Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Disposables
2.2. Lyophilizates, Extracts and Infusions
2.3. Cell Culture
2.4. Estimation of Cytotoxicity
2.5. Other Assays
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godos, J.; Bella, F.; Sciacca, S.; Galvano, F.; Grosso, G. Vegetarianism and breast, colorectal and prostate cancer risk: An overview and meta-analysis of cohort studies. J. Hum. Nutr. Diet. 2017, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Mousavi, Y.; Höckerstedt, K. Diet and breast cancer. Acta Oncol. 1992, 31, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Zupec-Kania, B. Toward a cancer-specific diet. Clin. Nutr. 2016, 35, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.T.H.B.M.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J. Beneficial effects of ketogenic diets for cancer patients: A realist review with focus on evidence and confirmation. Med. Oncol. 2017, 34, 132. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahmod, A.I.; Kamal, A.; Rashid, H.M.; Alashqar, A.M.D.; Khater, S.; Jamal, D.; Waly, M. Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2021, 43, 558–589. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Zuniga, K.B.; Chan, J.M.; Ryan, C.J.; Kenfield, S.A. Diet and lifestyle considerations for patients with prostate cancer. Urol. Oncol. 2020, 38, 105–117. [Google Scholar] [CrossRef]
- Inoue, M.; Tajima, K.; Mizutani, M.; Iwata, H.; Iwase, T.; Miura, S.; Hirose, K.; Hamajima, N.; Tominaga, S. Regular consumption of green tea and the risk of breast cancer recurrence: Follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Jpn. Cancer Lett. 2001, 167, 175–182. [Google Scholar] [CrossRef]
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef]
