Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pilot-Scale Vinification
2.2. Microbial Strains, Culture Conditions and Inoculum Preparation
2.3. Fermentation Modalities
2.4. Yeast and Bacterial Enumeration
2.5. Profiling of Wine Composition
2.6. Sensory Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Fermentation Kinetics and Trend of Titratable Acidity during the Pilot-Scale Vinification
3.2. Oenological Parameters of the Cabernet Sauvignon Wines
3.3. Volatile Composition of the Cabernet Sauvignon Wines
3.4. Multivariate Analysis of Cabernet Sauvignon Wine Parameters
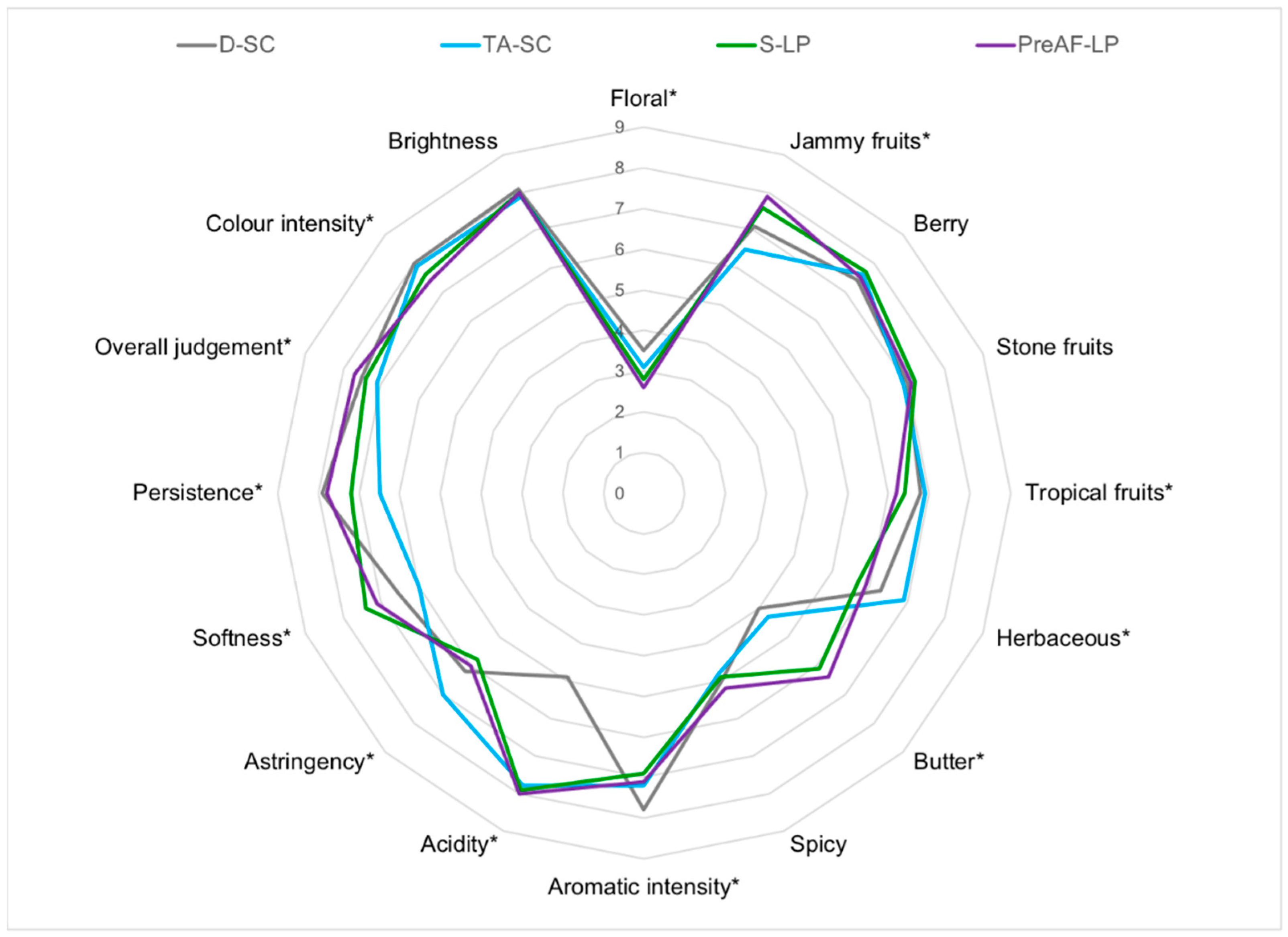
3.5. Sensory Profiling of the Cabernet Sauvignon Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, K.; Jiranck, V. Wine of reduced alcohol content: Consumer and society demand vs industry willingness and ability to deliver. In Conference: 1st International Symposium on Alcohol Level Reduction in Wine Oenoviti International Network; Institut des Sciences de la Vigne et du Vin: Villenave d’Ornon, France, 2013; pp. 98–104. [Google Scholar]
- Godden, P.; Wilkes, E.; Johnson, D. Trends in the composition of Australian wine 1984–2014. Aust. J. Grape Wine Res. 2015, 21, 741–753. [Google Scholar]
- Schultz, H.R.; Jones, G.V. Climate induced historic and future changes in viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Comuzzo, P.; Battistutta, F. Acidification and pH control in red wines. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–34. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and ph of wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar]
- Schelezki, O.J.; Suklje, K.; Boss, P.K.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on wine volatile composition and sensory properties. Food Chem. 2018, 259, 196–206. [Google Scholar]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021, 349, 129015. [Google Scholar]
- Vaquero, C.; Loira, I.; Antonia Banuelos, M.; Maria Heras, J.; Cuerda, R.; Morata, A. Industrial performance of several Lachancea thermotolerans strains for pH control in white wines from warm areas. Microorganisms 2020, 8, 830. [Google Scholar]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar]
- Lucio, O.; Pardo, I.; Maria Heras, J.; Krieger, S.; Ferrer, S. Influence of yeast strains on managing wine acidity using Lactobacillus plantarum. Food Control 2018, 92, 471–478. [Google Scholar]
- Onetto, C.A.; Bordeu, E. Pre-alcoholic fermentation acidification of red grape must using Lactobacillus plantarum. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 108, 1469–1475. [Google Scholar]
