Determination of 2-Pentanol Enantiomers via Chiral GC-MS and Its Sensory Evaluation in Baijiu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample
2.3. Stationary Phase Selection for Enantiomers of 2-Pentanol
2.4. Sample Pre-Treatment
2.4.1. DI
2.4.2. LLE
2.5. Separation and Quantification of 2-Pentanol Enantiomers in Baijiu by GC-MS
2.6. Sensory Analysis
2.7. Statistics and Analysis
3. Results and Discussion
3.1. Stationary Phase Selection for 2-Pentanol Enantiomers
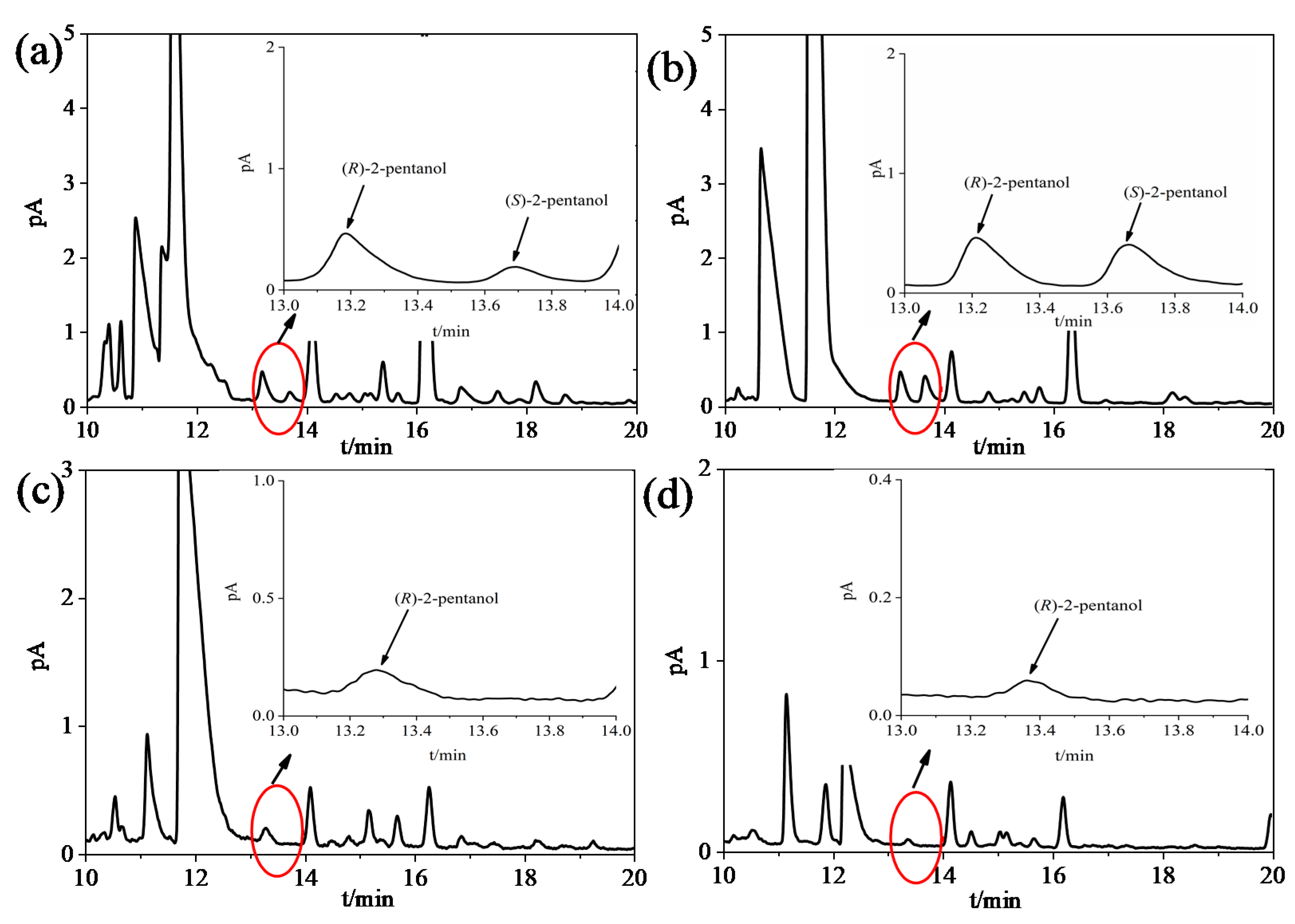
3.2. Effect of DI and LLE on the Enantiomeric Detection of 2-Pentanol in Baijiu
3.3. Separation and Quantification of 2-Pentanol Enantiomers in SSB, STB, LTB, and RTB
3.4. Sensory Analysis of Enantiomers of 2-Pentanol
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buffeteau, G.; Hornedo-Ortega, R.; Gabaston, J.; Daugey, N.; Palos-Pinto, A.; Thienpont, A.; Brotin, T.; Mérillon, J.-M.; Buffeteau, T.; Waffo-Teguo, P. Chiroptical and potential in vitro anti-inflammatory properties of viniferin stereoisomers from grapevine (Vitis vinifera L.). Food Chem. 2022, 393, 133359. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.-H. Chirality: An Important Phenomenon Regarding Biosynthesis, Perception, and Authenticity of Flavor Compounds. J. Agric. Food Chem. 2020, 68, 10265–10274. [Google Scholar] [CrossRef] [PubMed]
- Ebeler, S.E. Enantiomeric Analysis as a Tool for Authentication of Foods and Beverages; ACS Publications: Washington, DC, USA, 2006; Volume 952, pp. 39–49. [Google Scholar] [CrossRef]
- Kaunzinger, A.; Wüst, M.; Gröbmiller, H.; Burow, S.; Hemmrich, U.; Dietrich, A.; Beck, T.; Hener, U.; Mosandl, A.; Rapp, A. Enantiomer distribution of ethyl lactate—A new criterion for quality assurance of wine. Z. Für Lebensm. Unters. Und Forsch. 1996, 203, 499–500. [Google Scholar] [CrossRef]
- Langen, J.; Fischer, U.; Cavalar, M.; Coetzee, C.; Wegmann-Herr, P.; Schmarr, H.-G. Enantiodifferentiation of 1,2-propanediol in various wines as phenylboronate ester with multidimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 2425–2439. [Google Scholar] [CrossRef]
- Xu, H.; Dai, Y.; Qiu, S.; Sun, B.; Zeng, X. Distribution and Quantification of 1,2-Propylene Glycol Enantiomers in Baijiu. Foods 2021, 10, 3039. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Cifuentes, A. Chiral analysis in food science. TrAC Trends Anal. Chem. 2020, 123, 115761. [Google Scholar]
- Tominaga, T.; Niclass, Y.; Frérot, E.; Dubourdieu, D. Stereoisomeric distribution of 3-mercaptohexan-1-ol and 3-mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (Var. Sauvignon Blanc and Semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Chiral analysis of 3-sulfanylhexan-1-ol and 3-sulfanylhexyl acetate in wine by high-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2018, 998, 83–92. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wu, Y.; Zhao, D. Uncover the flavor code of strong-aroma baijiu: Research progress on the revelation of aroma compounds in strong-aroma baijiu by means of modern separation technology and molecular sensory evaluation. J. Food Compos. Anal. 2022, 109, 104499. [Google Scholar] [CrossRef]