- Bao, P.P.; Zhao, G.M.; Shu, X.O.; Peng, P.; Cai, H.; Lu, W.; Zheng, Y. Modifiable lifestyle factors and triple-negative breast cancer survival: A population-based prospective study. Epidemiology 2015, 26, 909–916. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razali, N.; Othman, N.; Ismail, N.H.; Bihud, N.V.; Ismail, N.H.; Yusof, F.Z.M. Goniothalamus lanceolatus extract inhibits the growth of human ovarian cancer cells through migration suppression and apoptosis induction. J. Appl. Sci. 2021, 11, 076–087. [Google Scholar]
- Le Blanc, M.; Russo, J.; Kudelka, A.P.; Smith, J.A. An in vitro study of inhibitory activity of gossypol. a cottonseed extract. in human carcinoma cell lines. Pharmacol. Res. 2002, 46, 551–555. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Furdak, P.; Sadowska-Bartosz, I. Delphinidin Increases the Sensitivity of Ovarian Cancer Cell Lines to 3-Bromopyruvate. Int. J. Mol. Sci. 2021, 22, 709. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2214. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tăbăran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef] [Green Version]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef]
- Bahrin, A.A.; Moshawih, S.; Dhaliwal, J.S.; Kanakal, M.M.; Khan, A.; Lee, K.S.; Goh, B.H.; Goh, H.P.; Kifli, N.; Ming, L.C. Cancer protective effects of plums: A systematic review. Biomed. Pharmacother. 2022, 146, 112568. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Kawahara, K.; Matsushita, S.; Hashiguchi, T.; Maruyama, I.; Kanekura, T. MK615, a Prunus mume Steb. Et Zucc (‘Ume’) extract, attenuates the growth of A375 melanoma cells by inhibiting the ERK1/2-Id-1 pathway. Phytother. Res. 2012, 26, 833–838. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Bohrer, B.M.; Munekata, P.E.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Improving oxidative stability of foods with apple-derived polyphenols. Compr. Rev. Food Sci. Food Saf. 2022, 21, 296–320. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G. Phenotypic Variation and Peel Contribution to Fruit Antioxidant Contents in European and Japanese Plums. Plants 2022, 11, 1338. [Google Scholar] [CrossRef]
- Annapurna, A.; Suhasin, G.; Akondi, R.B. Anti-cancer activity of Curcuma longa L. (turmeric). J. Pharm. Res. 2011, 4, 1274–1276. [Google Scholar]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Moran, N.E.; Thomas-Ahner, J.M.; Wan, L.; Zuniga, K.E.; Erdman, J.W., Jr.; Clinton, S.K. Tomatoes, Lycopene, and Prostate Cancer: What Have We Learned from Experimental Models? J. Nutr. 2022, 152, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaiij, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Parida, P.; Paul, S.; Basak, P. In vitro and in silico study of Aloe vera leaf extract against human breast cancer. Nat. Prod. Res. 2020, 34, 2363–2366. [Google Scholar] [CrossRef]
- Farshori, N.N.; Siddiqui, M.A.; Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Saquib, Q.; Ahmad, J.; Al-Khedhairy, A.A. Aloe vera-induced apoptotic cell death through ROS generation, cell cycle arrest, and DNA damage in human breast cancer cells. Biologia 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Pathak, D.R.; Stein, A.D.; He, J.P.; Noel, M.M.; Hembroff, L.; Nelson, D.A.; Vigneau, F.; Shen, T.; Scott, L.J.; Charzewska, J.; et al. Cabbage and Sauerkraut Consumption in Adolescence and Adulthood and Breast Cancer Risk among US-Resident Polish Migrant Women. Int. J. Environ. Res. Public Health 2021, 18, 10795. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Schiavano, G.F.; Zaffaroni, N.; De Marco, C.; Paiardini, M.; Cervasi, B.; Magnani, M. Mechanisms of action and antiproliferative properties of Brassica oleracea juice in human breast cancer cell lines. J. Nutr. 2005, 35, 1503–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.J.; Zhang, Y.; Nair, M.G. Tumor cell proliferation and cyclooxygenase inhibitory constituents in horseradish (Armoracia rusticana) and Wasabi (Wasabia japonica). J. Agric. Food Chem. 2005, 53, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Bo, P.; Lien, J.C.; Chen, Y.Y.; Yu, F.S.; Lu, H.F.; Yu, C.S.; Chou, Y.C.; Yu, C.C.; Chung, J.G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016, 44, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Challier, B.; Perarnau, J.M.; Viel, J.F. Garlic, onion and cereal fibre as protective factors for breast cancer: A French case-control study. Eur. J. Epidemiol. 1998, 14, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Desai, G.; Schelske-Santos, M.; Nazario, C.M.; Rosario-Rosado, R.V.; Mansilla-Rivera, I.; Ramírez-Marrero, F.; Nie, J.; Myneni, A.A.; Zhang, Z.F.; Freudenheim, J.L.; et al. Onion and Garlic Intake and Breast Cancer, a Case-Control Study in Puerto Rico. Nutr. Cancer 2020, 72, 791–800. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.X.; Ma, X.F. Inhibitory effects of onion (Allium cepa L.) extract on proliferation of cancer cells and adipocytes via inhibiting fatty acid synthase. Asian Pac. J. Cancer Prev. 2012, 13, 5573–5579. [Google Scholar] [CrossRef]