- Bae, S.; Fleet, G.H.; Heard, G.M. Lactic acid bacteria associated with wine grapes from several Australian vineyards. J. Appl. Microbiol. 2006, 100, 712–727. [Google Scholar]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a new biological tool to control malolactic fermentation: A review and an outlook. Beverages 2020, 6, 23. [Google Scholar]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Maria Heras, J.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT-Food Sci. Technol. 2016, 73, 334–341. [Google Scholar]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The microbial challenge of winemaking: Yeast-bacteria compatibility. FEMS Yeast Res. 2019, 19, foz040. [Google Scholar]
- Spano, G.; Massa, S. Environmental stress response in wine lactic acid bacteria: Beyond Bacillus subtilis. Crit. Rev. Microbiol. 2006, 32, 77–86. [Google Scholar]
- Devi, A.; Anu-Appaiah, K.A. Mixed malolactic co-culture (Lactobacillus plantarum and Oenococcus oeni) with compatible Saccharomyces influences the polyphenolic, volatile and sensory profile of Shiraz wine. LWT-Food Sci. Technol. 2021, 135, 110246. [Google Scholar]
- Devi, A.; Anu-Appaiah, K.A.; Lin, T.-F. Timing of inoculation of Oenococcus oeni and Lactobacillus plantarum in mixed malolactic culture along with compatible native yeast influences the polyphenolic, volatile and sensory profile of the Shiraz wines. LWT-Food Sci. Technol. 2022, 158, 113130. [Google Scholar]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter Lactobacillus plantarum M10: Impact on chemical and sensorial characteristics of Fiano wine. Microorganisms 2020, 8, 516. [Google Scholar]
- Maicas, S.; Gil, J.V.; Pardo, I.; Ferrer, S. Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Res. Int. 1999, 32, 491–496. [Google Scholar]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of co-inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the industrial production of Negroamaro wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar]
- Englezos, V.; Torchio, F.; Vagnoli, P.; Krieger-Weber, S.; Rantsiou, K.; Cocolin, L. Impact of Saccharomyces cerevisiae strain selection on malolactic fermentation by Lactobacillus plantarum and Oenococcus oeni. Am. J. Enol. Vitic. 2020, 71, 157–165. [Google Scholar]
- Paz, P.C.; Janny, R.J.; Hakansson, A. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar]
- Chen, Y.; Jiang, J.; Song, Y.; Zang, X.; Wang, G.; Pei, Y.; Song, Y.; Qin, Y.; Liu, Y. Yeast diversity during spontaneous fermentations and oenological characterisation of indigenous Saccharomyces cerevisiae for potential as wine starter cultures. Microorganisms 2022, 10, 1455. [Google Scholar]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar]
- Benucci, I.; Luziatelli, F.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ruzzi, M.; Esti, M. Pre-fermentative cold maceration in the presence of non-Saccharomyces strains: Effect on fermentation behaviour and volatile composition of a red wine. Aust. J. Grape Wine Res. 2018, 24, 267–274. [Google Scholar]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar]
- Gomez-Miguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar]