- Petronilho, S.; Coimbra, M.A.; Rocha, S.M. A critical review on extraction techniques and gas chromatography based determination of grapevine derived sesquiterpenes. Anal. Chim. Acta 2014, 846, 8–35. [Google Scholar] [CrossRef]
- Passos, C.P.; Petronilho, S.; Serôdio, A.F.; Neto, A.C.; Torres, D.; Rudnitskaya, A.; Nunes, C.; Kukurová, K.; Ciesarová, Z.; Rocha, S.M. HS-SPME Gas Chromatography Approach for Underivatized Acrylamide Determination in Biscuits. Foods 2021, 10, 2183. [Google Scholar] [CrossRef]
- Lytra, G.; Cameleyre, M.; Tempere, S.; Barbe, J.-C. Distribution and Organoleptic Impact of Ethyl 3-Hydroxybutanoate Enantiomers in Wine. J. Agric. Food Chem. 2015, 63, 10484–10491. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Tempere, S.; Marchand, S.; Moine, V.; De Revel, G. Ethyl 2-hydroxy-3-methylbutanoate enantiomers: Quantitation and sensory evaluation in wine. OENO One 2018, 52, 57–65. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Landais, Y.; Darriet, P.; Dubourdieu, D. Identification of a Sotolon Pathway in Dry White Wines. J. Agric. Food Chem. 2010, 58, 7273–7279. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.-C. 2-Methylbutyl acetate in wines: Enantiomeric distribution and sensory impact on red wine fruity aroma. Food Chem. 2017, 237, 364–371. [Google Scholar] [CrossRef]
- Lytra, G.; Franc, C.; Cameleyre, M.; Barbe, J.-C. Study of Substituted Ester Formation in Red Wine by the Development of a New Method for Quantitative Determination and Enantiomeric Separation of Their Corresponding Acids. J. Agric. Food Chem. 2017, 65, 5018–5025. [Google Scholar] [CrossRef]
- Stamatopoulos, P.; Brohan, E.; Prevost, C.; Siebert, T.E.; Herderich, M.; Darriet, P. Influence of Chirality of Lactones on the Perception of Some Typical Fruity Notes through Perceptual Interaction Phenomena in Bordeaux Dessert Wines. J. Agric. Food Chem. 2016, 64, 8160–8167. [Google Scholar] [CrossRef]
- Vyviurska, O.; Zvrškovcová, H.; Špánik, I. Distribution of enantiomers of volatile organic compounds in selected fruit distillates. Chirality 2017, 29, 14–18. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Sun, G.M.; Vickers, A.K.; Stremple, P. Gas Chromatographic Analysis of Chiral Aroma Compounds in Wine Using Modified Cyclodextrin Stationary Phases and Solid Phase Microextraction; ACS Publicationsym: Washington, DC, USA, 2001; Volume 794, pp. 45–56. [Google Scholar] [CrossRef]