- Zamri, N.; Hamid, H.A. Comparative Study of Onion (Allium cepa) and Leek (Allium ampeloprasum): Identification of Organosulphur Compounds by UPLC-QTOF/MS and Anticancer Effect on MCF-7 Cells. Plant Foods Hum. Nutr. 2019, 74, 525–530. [Google Scholar] [CrossRef]
- Tsubura, A.; Lai, Y.C.; Kuwata, M.; Uehara, N.; Yoshizawa, K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med. Chem. 2011, 11, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, F.; Tedeschi, P.; Grassilli, S.; Maietti, A.; Brandolini, V.; Bertagnol, V. Ethanol-based garlic extract prevents malignant evolution of non-invasive breast tumor cells induced by moderate hypoxia. Biomed. Pharmacother. 2021, 142, 112052. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Ursin, G.; Emory, T.H.; Lee, E.; Wang, R.; Torkelson, C.J.; Dostal, A.M.; Swenson, K.; Le, C.T.; Yang, C.S.; et al. A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. 2017, 10, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.K.; Xiao, H.M.; Xia, H.; Sun, G.J. Tea consumption and risk of breast cancer: A meta-analysis. Int. J. Clin. Pharmacol. Ther. 2018, 56, 617–619. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, L.; Wang, K.; Yan, Y.; He, J.; Ren, Y. Green tea consumption and risk of breast cancer: A systematic review and updated meta-analysis of case-control studies. Medicine 2019, 98, e16147. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.C.; Dong, D.S.; Bai, Y.; Xia, P. Meta-analysis of black tea consumption and breast cancer risk: Update 2013. Nutr. Cancer 2014, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nichols, H.B.; Troester, M.; Cai, J.; Bensen, J.T.; Sandler, D.P. Tea consumption and breast cancer risk in a cohort of women with family history of breast cancer. Int. J. Cancer 2020, 147, 876–886. [Google Scholar] [CrossRef]