- Carrasco-Quiroz, M.; Martinez-Gil, A.M.; Gutierrez-Gamboa, G.; Moreno-Simunovic, Y. Effect of rootstocks on volatile composition of Merlot wines. J. Sci. Food Agric. 2020, 100, 3517–3524. [Google Scholar]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; Lopes, M.A.d.B.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile compounds related to ‘stone fruit’ aroma attributes in Viognier and Chardonnay wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar]
- Kong, C.-L.; Li, A.-H.; Su, J.; Wang, X.-C.; Chen, C.-Q.; Tao, Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT-Food Sci. Technol. 2019, 109, 83–92. [Google Scholar]
- Cullere, L.; Ferreira, V.; Cacho, J. Analysis, occurrence and potential sensory significance of aliphatic aldehydes in white wines. Food Chem. 2011, 127, 1397–1403. [Google Scholar]
- Pons, M.; Dauphin, B.; La Guerche, S.; Pons, A.; Lavigne-Cruege, V.; Shinkaruk, S.; Bunner, D.; Richard, T.; Monti, J.-P.; Darriet, P. Identification of impact odorants contributing to fresh mushroom off-flavor in wines: Incidence of their reactivity with nitrogen compounds on the decrease of the olfactory defect. J. Agric. Food Chem. 2011, 59, 3264–3272. [Google Scholar]
- Saguir, F.M.; de Nadra, M.C.M. Effect of L-malic and citric acids metabolism on the essential amino acid requirements for Oenococcus oeni growth. J. Appl. Microbiol. 2002, 93, 295–301. [Google Scholar]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile composition and sensory properties of Shiraz wines as affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. In Annual Review of Food Science and Technology; Doyle, M.P., Klaenhammer, T.R., Eds.; Annual Reviews: San Mateo, CA, USA, 2013; Volume 4, pp. 1–20. [Google Scholar]
- Hu, K.; Zhao, H.; Kang, X.; Ge, X.; Zheng, M.; Hu, Z.; Tao, Y. Fruity aroma modifications in Merlot wines during simultaneous alcoholic and malolactic fermentations through mixed culture of S. cerevisiae, P. fermentans, and L. brevis. LWT-Food Sci. Technol. 2022, 154, 112711. [Google Scholar]
- Cooke, R.C.; van Leeuwen, K.A.; Capone, D.L.; Gawel, R.; Elsey, G.M.; Sefton, M.A. Odor detection thresholds and enantiomeric distributions of several 4-alkyl substituted gamma-lactones in Australian red wine. J. Agric. Food Chem. 2009, 57, 2462–2467. [Google Scholar]
- Brizuela, N.S.; Franco-Luesma, E.; Bravo-Ferrada, B.M.; Perez-Jimenez, M.; Semorile, L.; Tymczyszyn, E.E.; Pozo-Bayon, M.A. Influence of Patagonian Lactiplantibacillus plantarum and Oenococcus oeni strains on sensory perception of Pinot Nnoir wine after malolactic fermentation. Aust. J. Grape Wine Res. 2021, 27, 118–127. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.-C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar]
- Lytra, G.; Tempere, S.; de Revel, G.; Barbe, J.-C. Impact of perceptive interactions on red wine fruity aroma. J. Agric. Food Chem. 2012, 60, 12260–12269. [Google Scholar]
 ), TA-SC (
), TA-SC (  ), S-LP (▲), PreAF-LP (△).
), S-LP (▲), PreAF-LP (△).
 ), TA-SC (
), TA-SC (  ), S-LP (▲), PreAF-LP (△).
), S-LP (▲), PreAF-LP (△).