- Langen, J.; Wegmann-Herr, P.; Schmarr, H.G. Quantitative determination of α-ionone, β-ionone, and β-damascenone and enantiodifferentiation of α-ionone in wine for authenticity control using multidimensional gas chromatography with tandem mass spectrometric detection. Anal. Bioanal. Chem. 2016, 408, 6483–6496. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, W.; Shen, C.; Yang, J. Basic flavor types and component characteristics of Chinese traditional liquors: A review. J. Food Sci. 2020, 85, 4096–4107. [Google Scholar] [CrossRef]
- Hong, J.; Tian, W.; Zhao, D. Research progress of trace components in sesame-aroma type of baijiu. Food Res. Int. 2020, 137, 109695. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in Baijiu analysis by chromatography based technology—A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, X.; Ji, K.; Guo, K.; Li, Y.; Wu, C.; Xu, G. Characterization of flavor compounds in Chinese liquor Moutai by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 597, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.W.; Kong, J.L.; Xiao, Z.B.; Chen, F.; Ma, N.; Zhu, J.C. Characterization of odor-active compounds of various Chinese “Wuliangye” liquors by gas chromatography–olfactometry, gas chromatography–mass spectrometry and sensory evaluation. Int. J. Food Prop. 2017, 20, S735–S745. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- Lin, M.-H.; Ke, L.-Y.; Yao, D.-J. Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique. Micromachines 2019, 10, 725. [Google Scholar] [CrossRef]
- Malyemez, A.S.; Bayraktar, E.; Mehmetoğlu, Ü. Optimization of process parameters and kinetic modeling for the enantioselective kinetic resolution of (R, S)-2-pentanol. Turk. J. Biochem. 2017, 42, 600–608. [Google Scholar] [CrossRef]
- Zheng, Z.-X.; Lin, J.-M.; Chan, W.; Lee, A.W.M.; Huie, C.W. Separation of enantiomers in microemulsion electrokinetic chromatography using chiral alcohols as cosurfactants. Electrophoresis 2004, 25, 3263–3269. [Google Scholar] [CrossRef]
- Clark, D.D.; Boyd, J.M.; Ensign, S.A. The Stereoselectivity and Catalytic Properties of Xanthobacter autotrophicus 2-[(R)-2-Hydroxypropylthio] ethanesulfonate Dehydrogenase Are Controlled by Interactions between C-Terminal Arginine Residues and the Sulfonate of Coenzyme M. Biochemistry 2004, 43, 6763–6771. [Google Scholar] [CrossRef]
- Strohalm, H.; Dregus, M.; Wahl, A.; Engel, K.-H. Enantioselective Analysis of Secondary Alcohols and Their Esters in Purple and Yellow Passion Fruits. J. Agric. Food Chem. 2007, 55, 10339–10344. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yao, Z.; Xiao, Q.; Xiao, Z.; Ma, N.; Zhu, J. Characterization of the key aroma compounds in different light aroma type Chinese liquors by GC-olfactometry, GC-FPD, quantitative measurements, and aroma recombination. Food Chem. 2017, 233, 204–215. [Google Scholar] [CrossRef]
- GB/T 33406-2016; Guidelines for Determination of Liquor Flavor Substances Thresh Old. China Standards Press: Beijing, China, 2016.
