fruit. Foods 2020, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Liu, J.; Zhou, M.; Wu, X.; Chen, Q. Determination of Phenolic Compounds and Antioxidant Activities from Peel, Flesh, Seed of Guava (Psidium guajava L.). Electrophoresis 2018, 39, 1654–1662. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.T.; Le, V.V.M.; Sajeev, D.; Schilling, M.W.; Dinh, T.T.N. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Sci. 2020, 165, 108106. [Google Scholar] [CrossRef]
- Shahimi, S.; Mutalib, S.A.; Ismail, N.; Elias, A.; Hashim, H.; Kashim, M.I.A.M. Species-specific identification of porcine blood plasma in heat-treated chicken meatballs. Saudi J. Biol. Sci. 2021, 28, 2447–2452. [Google Scholar] [CrossRef]
- Pan, N.; Dong, C.; Du, X.; Kong, B.; Sun, J.; Xia, X. Effect of freeze-thaw cycles on the quality of quick-frozen pork patty with different fat content by consumer assessment and instrument-based detection. Meat Sci. 2021, 172, 108313. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Xia, X.; Sun, F.; Kong, B. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein. LWT Food Sci. Technol. 2021, 142, 111016. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Xiong, Y.; Ren, Y. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Ma, J.; Yin, X.; Wang, L.; Wu, W.; Xiong, G.; Sun, W.; Zhou, Y. Effects of low frequency magnetic field on myoglobin oxidation stability. Food Chem. 2020, 309, 125651. [Google Scholar] [CrossRef]
- Zhu, Z.; Fang, R.; Huang, M.; Wei, Y.; Zhou, G. Oxidation combined with Maillard reaction induced free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine formation during braised chicken processing. Food Sci. Hum. Wellness 2020, 9, 383–393. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Huang, S.; Yao, M.; Lei, Y.; Khan, I.; Huang, M.; Zhou, X. Formation of Nε-Carboxymethyllysine and Nε-Carboxyethyllysine in Prepared Chicken Breast by Pan Frying. J. Food Prot. 2019, 82, 2154–2160. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Panaskar, S.N.; Arvindekar, A.U. nhibition of advanced glycation end product formation by cymene—A common food constituent. J. Funct. Foods 2014, 6, 107–115. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, Y.; Qin, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Study of the formation of food hazard factors in fried fish nuggets. Food Chem. 2022, 373, 131562. [Google Scholar] [CrossRef]
- Biffin, T.E.; Smith, M.A.; Bush, R.D.; Collins, D.; Hopkins, D.L. The effect of electrical stimulation and tenderstretching on colour and oxidation traits of alpaca (vicunga pacos) meat. Meat Sci. 2019, 156, 125–130. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L.; Ji, H.; Liu, S. Effect of vacuum impregnation of red sea bream (Pagrosomus major) with herring AFP combined with CS@Fe3O4 nanoparticles during freeze-thaw cycles. Food Chem. 2019, 291, 139–148. [Google Scholar] [CrossRef]
- Banerjee, S. Inhibition of mackerel (Scomber scombrus) muscle lipoxygenase by green tea polyphenols. Food Res. Int. 2006, 39, 486–491. [Google Scholar] [CrossRef]
- Nantitanon, W.; Okonogi, S. Comparison of antioxidant activity of compounds isolated from guava leaves and a stability study of the most active compound. Drug Discov. Ther. 2012, 6, 38–43. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalis, S.; Wojdyła, M.W.; Florowski, T.; Oszmiański, J. Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage. LWT Food Sci. Technol. 2020, 130, 109718. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Dong, C.; Ren, Y.; Pan, N.; Kong, B.; Liu, H.; Xia, X. Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT Food Sci. Technol. 2020, 139, 110570. [Google Scholar] [CrossRef]
- Guan, J.-J.; Qiu, A.-Y.; Liu, X.-Y.; Hua, Y.-F.; Ma, Y.-H. Microwave improvement of soy protein isolate—Saccharide graft reactions. Food Chem. 2005, 97, 577–585. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Pan, N.; Wang, S.; Xia, X.; Zhang, D. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, L.; Xiang, R.; Liu, X.; Zhu, M. Effects of mulberry polyphenols on oxidation stability of sarcoplasmic and myofibrillar proteins in dried minced pork slices during processing and storage. Meat Sci. 2020, 160, 107973. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, S.; Khan, I.; Cheng, Y.; Yu, Y.; Zhang, C.; Huang, J.; Huang, M.; Zhou, X. The effect of oxidation and Maillard reaction on formation of Nε-carboxymethyllysine and Nε-carboxyethyllysine in prepared chicken breast. CyTA-J. Food 2019, 17, 685–694. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Huang, S.; Dong, X.; Zhang, Y.; Huang, M.; Zheng, Y. Effects of oxidation and precursors (lysine, glyoxal and Schiff base) on the formation of Nε-carboxymethyl-lysine in aged, stored and thermally treated chicken meat. Food Sci. Hum. Wellness 2022, 11, 1252–1258. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 625–651. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Requena, J.R.; Jenkins, A.J. The advanced glycation end product, Nε-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Zhu, H.; Yang, F.; Xiao, K.; Zhang, L.; Zhang, M.; Peng, Y.; Wang, C.; Li, D.; et al. Lactobacillus fermentum as a new inhibitor to control advanced glycation end-product formation during vinegar fermentation. Food Sci. Hum. Wellness 2022, 11, 1409–1418. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, J.; Wang, M. Phloretin and its methylglyoxal adduct: Implications against advanced glycation end products-induced inflammation in endothelial cells. Food Chem. Toxicol. 2019, 129, 291–300. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, L.; Sun, D. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Wu, C.-H.; Huang, S.-M.; Lin, J.-A.; Yen, G.-C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.-W.; Ma, J.; Chen, B.; Ho, C.-T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef]
- Villa, M.; Parravano, M.; Micheli, A.; Gaddini, L.; Matteucci, A.; Mallozzi, C.; Facchiano, F.; Malchiodi-Albedi, F.; Pricci, F. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: Correlation with in vitro and in vivo non-enzymatic glycation. Metabolism 2017, 71, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Totlani, V.M.; Peterson, D.G. Epicatechin carbonyl-trapping reactions in aqueous maillard systems: Identification and structural elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef]
- Navarro, M.; Morales, F.J. Mechanism of reactive carbonyl species trapping by hydroxytyrosol under simulated physiological conditions. Food Chem. 2015, 175, 92–99. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, M.; Li, Y.; Kong, B.; Liu, Q.; Sun, F.; Yu, W.; Xia, X. Effect of hot air gradient drying on quality and appearance of beef jerky. LWT Food Sci. Technol. 2021, 150, 111974. [Google Scholar] [CrossRef]
- Zhao, M.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.; Sun, F.; Li, Y.; Xia, X. Monitoring dynamic changes in chicken freshness at 4 °C and 25 °C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef]
- Cheng, S.; He, Y.; Zeng, T.; Wang, D.; He, J.; Xia, Q.; Zhou, C.; Pan, D.; Cao, J. Heat stress induces various oxidative damages to myofibrillar proteins in ducks. Food Chem. 2022, 390, 133209. [Google Scholar] [CrossRef]
- Yu, L.; Li, Q.; Li, Y.; Yang, Y.; Guo, C.; Li, M. Impact of frozen storage duration of raw pork on the formation of advanced glycation end-products in meatballs. LWT Food Sci. Technol. 2021, 146, 111481. [Google Scholar] [CrossRef]


| Ingredients | Chicken Meatballs Groups | ||
|---|---|---|---|
| CON | GLP | TP | |
| Chicken (g) | 100 | 100 | 100 |
| Pork fat (g) | 20 | 20 | 20 |
| Salt (g) | 2 | 2 | 2 |
| Potato starch (g) | 6 | 6 | 6 |
| Composite phosphate (g) | 0.4 | 0.4 | 0.4 |
| Polyphenols powder (mg) | - | 9 | 3 |
| Water (mL) | 20 | 20 | 20 |
| Frozen Storage Time | Samples | Pentosidine | Fluorescent AGEs | ||
|---|---|---|---|---|---|
| Wavelength (nm) | Fluorescence Intensity (a.u.) | Wavelength (nm) | Fluorescence Intensity (a.u.) | ||
| 0 months | CON | 331 | 128,715 | 368 | 75,886 |
| GLP | 331 | 124,415 | 368 | 73,804 | |
| TP | 330 | 125,327 | 368 | 75,029 | |
| 1 months | CON | 333 | 207,354 | 368 | 131,320 |
| GLP | 333 | 179,418 | 368 | 125,920 | |
| TP | 333 | 174,050 | 368 | 119,465 | |
| 2 months | CON | 330 | 258,223 | 368 | 164,285 |
| GLP | 330 | 230,923 | 368 | 133,297 | |
| TP | 330 | 224,132 | 368 | 127,638 | |
| 3 months | CON | 332 | 320,950 | 368 | 191,465 |
| GLP | 333 | 288,865 | 368 | 170,800 | |
| TP | 332 | 285,138 | 368 | 164,615 | |
| 6 months | CON | 332 | 680,509 | 368 | 328,147 |
| GLP | 332 | 541,299 | 368 | 302,961 | |
| TP | 332 | 509,650 | 368 | 300,140 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, Y.; Bai, X.; Feng, J.; Xia, X.; Li, F. Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (−18 °C) and Its Mechanism Analysis. Foods 2022, 11, 2509. https://doi.org/10.3390/foods11162509
Zhao M, Li Y, Bai X, Feng J, Xia X, Li F. Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (−18 °C) and Its Mechanism Analysis. Foods. 2022; 11(16):2509. https://doi.org/10.3390/foods11162509
Chicago/Turabian StyleZhao, Mengna, Ying Li, Xue Bai, Jia Feng, Xiufang Xia, and Fangfei Li. 2022. "Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (−18 °C) and Its Mechanism Analysis" Foods 11, no. 16: 2509. https://doi.org/10.3390/foods11162509
APA StyleZhao, M., Li, Y., Bai, X., Feng, J., Xia, X., & Li, F. (2022). Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (−18 °C) and Its Mechanism Analysis. Foods, 11(16), 2509. https://doi.org/10.3390/foods11162509