- Santos, R.A.; Andrade, E.D.S.; Monteiro, M.; Fialho, E.; Silva, J.L.; Daleprane, J.B.; Ferraz da Costa, D.C. Green Tea (Camellia sinensis) Extract Induces p53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods 2021, 10, 3154. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Passi, N.; Maheshwari, R.K. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol. Ther. 2007, 6, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Koňariková, K.; Ježovičová, M.; Keresteš, J.; Gbelcová, H.; Ďuračková, Z.; Žitňanová, I. Anticancer effect of black tea extract in human cancer cell lines. Springerplus 2015, 4, 127. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Inhibitory Effects of the Four Main Theaflavin Derivatives Found in Black Tea on Ovarian Cancer Cells. Anticancer Res. 2016, 36, 643–651. [Google Scholar] [PubMed]
- Ćurčić Milutinović, M.; Stanković, M.; Cvetkovic, D.; Topuzovic, M.; Mihailovic, V.; Marković, S. Antioxidant and anticancer properties of leaves and seed cones from European yew (Taxus baccata L.). Arch. Biol. Sci. 2015, 67, 525–534. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Jiménez-González, V.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Díaz-Ortega, P.; García, F.; Aparicio, A.; López-Lázaro, M. Screening for Selective Anticancer Activity of 65 Extracts of Plants Collected in Western Andalusia, Spain. Plants 2021, 10, 2193. [Google Scholar] [CrossRef] [PubMed]
- Nazarewicz, R.R.; Bikineyeva, A.; Dikalov, S.I. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J. Biomol. Screen. 2013, 18, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Deng, Y.; Lu, B.M.; Liu, Y.X.; Li, J.; Bao, J.K. Green tea catechins: A fresh flavor to anticancer therapy. Apoptosis 2014, 19, 1–18. [Google Scholar] [CrossRef]