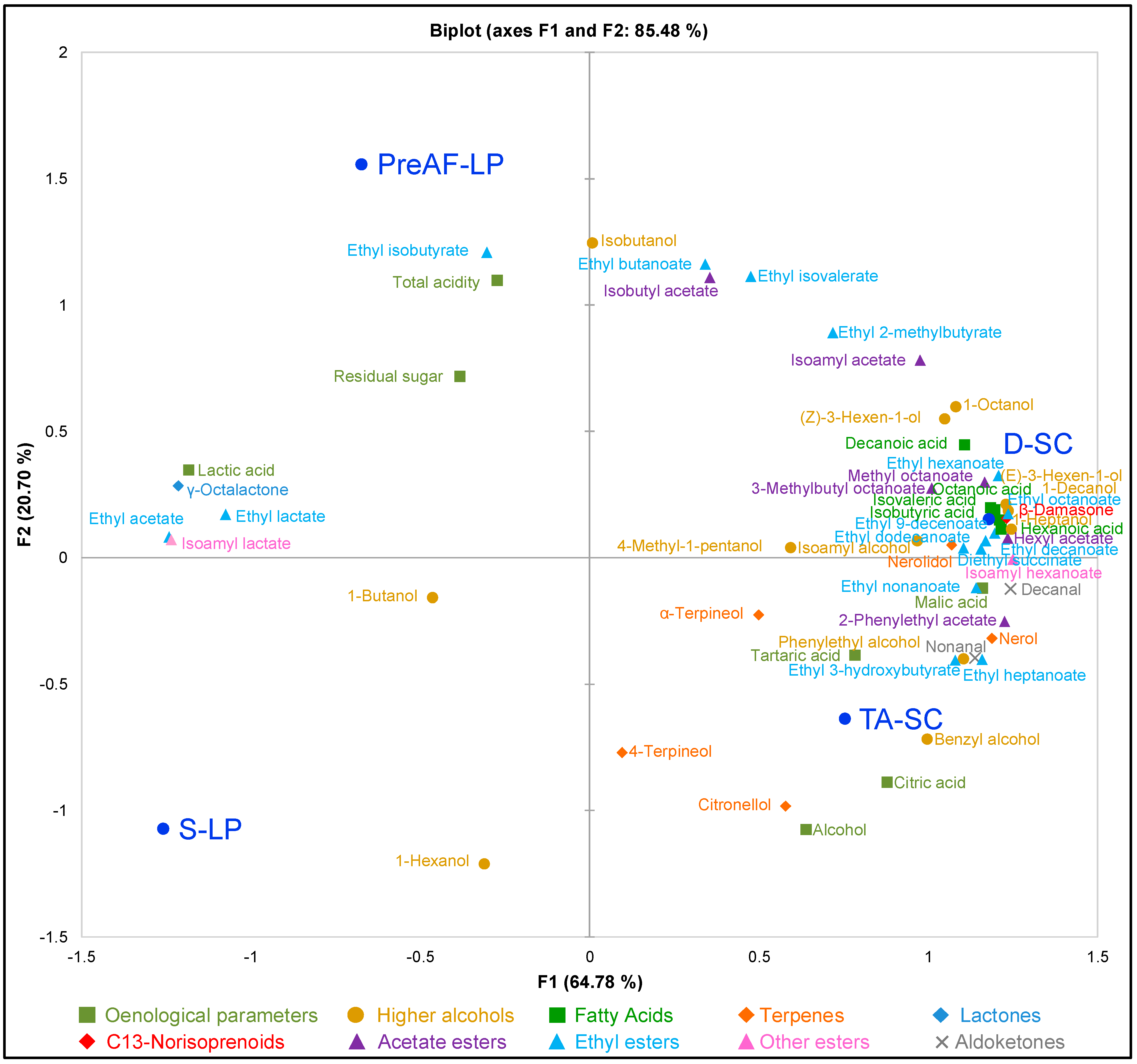
| Parameters | D-SC | TA-SC | S-LP | PreAF-LP |
|---|---|---|---|---|
| Sugar (g/L) | 2.19 ± 0.11 b | 1.06 ± 0.23 a | 1.94 ± 0.17 b | 2.26 ± 0.07 b |
| Lactic acid (g/L) | 0.40 ± 0.13 a | 0.23 ± 0.13 a | 2.04 ± 0.01 b | 2.05 ± 0.03 b |
| Malic acid (g/L) | 1.53 ± 0.13 c | 1.78 ± 0.04 d | 0.81 ± 0.03 a | 1.04 ± 0.05 b |
| Citric acid (g/L) | 0.31 ± 0.03 b | 0.33 ± 0.03 b | 0.27 ± 0.01 b | 0.20 ± 0.01 a |
| Tartaric acid (g/L) | 2.93 ± 0.06 a | 4.06 ± 0.04 b | 2.79 ± 0.12 a | 2.83 ± 0.01 a |
| Acetic acid (g/L) | 0.23 ± 0.01 a | 0.26 ± 0.01 a | 0.37 ± 0.01 ab | 0.50 ± 0.01 b |
| ATitratable acidity (g/L) | 6.62 ± 0.19 a | 7.72 ± 0.06 b | 7.58 ± 0.13 b | 8.04 ± 0.05 c |
| pH | 3.58 ± 0.01 c | 3.55 ± 0.01 b | 3.57 ± 0.01 bc | 3.51 ± 0.01 a |
| BVolatile acidity (g/L) | 0.31 ± 0.03 a | 0.32 ± 0.07 a | 0.40 ± 0.01 a | 0.54 ± 0.08 a |
| Ethanol (% v/v) | 12.65 ± 0.21 b | 12.85 ± 0.21 b | 12.55 ± 0.07 b | 11.60 ± 0.14 a |
| Compounds | Aroma Threshold | D-SC | TA-SC | S-LP | PreAF-LP | Odour Description | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | OAV | Concentration | OAV | Concentration | OAV | Concentration | OAV | |||
| Isoamyl acetate | 30 [30] | 2277.34 ± 23.14 c | 75.9 | 1833.62 ± 95.75 b | 61.1 | 1020.43 ± 34.48 a | 34.0 | 1976.14 ± 87.67 b | 65.9 | Banana [30] |
| Isobutyl acetate | 1600 [31] | 185.84 ± 5.42 b | 0.1 | 108.29 ± 1.34 a | <0.1 | 97.80 ± 5.82 a | <0.1 | 191.18 ± 8.17 b | 0.1 | Banana [31] |
| Hexyl acetate | 670 [30] | 11.86 ± 0.16 c | <0.1 | 7.82 ± 0.74 b | <0.1 | 4.25 ± 0.31 a | <0.1 | 5.37 ± 0.22 a | <0.1 | Fruity, floral [30] |
| 2-Phenylethyl acetate | 250 [31] | 31.31 ± 3.67 b | 0.1 | 29.32 ± 1.39 b | 0.1 | 11.92 ± 0.35 a | <0.1 | 12.44 ± 0.46 a | <0.1 | Honey, floral, fruity [31] |
| Σ Acetate esters | 2506.35 ± 32.39 d | 1979.05 ± 99.22 b | 1134.40 ± 40.96 a | 2185.13 ± 96.52 c | ||||||
| Ethyl acetate | 7500 [31] | 55,398.05 ± 609.17 a | 7.4 | 55,799.40 ± 382.54 a | 7.4 | 66,976.00 ± 958.55 b | 8.9 | 64,385.25 ± 4396.44 b | 8.6 | Fruity, nail polish, balsamic [33] |
| Ethyl butanoate | 20 [30] | 106.29 ± 3.73 b | 5.3 | 103.35 ± 1.23 b | 5.2 | 77.64 ± 4.68 a | 3.9 | 127.72 ± 7.28 c | 6.4 | Strawberry, lactic [30] |
