Antioxidant Activity, Functional Properties, and Cytoprotective Effects on HepG2 Cells of Tree Peony (Paeonia suffruticosa Andr.) Seed Protein Hydrolysate as Influenced by Molecular Weights Fractionation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tree Peony Seed Protein Hydrolysate (TPSPH) and Five Fractions
2.3. Determination of Amino Acid Composition of TPSPH
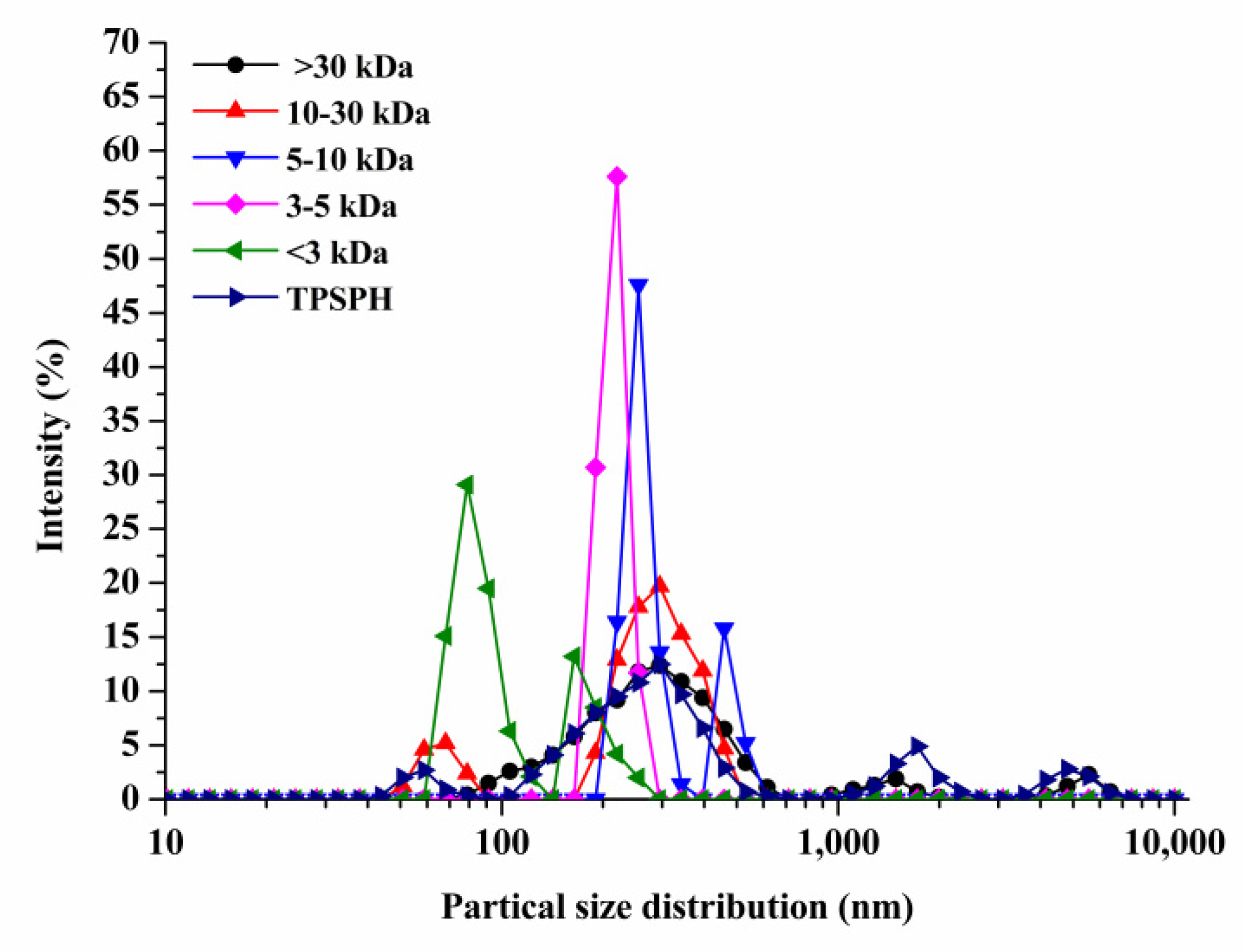
2.4. Determination of Particle Size Distribution of TPSPH
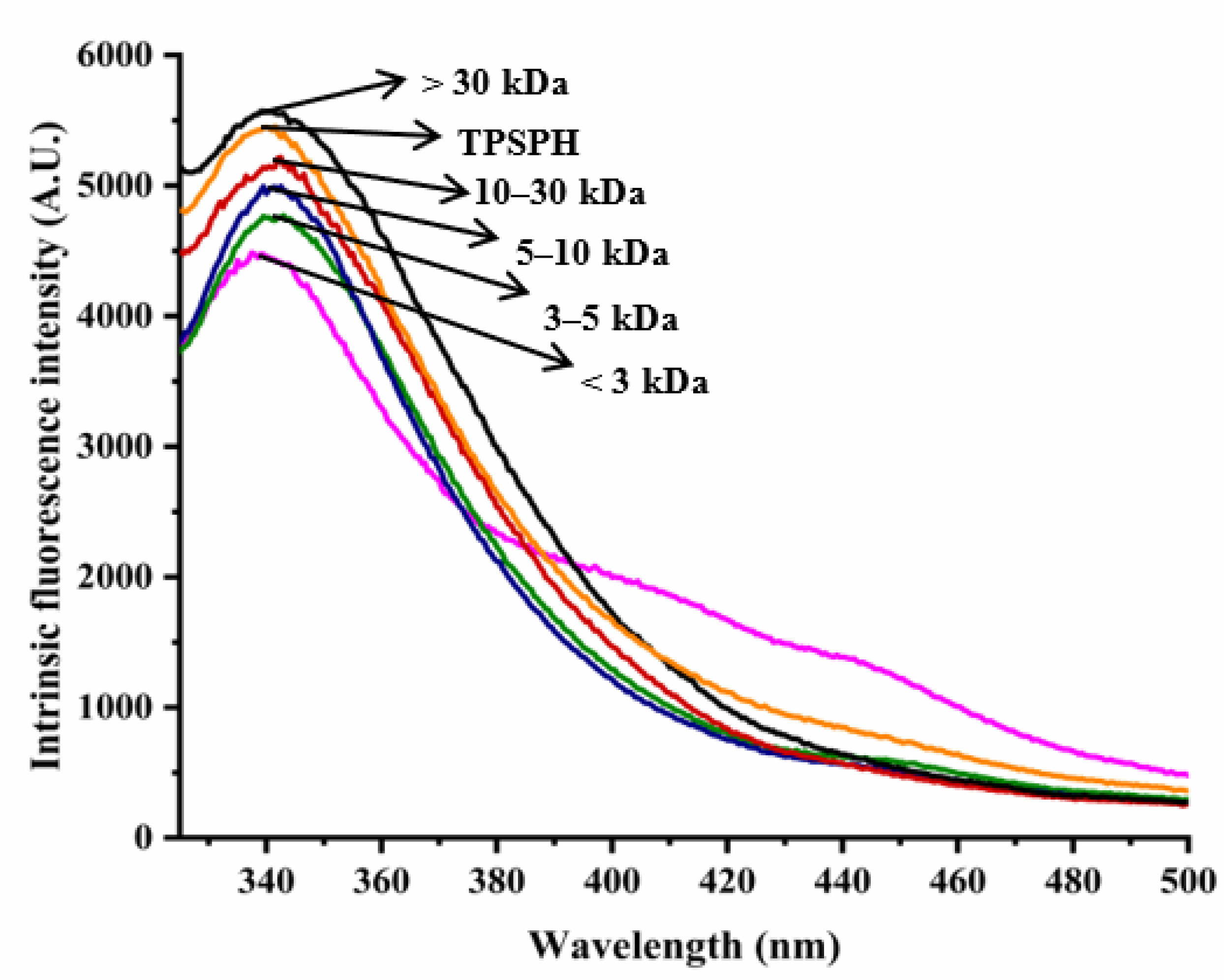
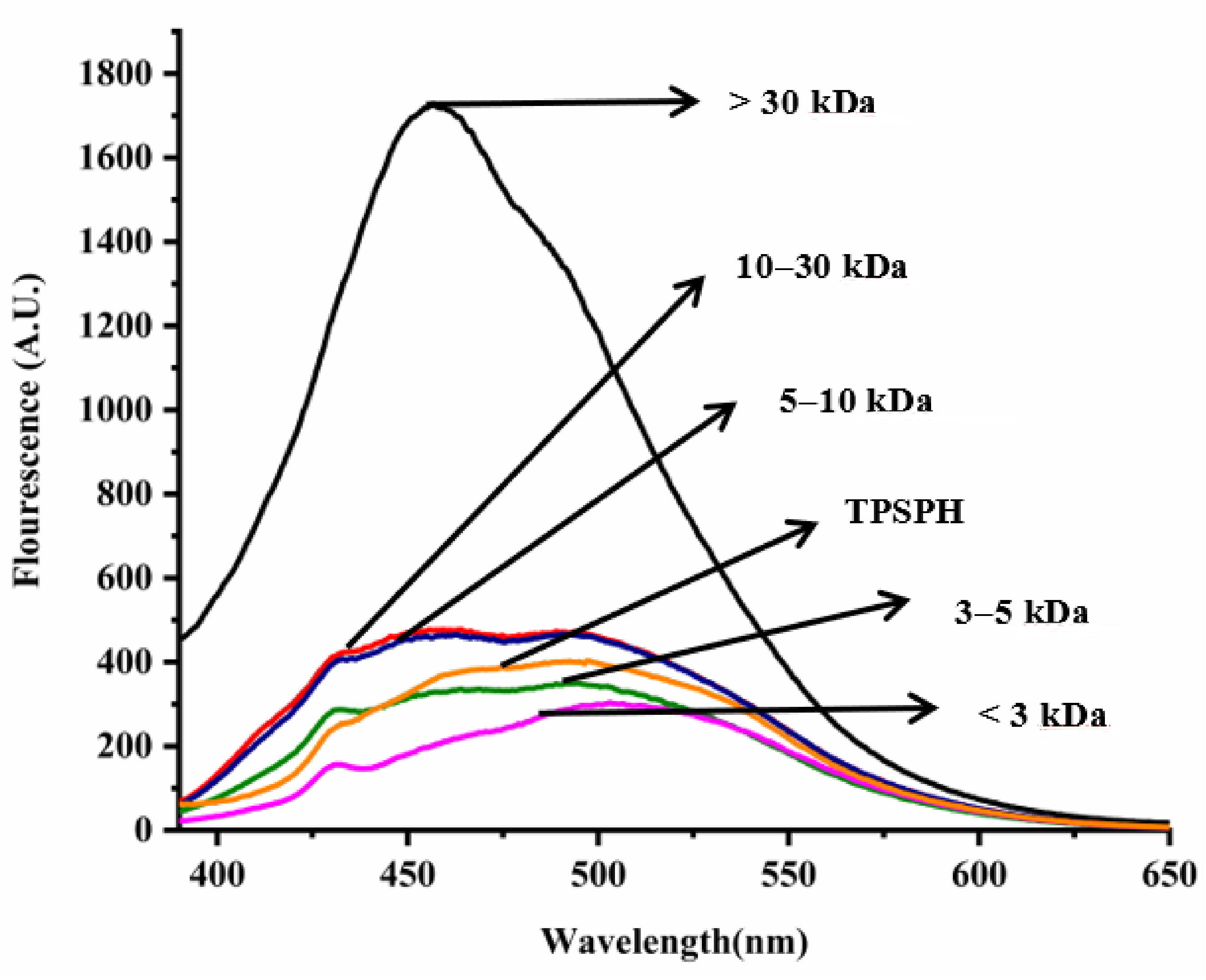
2.5. Determination of Intrinsic Fluorescence Spectroscopy of TPSPH
2.6. Determination of Surface Hydrophobicity of TPSPH
2.7. Determination of Functional Properties of TPSPH
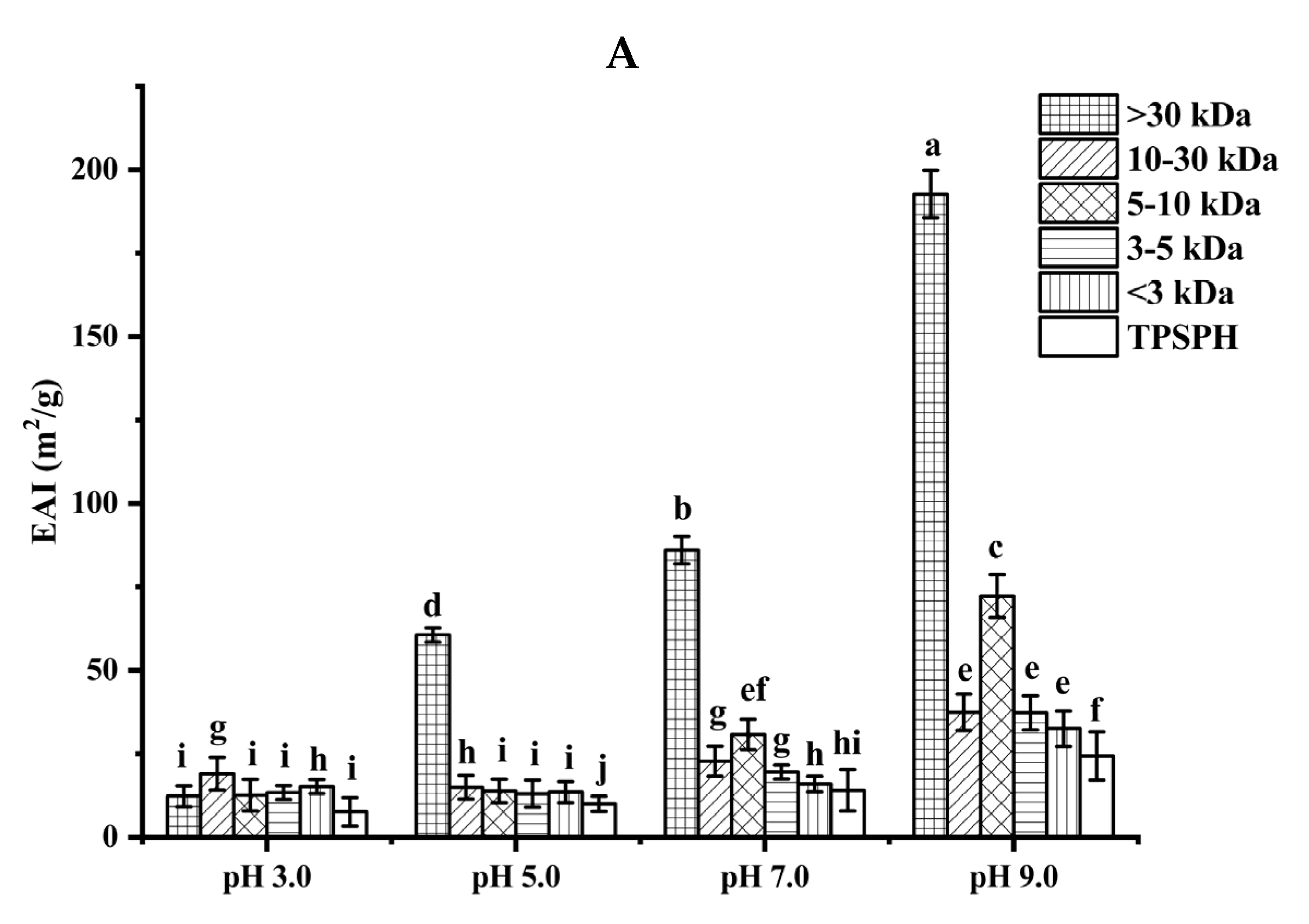
2.7.1. Emulsifying Properties
2.7.2. Foaming Properties
2.8. Determination of Antioxidant Activity of TPSPH
2.8.1. DPPH Radical Scavenging Activity
2.8.2. Hydroxyl Radical Scavenging Activity
2.8.3. Ferrous Chelating Activity
2.8.4. The β-carotene Bleaching Assay