- Duan, J.X.; Yang, S.Q.; Li, H.H.; Qin, D.; Shen, Y.; Li, H.H.; Sun, J.Y.; Zheng, F.P.; Sun, B.G. Why the key aroma compound of soy sauce aroma type baijiu has not been revealed yet? LWT 2022, 154, 112735. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, K.; Zou, W.; Hou, Y. Three main flavour types of Chinese Baijiu: Characteristics, research, and perspectives. J. Inst. Brew. 2021, 127, 317–326. [Google Scholar] [CrossRef]
- Hong, J.; Wang, J.; Zhang, C.; Zhao, Z.; Tian, W.; Wu, Y.; Chen, H.; Zhao, D.; Sun, J. Unraveling variation on the profile aroma compounds of strong aroma type of Baijiu in different regions by molecular matrix analysis and olfactory analysis. RSC Adv. 2021, 11, 33511–33521. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Fan, W.L.; Xu, Y. Characterization of the key odorants in light aroma type Chinese liquor by gas chromatography–olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2014, 62, 5796–5804. [Google Scholar] [CrossRef]
- Song, X.; Wang, G.; Zhu, L.; Zheng, F.; Ji, J.; Sun, J.; Li, H.; Huang, M.; Zhao, Q.; Zhao, M.; et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT 2021, 146, 111427. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of Chinese rice wine by gas chromatography–olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Hu, X.; Li, Y. The impact of environmental factors on the environmental bacterial diversity and composition in the Jiang-flavoured Baijiu production region. LWT Food Sci. Technol. 2021, 149, 111784. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Brenna, M.E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Wang, X.X.; Fan, W.L.; Xu, Y. Comparison on aroma compounds in Chinese soy sauce and strong aroma type liquors by gas chromatography–olfactometry, chemical quantitative and odor activity values analysis. Eur. Food Res. Technol. 2014, 239, 813–825. [Google Scholar] [CrossRef]


| Methodologies | Samples | Chiral Compounds |
|---|---|---|
| DI-GC, DI-GC-FID | Wine [4], Baijiu [6] | ethyl lactate, 1,2-propanediol |
| LLE-GC-MS, LLE-MDGC-TOF-MS | Wine [13,14], white wine [8,15], red wine [16,17], Bordeaux dessert wines [18] | 3-mercapto-1-hexanol, 3-mercaptohexyl Acetate, 2-methylbutyl acetate, 2-methylbutyric acid, 3-hydroxybutyric acid, 2-hydroxy-3-methylbutyric acid, 2-hydroxy-4-methylvaleric acid, 2-nonen-4-olide, γ-nonalactone, 3-methyl-4-octanolide, ethyl 3-Hydroxybutanoate, ethyl 2-hydroxy-3-methylbutanoate, (3-hydroxy-4, 5-dimethyl-2 (5H)-furanone) |
| SPME-GC-MS, SPME-GC | fruit brandy [19], wine [20,21] | linalool, trans-linalool oxide, cis-linalool oxide, limonene, α-terpineol, nerolidol, β-citronellol, γ-decalactone, γ-dodecalactone, 2-Butanol, 2,3-Butanediol, α-ionone |