| Ethyl hexanoate | 14 [30] | 903.99 ± 43.24 c | 64.6 | 821.06 ± 51.74 c | 58.6 | 477.62 ± 37.73 a | 34.1 | 684.05 ± 26.34 b | 48.9 | Apple peel, fruit [30] |
| Ethyl heptanoate | 220 [31] | 6.07 ± 0.30 c | <0.1 | 4.91 ± 0.64 b | <0.1 | 3.32 ± 0.30 a | <0.1 | 2.6 ± 0.15 a | <0.1 | Fruity, pineapple [31] |
| Ethyl octanoate | 5 [31] | 862.82 ± 71.22 d | 172.6 | 546.01 ± 88.56 c | 109.2 | 146.91 ± 1.16 a | 29.4 | 342.8 ± 17.11 b | 68.6 | Pear, apricot [31] |
| Ethyl nonanoate | 1200 [30] | 4.69 ± 0.08 c | <0.1 | 4.03 ± 0.15 b | <0.1 | 3.54 ± 0.03 a | <0.1 | 3.51 ± 0.06 a | <0.1 | Waxy, fruity, rose, rum [30] |
| Ethyl decanoate | 200 [31] | 130.85 ± 15.01 c | 0.7 | 99.81 ± 14.89 b | 0.5 | 38.26 ± 0.14 a | 0.2 | 59.99 ± 0.21 a | 0.3 | Fruity, fatty [31] |
| Ethyl 3-hydroxybutyrate | 20,000 [33] | 514.45 ± 27.25 b | <0.1 | 583.44 ± 7.35 c | <0.1 | 385.1 ± 20.67 a | <0.1 | 383.11 ± 1.38 a | <0.1 | Grape |
| Ethyl lactate | 146,000 [30] | 22,375.3 ± 335.03 a | 0.2 | 86,150.35 ± 5320.34 b | 0.6 | 114,821.5 ± 737.51 c | 0.8 | 126,198.7 ± 19.66 c | 0.9 | Fruity, buttery [43] |
| Ethyl dodecanoate | 500 [30] | 101.79 ± 3.13 c | 0.2 | 90.36 ± 1.01 b | 0.2 | 84.28 ± 0.08 a | 0.2 | 85.59 ± 0.83 a | 0.2 | Sweet, floral, fruity, buttery [30] |
| Diethyl succinate | 1,250,000 [30] | 633.88 ± 92.01 bc | <0.1 | 691.51 ± 16.79 c | <0.1 | 444.41 ± 2.62 ab | <0.1 | 533.68 ± 27.01 a | <0.1 | Wine, fruity [30] |
| Ethyl isobutyrate | 15 [34] | 206.43 ± 3.06 b | 13.8 | 160.38 ± 1.68 a | 10.7 | 173.24 ± 11.77 a | 11.5 | 288.74 ± 14.44 c | 19.2 | Fruity, strawberry, lemon [33] |
| Ethyl 2-methylbutyrate | 18 [31] | 28.82 ± 0.97 b | 1.6 | 30.46 ± 0.94 b | 1.7 | 17.54 ± 1.72 a | 1.0 | 32.20 ± 2.16 b | 1.8 | Apple, berry, sweet, cider, anise [33] |
| Ethyl isovalerate | 3 [31] | 37.55 ± 6.58 b | 12.5 | 35.64 ± 0.56 b | 11.9 | 18.08 ± 1.66 a | 6.0 | 47.72 ± 1.85 c | 15.9 | Banana, sweet, fruity [33] |
| Ethyl 9-decenoate | 100 [36] | 40.54 ± 1.71 c | 0.4 | 34.07 ± 1.5 b | 0.3 | 26.6 ± 0.11 a | 0.3 | 29.34 ± 0.08 a | 0.3 | Fruity, fatty [30] |