2.9. Determination of Cytoprotective Effect of TPSPH on Cell Damage Induced by H2O2
2.9.1. Cell Culture and Cytotoxicity Assay
2.9.2. Cytoprotective Effect
2.9.3. Determination of Intracellular ROS Generation in HepG2 Cells
2.9.4. Determination of MDA, LDH, SOD, and CAT Levels in HepG2 Cells
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of Molecular Weight Fractionation on Amino Acid Compositions of TPSPH
3.2. Effects of Molecular Weight Fractionation on Particle Size Distribution of TPSPH
3.3. Effects of Molecular Weight Fractionation on Intrinsic Fluorescence Spectroscopy of TPSPH
3.4. Effects of Molecular Weight Fractionation on Surface Hydrophobicity of TPSPH
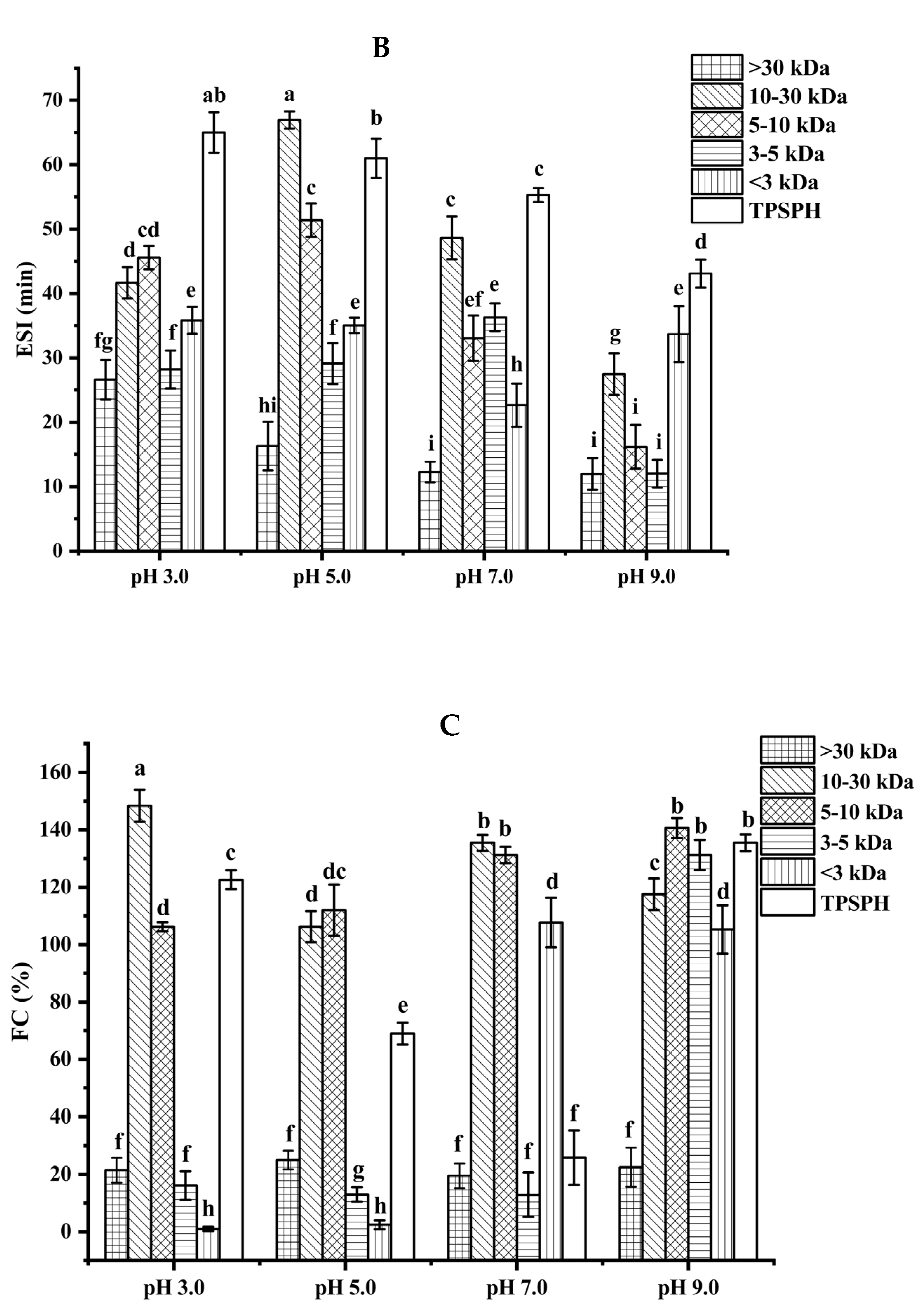
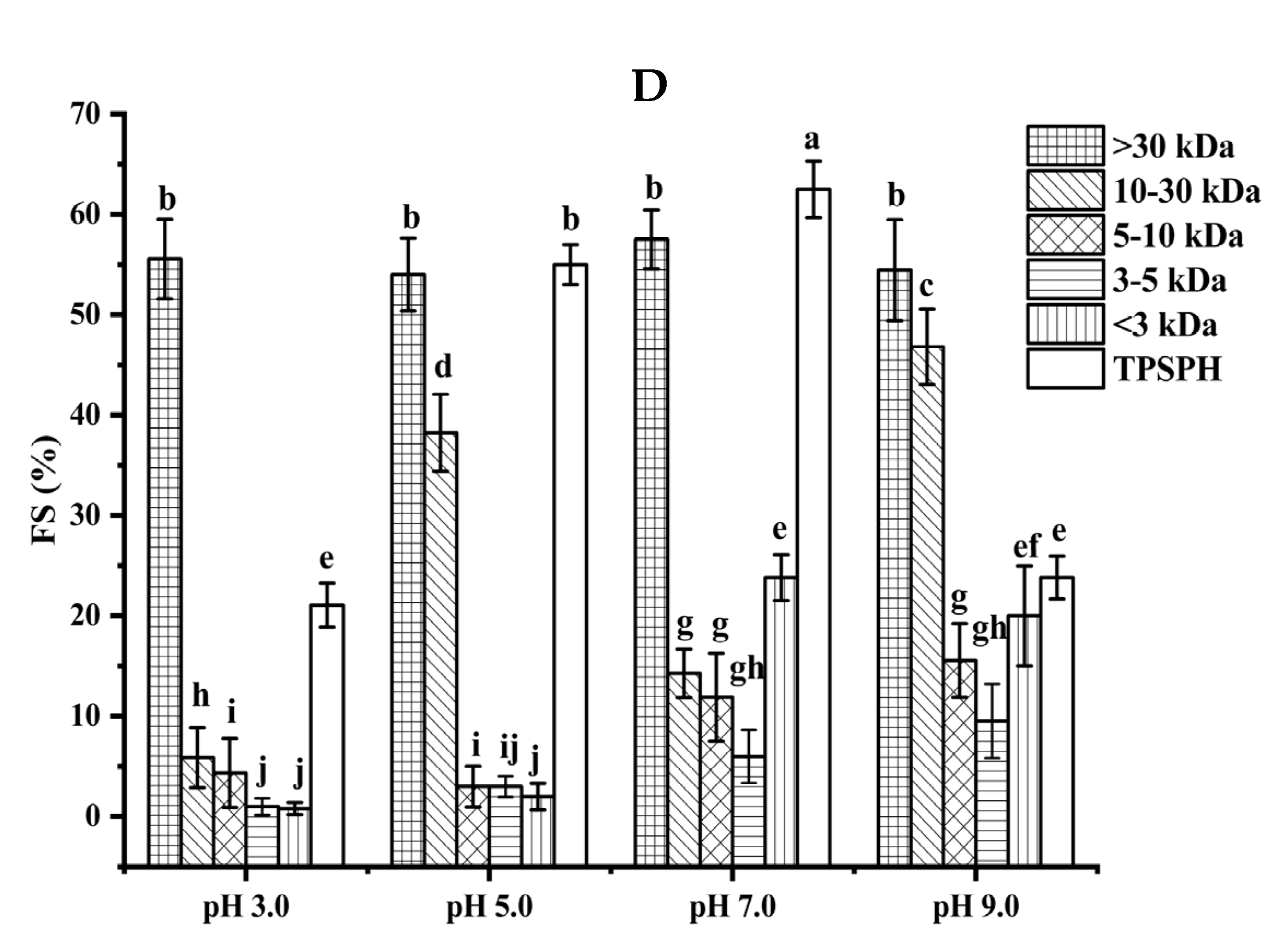
3.5. Effects of Molecular Weight Fractionation on Functional Properties of TPSPH
3.5.1. Emulsifying Properties
3.5.2. Foaming Properties
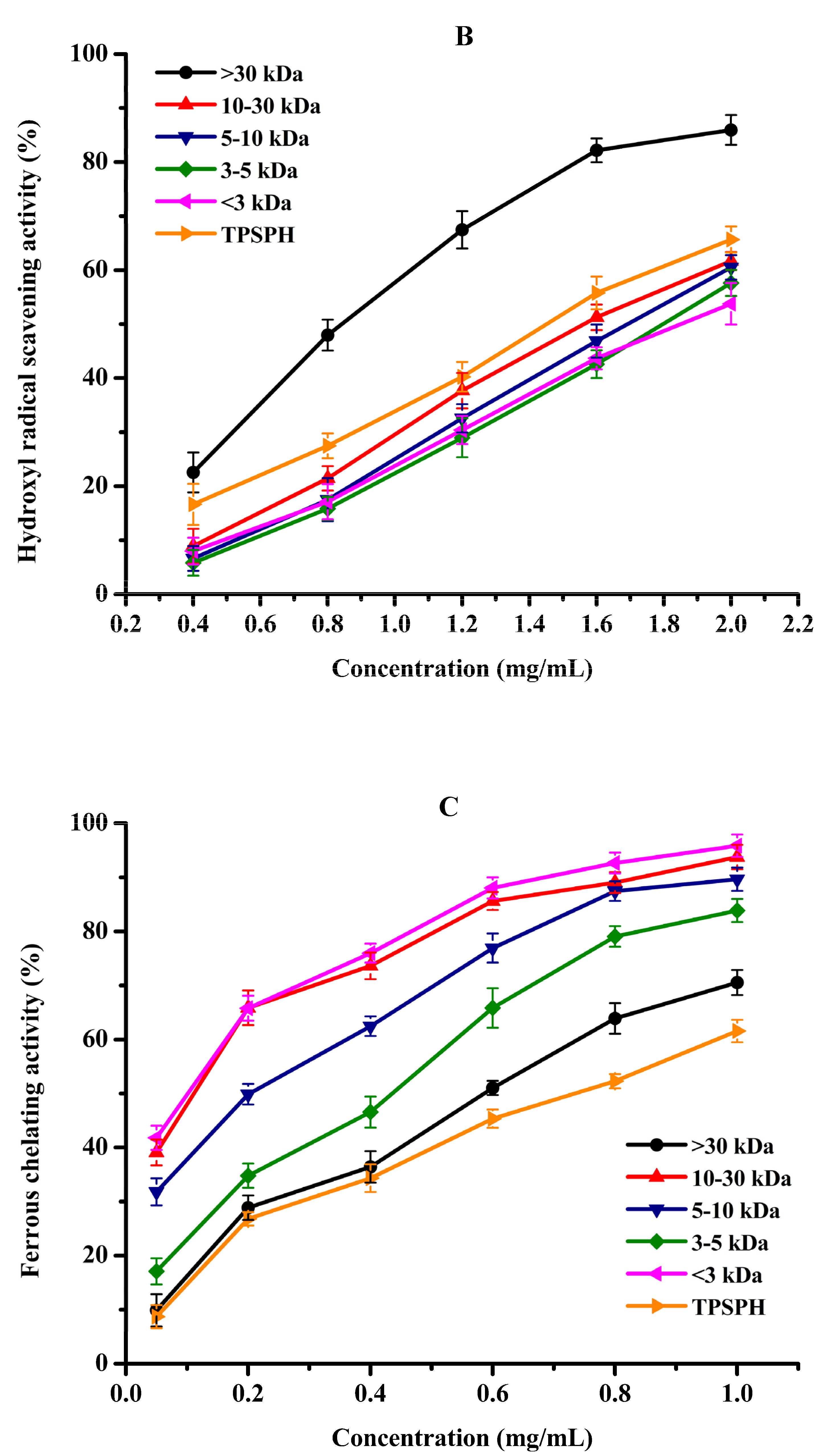
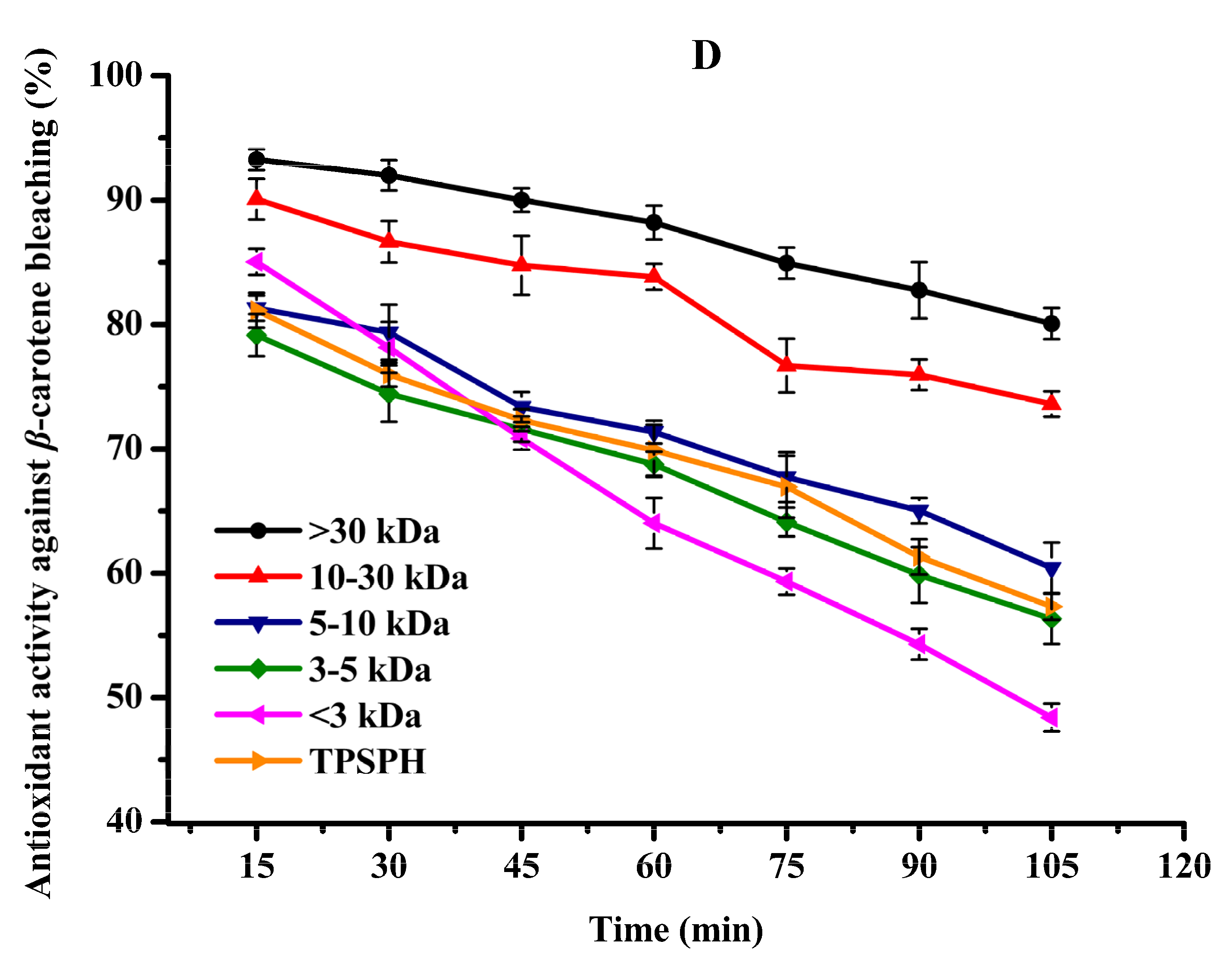
3.6. Effects of Molecular Weight Fractionation on Antioxidant Activity of TPSPH