| Chromatographic Column Names | Stationary Phase | Specification |
|---|---|---|
| Beta DEX™ 120 | SPB-35 poly (35% phenyl/65% methylsiloxane) containing 20% permethylated β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| Astec CHIRADEXL® B-TA | 2,6-di-O-pentyl-3-trifluoroacetyl derivative of β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| Astec CHIRALDEX™ B-DM | 2,3-di-O-methyl-6-t-butyl silyl derivative of β-cyclodextrin | 50 m × 0.25 mm × 0.12 µm |
| Astec CHIRALDEX® G-TA | 2,6-di-O-pentyl-3-trifluoroacetyl derivative of γ-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| CYCLOSIL-B | hepta-(2,3-di-O-methyl-6-O-tert-butyldimethylsilyl)-β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| HP-CHIRAL-20B | (35%-phenyl)-methyl polysiloxane-β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| MEGA-DEX DAC Beta | diacetyl tertbutylsilyl-β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| MEGA-DEX DET Beta | diethyl-TBS-β-cyclodextrin | 30 m × 0.25 mm × 0.25 µm |
| Pre-Process | Compounds | Linearity Range (mg/L) | R2 | RSD (%) | LODs(mg/L) | Recovery Rate (%) |
|---|---|---|---|---|---|---|
| DI | (R)-2-Pentanol | 2.60~253.70 | 0.9997 | 0.71~9.75% | 0.65 | 90.69~105.28% |
| (S)-2-Pentanol | 1.30~126.85 | 0.9991 | 0.00~8.75% | 0.35 | 92.02~98.97% | |
| LLE | (R)-2-Pentanol | 0.08~79.28 | 0.9998 | 2.56~6.64% | 0.03 | 76.76~100.03% |
| (S)-2-Pentanol | 0.03~29.73 | 0.9998 | 2.62~5.19% | 0.02 | 74.60~121.47% |
| Samples | (R)-2-Pentanol (mg/L) | (S)-2-Pentanol (mg/L) | ee | R:S |
|---|---|---|---|---|
| SSB | ||||
| BDC | 2.06 ± 0.77 a | 0.29 ± 0.03 a | 75.32% | 88:12 |
| DYT | - | - | - | - |
| GZJSJ | 3.85 ± 1.00 ab | 1.40 ± 0.33 a | 46.67% | 73:27 |
| GBYJJ | - | - | - | - |
| DYTGBJ | - | - | - | - |
| GT | - | - | - | - |
| JSHS1951 | 6.10 ± 0.43 ab | 2.28 ± 0.19 a | 45.58% | 73:27 |
| JSHSJ | 3.06 ± 0.10 ab | 1.40 ± 0.02 a | 37.22% | 69:31 |
| JSJ1998 | 2.56 ± 0.27 ab | 1.00 ± 0.11 a | 43.82% | 72:28 |
| LM | 1.92 ± 0.00 a | 0.54 ± 0.05 a | 56.10% | 78:22 |
| LJ | 3.02 ± 0.06 ab | 2.53 ± 0.32 a | 8.83% | 54:46 |
| MT43 | 4.22 ± 0.31 ab | 1.00 ± 0.04 a | 61.69% | 81:19 |
| MTCX | 0.68 ± 0.08 a | - | - | - |
| MTWZJ | 6.18 ± 0.25 ab | 1.23 ± 0.08 a | 66.80% | 83:17 |
| QJ1H | - | - | - | - |
| QHL | 3.51 ± 0.01 ab | 2.93 ± 0.07 a | 9.01% | 55:45 |
| TCSP | 3.41 ± 0.35 ab | - | - | - |
| XJYZ | 1.52 ± 0.28 a | 0.91 ± 0.19 a | 25.10% | 63:37 |
| ZJ | - | - | - | - |
| STB | ||||
| DK | 0.74 ± 0.06 a | 0.36 ± 0.06 a | 34.55% | 67:33 |
| GJDQ | 0.89 ± 0.06 a | 0.38 ± 0.07 a | 40.16% | 70:30 |
| GJ1573 | 4.15 ± 0.05 ab | 3.39 ± 0.46 ab | 10.08% | 55:45 |
| LZLJ-JPTQ | 1.20 ± 0.05 a | 0.38 ± 0.05 a | 51.90% | 76:24 |