| Σ Ethyl esters | 81,351.52 ± 1212.49 a | 145,154.78 ± 5889.92 b | 183,694.04 ± 1778.73 c | 193,204.95 ± 4515.00 d | ||||||
| Methyl octanoate | 4 [30] | 4.04 ± 0.19 c | 1.0 | 2.72 ± 0.34 b | 0.7 | 1.26 ± 0.03 a | 0.3 | 2.20 ± 0.06 b | 0.6 | Orange [30] |
| Isoamyl hexanoate | NF | 6.40 ± 0.21 b | ND | 6.24± 0.28 b | ND | 5.17 ± 0.03 a | ND | 5.48 ± 0.05 a | ND | Apple, green, pineapple |
| 3-Methylbutyl octanoate | 125 [30] | 12.06 ± 2.14 c | <0.1 | 8.01 ± 0.07 b | <0.1 | 2.25 ± 0.11 a | <0.1 | 4.77 ± 0.06 a | <0.1 | Sweet, fruity, pineapple, coconut [30] |
| Isoamyl lactate | 200 [32] | 42.07 ± 2.1 a | 0.2 | 36.93 ± 0.12 a | 0.2 | 370.22 ± 8.48 c | 1.9 | 287.17 ± 2.98 b | 1.4 | Cream, nut [32] |
| Σ Other esters | 64.57 ± 24.64 a | 53.90 ± 0.81 a | 378.90 ± 8.65 c | 299.62 ± 3.15 b | ||||||
| 1-Hexanol | 8000 [30] | 1786.34 ± 5.66 b | 0.2 | 1979.48 ± 99.07 c | 0.2 | 2214.96 ± 3.90 d | 0.3 | 1558.28 ± 13.77 a | 0.2 | Green, grass [30] |
| (E)-3-Hexen-1-ol | 1000 [35] | 122.83 ± 5.88 b | 0.1 | 146.33 ± 12.54 c | 0.1 | 77.65 ± 4.92 a | 0.1 | 115.23 ± 0.82 b | 0.1 | Herbaceous, green [35] |
| (Z)-3-Hexen-1-ol | 400 [37] | 272.50 ± 2.62 c | 0.7 | 213.96 ± 15.38 b | 0.5 | 167.49 ± 4.00 a | 0.4 | 218.19 ± 1.80 b | 0.5 | Green, cypress [37] |
| 1-Butanol | 150,000 [31] | 1437.36 ± 9.67 a | <0.1 | 2034.81 ± 38.34 c | <0.1 | 1819.35 ± 41.05 b | <0.1 | 1873.73 ± 78.44 b | <0.1 | Fusel alcohol [31] |
| Isobutanol | 40,000 [31] | 240,292 ± 8763.88 b | 6.0 | 219,126 ± 371.94 a | 5.5 | 214,157 ± 6051.42 a | 5.4 | 264,662.50 ± 3293.00 c | 6.6 | Fusel alcohol [31] |
| Isoamyl alcohol | 30,000 [31] | 25,219.50 ± 1914.14 b | 0.8 | 27,527.20 ± 12,880.66 c | 0.9 | 22,637.00 ± 3289.46 a | 0.8 | 24,621.95 ± 4541.75 b | 0.8 | Whisky, nail polish [31] |
| 4-Methyl-1-pentanol | 50,000 [37] | 66.66 ± 3.31 a | <0.1 | 89.83 ± 3.77 c | <0.1 | 60.78 ± 0.28 ab | <0.1 | 72.97 ± 0.43 b | <0.1 | Almond, toasted [37] |
| 3-Methyl-1-pentanol | 500 [31] | 167.26 ± 0.09 a | 0.3 | 255.75 ± 9.50 b | 0.5 | 168.49 ± 4.16 a | 0.3 | 174.85 ± 0.91 a | 0.3 | Soil, mushroom [31] |
| 1-Heptanol | 200–300 [31] | 58.16 ± 1.05 c | 0.1–1 | 50.52 ± 2.86 b | 0.1–1 | 39.37 ± 1.07 a | 0.1–1 | 43.73 ± 0.85 a | 0.1–1 | Lemon, orange, copper [31] |