- Borek-Dohalska, L.; Stiborova, M. Taxanes-Anticancer Drugs with Unique Mechanism of Action. Chem. Listy 2000, 94, 226–229. [Google Scholar]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Ma, F.; An, Z.; Yue, Q.; Zhao, C.; Zhang, S.; Sun, X.; Li, K.; Zhao, L.; Su, L. Effects of brassinosteroids on cancer cells: A review. J. Biochem. Mol. Toxicol. 2022, 36, e23026. [Google Scholar] [CrossRef]
- Zhabinskii, V.N.; Khripach, N.B.; Khripach, V.A. Steroid plant hormones: Effects outside plant kingdom. Steroids 2015, 97, 87–97. [Google Scholar] [CrossRef]
- Zhang, Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat. Res. 2004, 555, 173–190. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A review on the phytochemical composition and potential medicinal uses of horseradish (Armoracia rusticana) root. Food Rev. Int. 2013, 29, 261–275. [Google Scholar] [CrossRef]
- Ortecho-Zuta, U.; de Oliveira Duque, C.C.; Leite, M.L.; Bordini, E.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A.; Soares, D.G. Effects of Enzymatic Activation of Bleaching Gels on Hydrogen Peroxide Degradation Rates, Bleaching Effectiveness, and Cytotoxicity. Oper. Dent. 2019, 44, 414–423. [Google Scholar] [CrossRef]
- Iciek, M.; Kwiecień, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.X.; Zhu, K.X.; Fan, J.G.; Qiao, L. Garlic-derived allyl sulfides in cancer therapy. Anti-Cancer Agents Med. Chem. 2014, 14, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Speck, R.M.; DeMichele, A.; Farrar, J.T.; Hennessy, S.; Mao, J.J.; Stineman, M.G.; Barg, F.K. Taste alteration in breast cancer patients treated with taxane chemotherapy: Experience, effect, and coping strategies. Support. Care Cancer 2013, 21, 549–555. [Google Scholar] [CrossRef]