3.6.1. DPPH Radical Scavenging Activity
3.6.2. Hydroxyl Radical Scavenging Activity
3.6.3. Ferrous Chelating Activity
3.6.4. Antioxidant Activity in β-Carotene Bleaching
3.7. Effects of Molecular Weight Fractionation on Protective Effects of TPSPH on HepG2 Cells
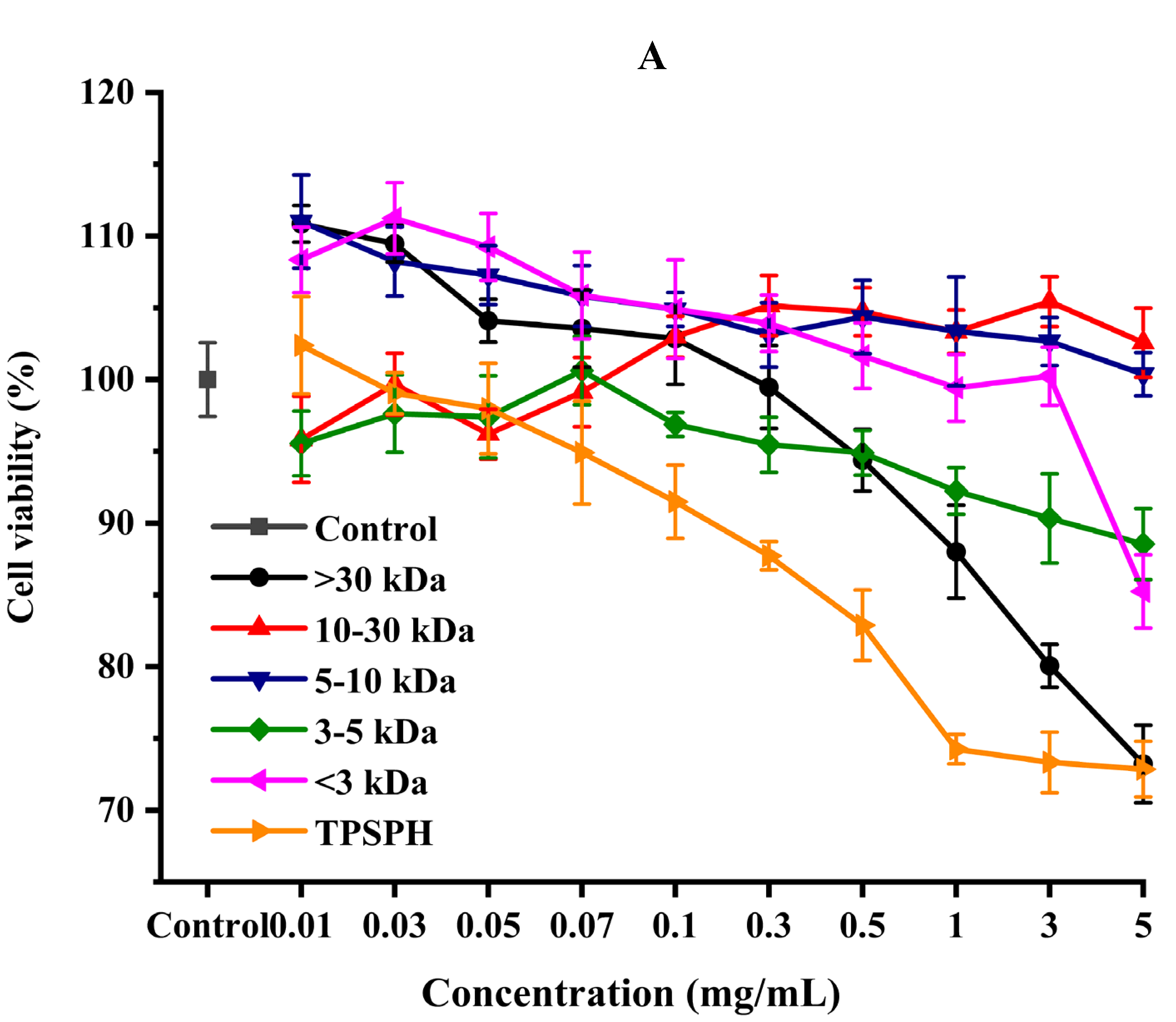
3.7.1. Cytotoxic Effect
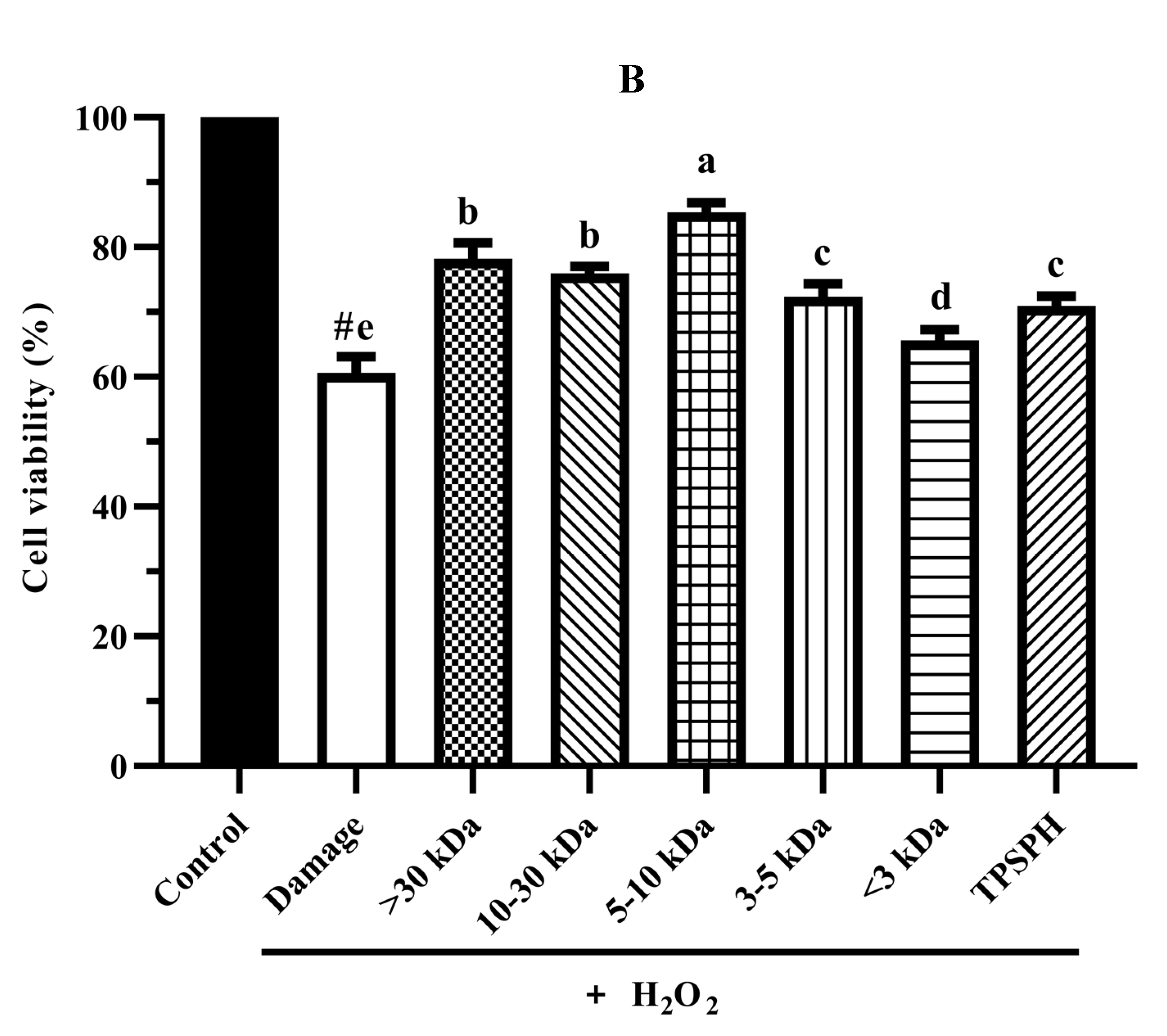
3.7.2. Cytoprotective Effect
3.7.3. The ROS Content in HepG2 Cells
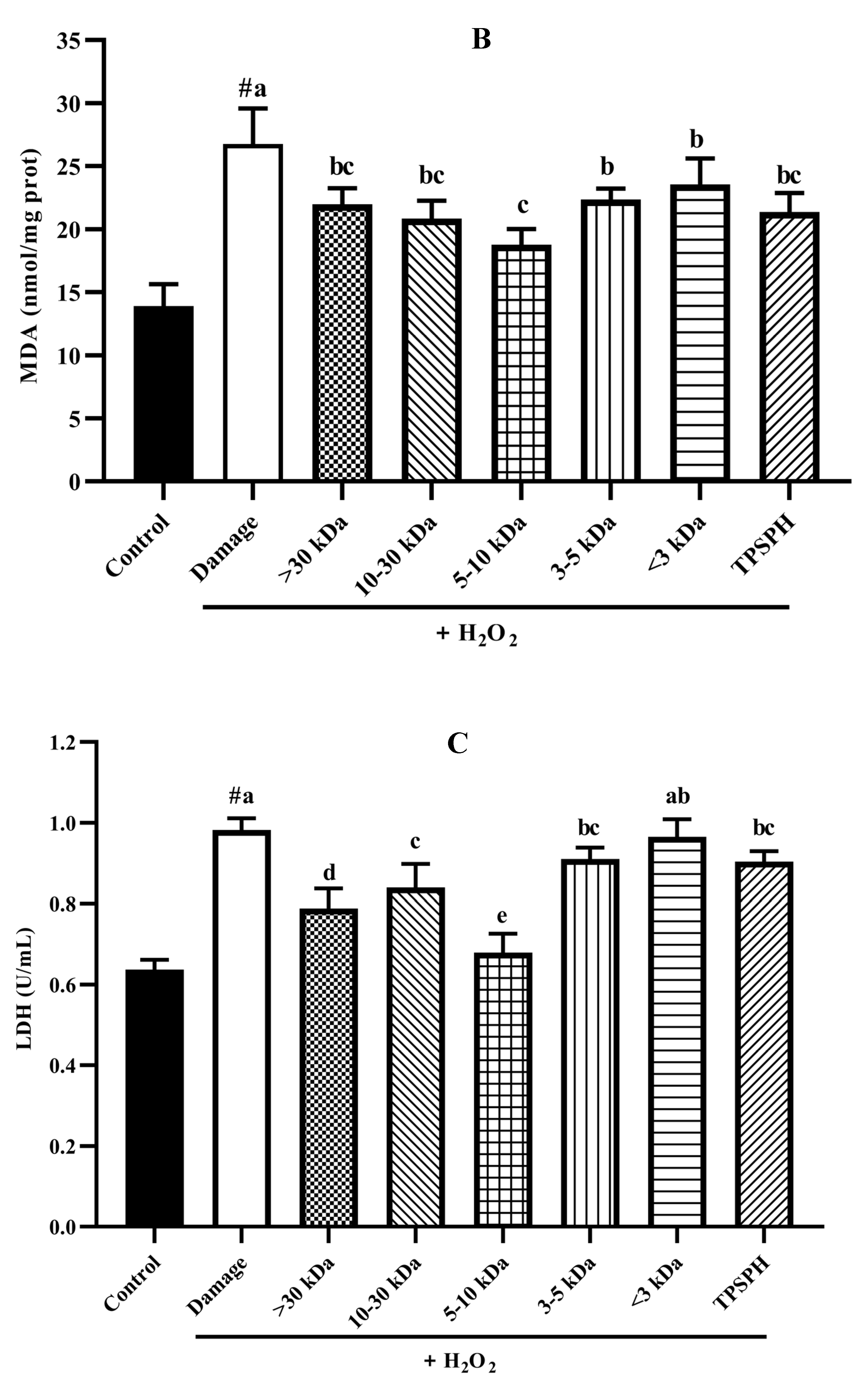
3.7.4. The Content of LDH and MDA in HepG2 Cells
3.7.5. Activity of Antioxidant Enzymes in HepG2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full names |
| TPSPH | tree peony seed protein hydrolysate |
| MW | molecular weight |
| ANS | 1-anilino-8-naphthalene-sulphonate |
| EAI | emulsifying activity index |
| ESI | emulsion stability index |
| FC | foaming capacity |
| FS | foam stability |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| GSH | glutathione |
| IC50 | 50% inhibitory concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylthiazolium bromide |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | fetal bovine serum |