| 1-Octanol | 0.7 [30] | 18.03 ± 0.42 c | 25.8 | 16.27 ± 1.46 bc | 23.2 | 3.81 ± 0.14 a | 5.4 | 14.29 ± 0.67 b | 20.4 | Chemical, metal, burnt [30] |
| 1-Octen-3-ol | 40 [39] | 4.59 ± 0.05 a | 0.1 | 6.09 ± 2.38 a | 0.2 | 4.42 ± 0.21 a | 0.1 | 4.25 ± 1.35 a | 0.1 | Mushroom [39] |
| 1-Decanol | 500 [30] | 2.74 ± 0.33 a | <0.1 | 2.45 ± 0.19 b | <0.1 | 1.56 ± 0.02 a | <0.1 | 1.98 ± 0.02 ab | <0.1 | Fat [30] |
| Benzyl alcohol | 200,000 [31] | 158.81 ± 18.79 bc | <0.1 | 174.62 ± 15.64 c | <0.1 | 131.11 ± 2.5 ab | <0.1 | 109.08 ± 5.18 a | <0.1 | Almond [31] |
| Phenylethyl alcohol | 14,000 [31] | 62,278.00 ± 1426.38 b | 4.4 | 78,121.80 ± 2987.81 c | 5.6 | 38,050.10 ± 157.68 a | 2.7 | 37,875.55 ± 2014.48 a | 2.7 | Floral, rose [31] |
| Σ Higher alcohols | 331,884.78 ± 12,152.27 b | 329,745.11 ± 16,441.54 b | 279,533.09 ± 9560.81 a | 331,346.58 ± 7944.17 b | ||||||
| Octanoic acid | 500 [30] | 2282.31 ± 635.02 c | 4.6 | 2048.17 ± 181.76 bc | 4.1 | 502.56 ± 3.08 a | 1.0 | 1263.75 ± 151.54 ab | 2.5 | Butter, almond [30] |
| Decanoic acid | 1000 [30] | 50.41 ± 2.82 b | <0.1 | 51.78 ± 1.74 b | <0.1 | 28.20 ± 0.49 a | <0.1 | 44.25 ± 7.08 b | <0.1 | Rancid, fat [30] |
| Hexanoic acid | 420 [30] | 2877.46 ± 493.75 b | 6.9 | 2482.3 ± 246.27 b | 5.9 | 1121.08 ± 13.41 a | 2.7 | 1663.79 ± 116.52 a | 4.0 | Leafy, wood, varnish [30] |
| Isobutyric acid | 200,000 [37] | 6796.09 ± 949.58 c | <0.1 | 5712.2 ± 335.31 bc | <0.1 | 3447.21 ± 149.45 a | <0.1 | 4449.45 ± 281.37 ab | <0.1 | Cheese, butter, rancid [37] |
| Isovaleric acid | 33.4 [37] | 18.31 ± 1.33 c | 0.5 | 19.52 ± 0.71 c | 0.6 | 6.20 ± 0.00 a | 0.2 | 12.60 ± 0.24 b | 0.4 | Fatty, sweet [37] |
| Σ Fatty acids | 12,024.58 ± 2082.50 c | 10,313.97 ± 765.79 bc | 5105.25 ± 166.43 a | 7433.84 ± 556.75 ab | ||||||
| Nonanal | 2.5 [30] | 0.71 ± 0.06 b | 0.3 | 0.54 ± 0.06 b | 0.2 | 0.20 ± 0.01 a | <0.1 | 0.08 ± 0.21 a | <0.1 | Fat, citrus, green [30] |
| Decanal | 1.25 [38] | 0.93 ± 0.10 b | 0.7 | 0.80 ± 0.08 b | 0.6 | 0.32 ± 0.05 a | 0.3 | 0.39 ± 0.11 a | 0.3 | Green [38] |
| Σ Aldoketones | 1.64 ± 0.16 b | 1.34 ± 0.14 b | 0.52 ± 0.06 a | 0.47 ± 0.32 a | ||||||