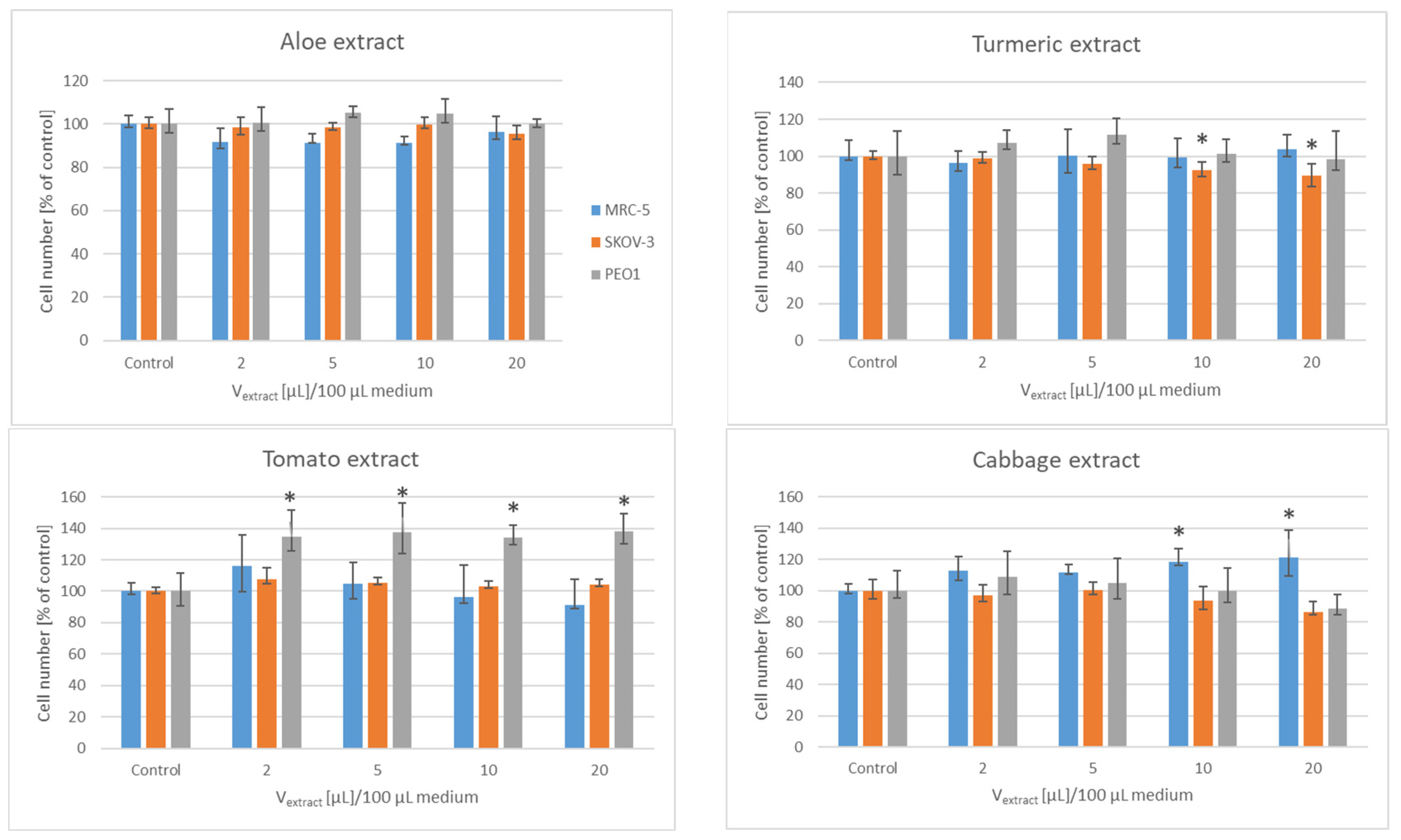
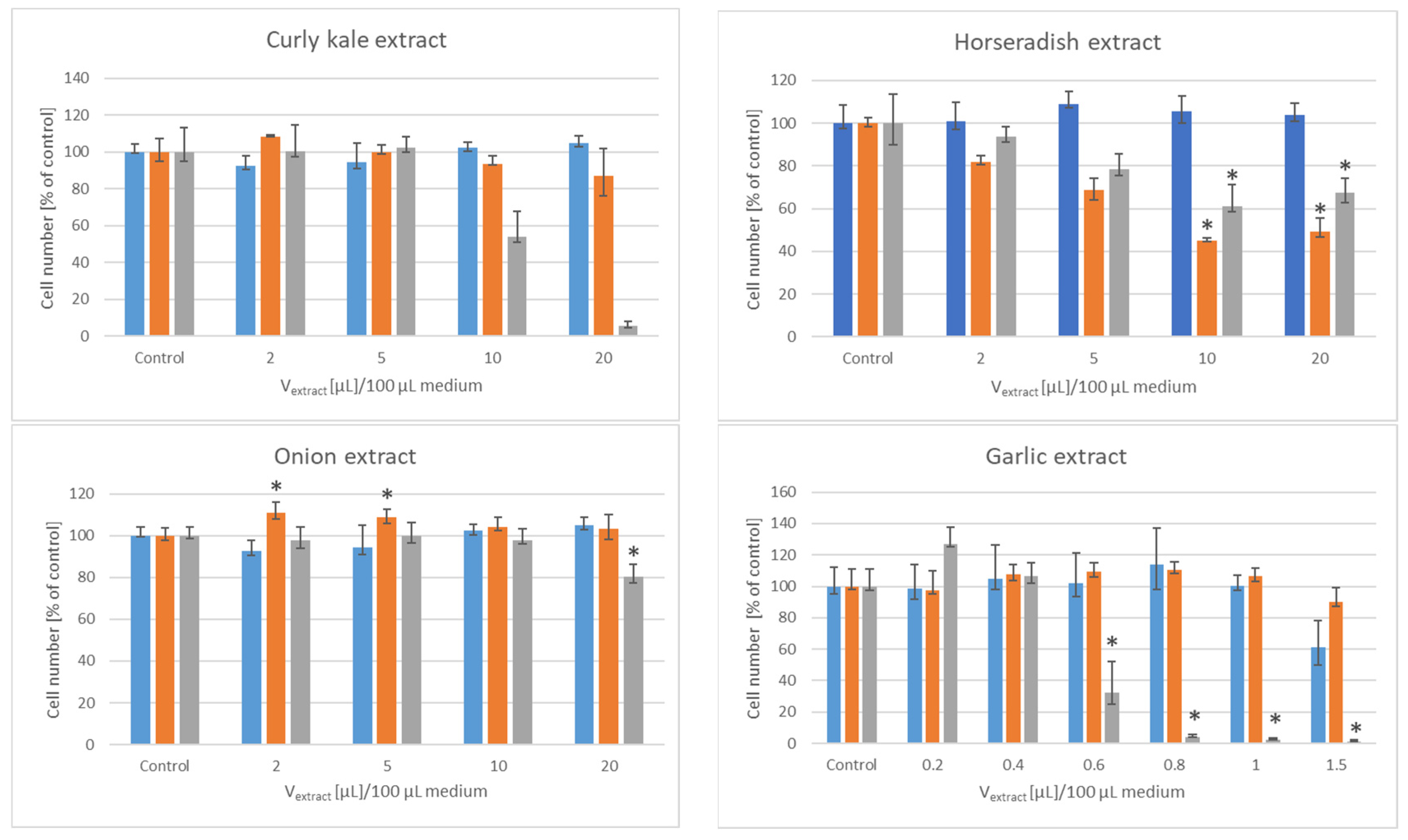
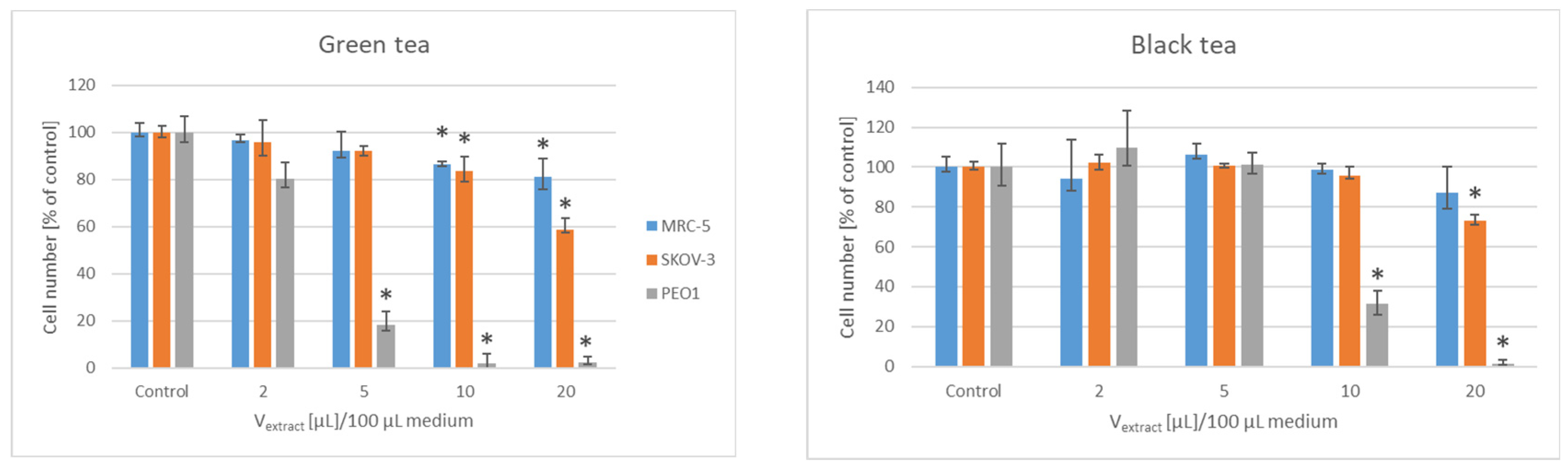
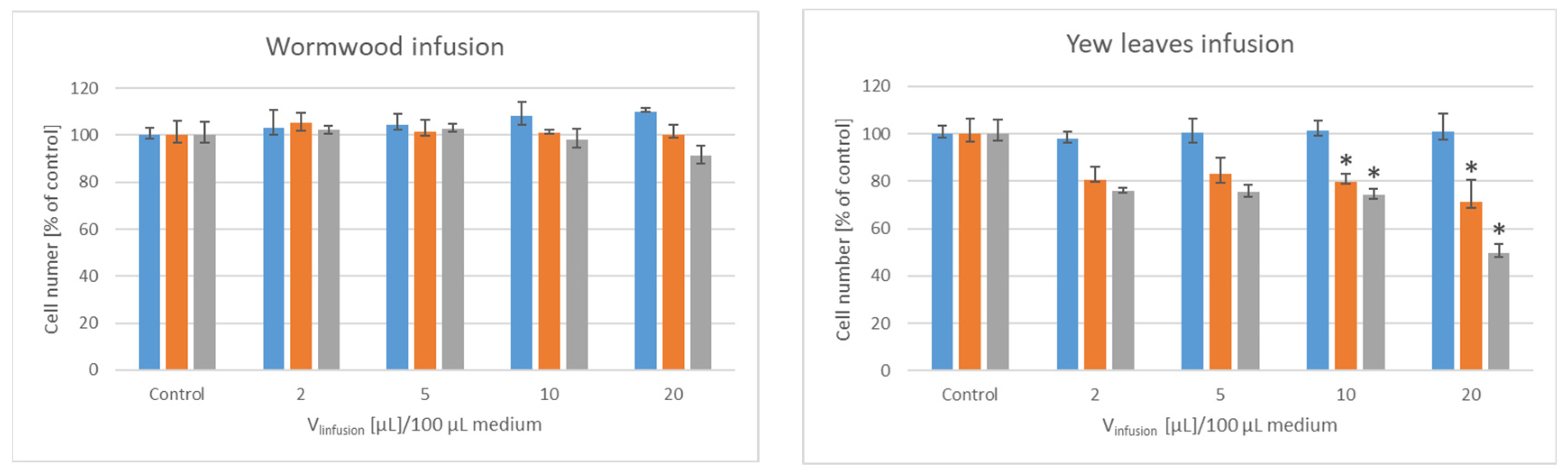
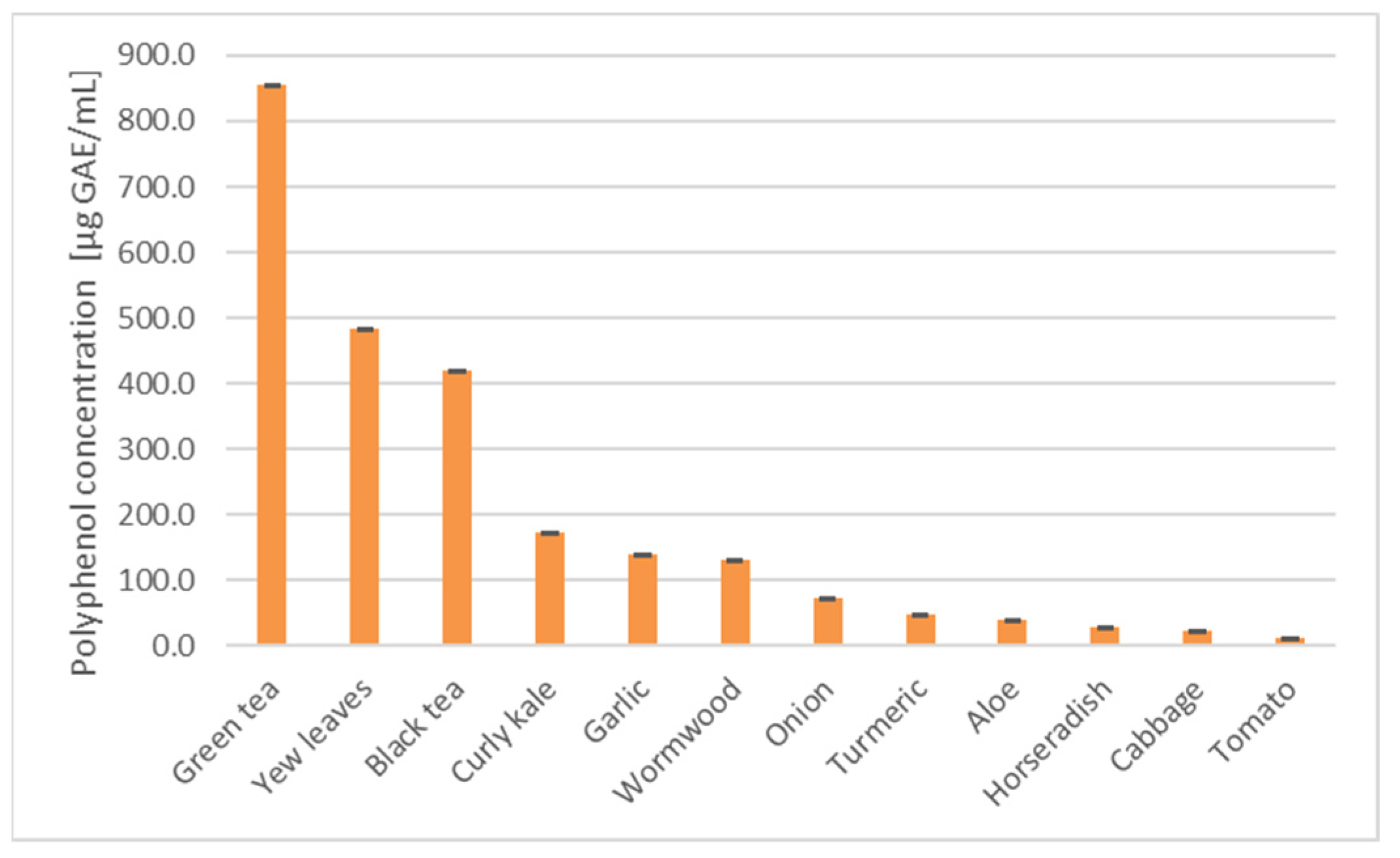


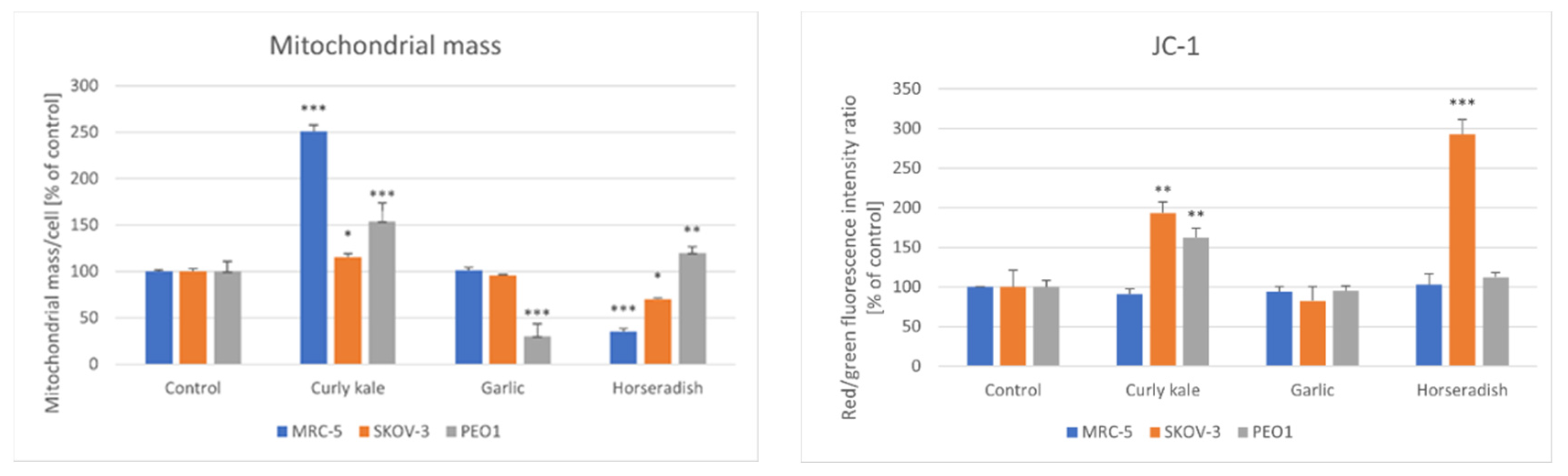
| Lyophilizate/ Extract/Infusion | IV50 [µL]/Mass of Initial Material PEO1 | SKOV-3 |
|---|---|---|
| Bilberry | ND | ND |
| Apple | ND | ND |
| Apple peel | ND | ND |
| Plum | ND | ND |
| Plum peel | ND | ND |
| Aloe | ND | ND |
| Turmeric | ND | ND |
| Tomato | ND | ND |
| Cabbage | ND | ND |
| Curly kale | 10.6 | ND |
| Horseradish | 14.2 | ND |
| Onion | 67.4 (6.74 μg) E | ND |
| Garlic | 1.1 (111 ng) | 3.3 (333 ng) |
| Green tea | 3.3 (33.3 ng) | 25.3 (253 ng) E |
| Black tea | 6.5 (65 ng) | 21.1 (211 ng) |
| Wormwood | ND | ND |
| Yew leaves | 19.9 (199 ng) | 275.3 (2.753 mg) E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdak, P.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells. Foods 2022, 11, 2518. https://doi.org/10.3390/foods11162518
Furdak P, Pieńkowska N, Bartosz G, Sadowska-Bartosz I. Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells. Foods. 2022; 11(16):2518. https://doi.org/10.3390/foods11162518
Chicago/Turabian StyleFurdak, Paulina, Natalia Pieńkowska, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2022. "Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells" Foods 11, no. 16: 2518. https://doi.org/10.3390/foods11162518
APA StyleFurdak, P., Pieńkowska, N., Bartosz, G., & Sadowska-Bartosz, I. (2022). Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells. Foods, 11(16), 2518. https://doi.org/10.3390/foods11162518