| LZLJ-EQ | 0.62 ± 0.03 a | - | - | - |
| LZLJ-TEQ | 1.74 ± 0.14 a | 1.24 ± 0.09 a | 16.78% | 58:42 |
| LZLJ-TQJNB | 1.55 ± 0.12 a | 1.04 ± 0.07 a | 19.69% | 60:40 |
| LZLJ-TOUQ | 1.04 ± 0.13 a | 0.33 ± 0.07 a | 51.82% | 76:24 |
| SJF | 16.94 ± 2.58 c | 9.74 ± 1.87 d | 26.99% | 63:37 |
| WLY | 49.30 ± 10.54 d | 24.43 ± 3.97 e | 33.73% | 67:33 |
| XFCJ | - | - | - | - |
| YHMZL | 6.96 ± 1.60 ab | 6.34 ± 1.82 bc | 4.66% | 52:48 |
| LTB | ||||
| BF | 0.85 ± 0.05 a | - | - | - |
| FJ10 | 0.69 ± 0.03 a | - | - | - |
| FJ20 | 0.65 ± 0.04 a | - | - | - |
| FJBF | 0.48 ± 0.03 a | 0.03 ± 0.01 a | 88.24% | 94:6 |
| FJQH20 | 0.78 ± 0.08 a | - | - | - |
| FJQXMR | 0.62 ± 0.04 a | 0.04 ± 0.01 a | 87.88% | 94:6 |
| FPLJ | 0.44 ± 0.06 a | - | ||
| HXEGT | - | - | - | - |
| JXB | 0.84 ± 0.19 a | - | ||
| LBFJ | 0.66 ± 0.02 a | - | - | - |
| YTXZC1988 | 0.41 ± 0.00 a | - | - | - |
| NLSEGT | 0.78 ± 0.07 a | - | - | - |
| NLSCNBJ | - | - | - | - |
| RTB GLSH | ||||
| 1.04 ± 0.08 a | - | - | - | |
| XSJ | 0.98 ± 0.06 a | - | - | - |
| CLS | 1.00 ± 0.05 a | - | - | - |
| LGL | 1.34 ± 0.41 a | - | - | - |
| Mean Concentration (mg/L) ± Standard Deviation | |||||
|---|---|---|---|---|---|
| Aroma-Types | Number | RS | R | S | R:S |
| SSB | 13 | 4.65 ± 0.46 | 3.24 ± 0.30 | 1.41 ± 0.13 | 72:28 |
| STB | 11 | 12.50 ± 1.13 | 7.74 ± 1.40 | 4.76 ± 0.85 | 64:36 |
| LTB | 11 | 0.70 ± 0.04 | 0.68 ± 0.07 | 0.02 ± 0.01 | 94:6 |
| RTB | 4 | 1.09 ± 0.15 | 1.09 ± 0.15 | - | 100:0 |
| Olfactory Threshold (mg/L) | ||||
|---|---|---|---|---|
| Compound | Odor Description | Odor Description [45] | In Pure Water | In 46% Ethanol Solution |
| (R)-2-Pentanol | Paint, rubber, grease | Light, seedy, sharp | 12.62 | 163.30 |
| (S)-2-Pentanol | Mint, plastic, pungent | Heavy, wild berry, ripe, dusty, astringent | 3.03 | 78.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Qiu, S.; Dai, Y.; Tian, L.; Wei, C. Determination of 2-Pentanol Enantiomers via Chiral GC-MS and Its Sensory Evaluation in Baijiu. Foods 2022, 11, 2584. https://doi.org/10.3390/foods11172584
Hu L, Qiu S, Dai Y, Tian L, Wei C. Determination of 2-Pentanol Enantiomers via Chiral GC-MS and Its Sensory Evaluation in Baijiu. Foods. 2022; 11(17):2584. https://doi.org/10.3390/foods11172584
Chicago/Turabian StyleHu, Lisha, Shuyi Qiu, Yifeng Dai, Luqin Tian, and Chaoyang Wei. 2022. "Determination of 2-Pentanol Enantiomers via Chiral GC-MS and Its Sensory Evaluation in Baijiu" Foods 11, no. 17: 2584. https://doi.org/10.3390/foods11172584
APA StyleHu, L., Qiu, S., Dai, Y., Tian, L., & Wei, C. (2022). Determination of 2-Pentanol Enantiomers via Chiral GC-MS and Its Sensory Evaluation in Baijiu. Foods, 11(17), 2584. https://doi.org/10.3390/foods11172584