| β-Damascenone | 0.05 [30] | 12.61 ± 0.68 d | 252.2 | 11.29 ± 0.37 c | 225.8 | 7.75 ± 0.09 a | 155.0 | 9.38 ± 0.17 b | 187.6 | Apple, rose, honey [30] |
| Σ C13-Norisoprenoids | 12.61 ± 0.68 d | 11.29 ± 0.37 c | 7.75 ± 0.09 a | 9.38 ± 0.17 b | ||||||
| α-Terpineol | 250 [30] | 20.11 ± 2.53 c | <0.1 | 1.05 ± 0.10 a | <0.1 | 9.00 ± 0.10 b | <0.1 | 1.04 ± 0.22 a | <0.1 | Oil, anise, spicy [30] |
| 4-Terpineol | 250 [37] | 2.11 ± 0.05 b | <0.1 | 1.13 ± 0.33 a | <0.1 | 2.10 ± 0.05 b | <0.1 | 0.76 ± 0.04 a | <0.1 | Flowery [37] |
| Citronellol | 100 [30] | 2.76 ± 2.41 ab | <0.1 | 5.39 ± 0.17 b | <0.1 | 3.00 ± 0.07 ab | <0.1 | 0.53 ± 0.24 a | <0.1 | Citrus [30] |
| Nerol | 500 [32] | 3.90 ± 0.25 c | <0.1 | 3.44 ± 0.10 b | <0.1 | 2.48 ± 0.01 a | <0.1 | 2.30 ± 0.03 a | <0.1 | Violets, floral [32] |
| Nerolidol | 700 [37] | 3.29 ± 0.15 c | <0.1 | 2.49± 0.03 b | <0.1 | 2.16 ± 0.00 a | <0.1 | 2.23 ± 0.03 a | <0.1 | Rose, apple, citrus [37] |
| Σ Terpenes | 32.17 ± 5.39 c | 13.5 ± 0.73 ab | 18.74 ± 0.23 b | 6.86 ± 0.56 a | ||||||
| γ-Octalactone | 400 [34] | 26.02 ± 0.03 a | <0.1 | 26.01 ± 0.01 a | <0.1 | 46.72 ± 0.24 b | 0.1 | 46.22 ± 1.68 b | 0.1 | Coconut [44] |
| Σ Lactones | 26.02 ± 0.03 a | 26.01 ± 0.01 a | 46.72 ± 0.24 b | 46.22 ± 1.68 b | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhang, W.; Wu, Y.; Shi, X.; Yang, X.; Song, Y.; Qin, Y.; Ye, D.; Liu, Y. Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification. Foods 2022, 11, 2511. https://doi.org/10.3390/foods11162511
Jiang J, Zhang W, Wu Y, Shi X, Yang X, Song Y, Qin Y, Ye D, Liu Y. Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification. Foods. 2022; 11(16):2511. https://doi.org/10.3390/foods11162511
Chicago/Turabian StyleJiang, Jiao, Wenjing Zhang, Yitian Wu, Xuerong Shi, Xiaobing Yang, Yuyang Song, Yi Qin, Dongqing Ye, and Yanlin Liu. 2022. "Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification" Foods 11, no. 16: 2511. https://doi.org/10.3390/foods11162511
APA StyleJiang, J., Zhang, W., Wu, Y., Shi, X., Yang, X., Song, Y., Qin, Y., Ye, D., & Liu, Y. (2022). Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification. Foods, 11(16), 2511. https://doi.org/10.3390/foods11162511