| BCA | bicinchoninic acid |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DCFH | 2′,7′-dichlorodihydrofluorescein |
| DCF | 2′,7′-dichlorofluorescein |
| ROS | reactive oxygen species |
| MDA | malonaldehyde |
| LDH | lactate dehydrogenase |
| SOD | superoxide dismutase |
| CAT | catalase |
References
- Deng, R.X.; Gao, J.Y.; Yi, J.P.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Li, Y.Q.; Wang, Z.S.; Sun, G.J.; Qi, X.M.; Mo, H.Z. Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem. 2018, 240, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.B.; Wang, S.Y.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, C.Y.; Wang, S.T.; Li, Y.Q.; Mo, H.Z.; He, J.X. Physicochemical properties and antioxidant activities of tree peony (Paeonia suffruticosa Andr.) seed protein hydrolysates obtained with different proteases. Food Chem. 2021, 345, 128765. [Google Scholar] [CrossRef]
- Coelho, M.S.; Aquino, S.D.A.; Latorres, J.M.; Salas-Mellado, M.D.L.M. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll. 2019, 91, 19–25. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Antioxidant potential of date (Phoenix dactylifera L.) seed protein hydrolysates and carnosine in food and biological systems. J. Agric. Food Chem. 2015, 63, 864–871. [Google Scholar] [CrossRef]
- Samaei, S.P.; Martini, S.; Tagliazucchi, D.; Gianotti, A.; Babinil, E. Antioxidant and angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from alcalase protein hydrolysate fractions of hemp (Cannabis sativa L.) bran. J. Agric. Food. Chem. 2021, 69, 9220–9228. [Google Scholar] [CrossRef]
- Xie, J.H.; Du, M.X.; Shen, M.Y.; Wu, T.; Lin, L.H. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Rahimi, R.; Gavlighi, H.A.; Sarteshnizi, R.A.; Barzegar, M.; Udenigwe, C.C. In vitro antioxidant activity and antidiabetic effect of fractionated potato protein hydrolysate via ultrafiltration and adsorption chromatography. LWT Food Sci. Technol. 2022, 154, 112765. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhou, J.; Feng, Y.Q.; Duan, Y.Q.; Zhang, H.H.; Ma, H.L. Slit divergent ultrasound pretreatment assisted watermelon seed protein enzymolysis and the antioxidant activity of its hydrolysates in vitro and in vivo. Food Chem. 2020, 328, 127135. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Chen, L. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Tong, X.H.; Sui, X.N.; Wang, Z.J.; Qi, B.K.; Li, Y.; Jiang, L.Z. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res. Int. 2018, 111, 256–264. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, Y.Q.; Sun, G.J.; Wang, C.Y.; Liang, Y.; Zhao, X.Z.; He, J.X.; Mo, H.Z. Influence of ultrasound treatment on the physicochemical and antioxidant properties of mung bean protein hydrolysate. Ultrason. Sonochem. 2022, 84, 105964. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food. Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- De Avellar, I.G.J.D.; Magalhaes, M.M.M.; Silva, A.B.; Souza, L.L.; Leitao, A.C.; Hermes-Lima, M. Reevaluating the role of 1,10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage. Biochimi. Biophysi. Acta Gen. Subj. 2004, 1675, 46–53. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Hu, Y.M.; Lu, S.Z.; Li, Y.S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Ye, H.D.; Du, M.X.; Yu, Q.; Chen, Y.; Shen, M.Y. Mung bean protein hydrolysates protect mouse liver cell line Nctc-1469 cell from hydrogen peroxide-induced cell injury. Foods 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Dai, L.; Sun, Q.J.; McClements, D.J.; Xu, X.F. Effect of molecular weight on the interfacial and emulsifying characteristics of rice glutelin hydrolysates. Food Hydrocoll. 2022, 128, 107560. [Google Scholar] [CrossRef]
- Singh, T.P.; Siddiqi, R.A.; Sogi, D.S. Enzymatic modification of rice bran protein: Impact on structural, antioxidant and functional properties. LWT Food Sci. Technol. 2021, 138, 110648. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidic, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Xie, H.X.; Huang, J.M.; Woo, M.W.; Hu, J.W.; Xiong, H.; Zhao, Q. Effect of cold and hot enzyme deactivation on the structural and functional properties of rice dreg protein hydrolysates. Food Chem. 2021, 345, 128784. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ma, M.T.; Yu, Z.P.; Du, S.K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Fathollahy, I.; Farmani, J.; Kasaai, M.R.; Hamishehkar, H. Characteristics and functional properties of Persian lime (Citrus latifolia) seed protein isolate and enzymatic hydrolysates. LWT Food Sci. Technol. 2021, 140, 110765. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J. Am. Oil Chem. Soc. 2011, 88, 381–389. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Sierra, L.; Fan, H.; Zapata, J.; Wu, J.P. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT Food Sci. Technol. 2021, 146, 111631. [Google Scholar] [CrossRef]
- Wiriyaphan, A.C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Zhang, Y.F.; Zhuang, Y.L. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Zhan, Q.P.; Wang, Q.; Liu, Q.; Guo, Y.F.; Gong, F.; Hao, L.H.; Wu, H.; Dong, Z. The antioxidant activity of protein fractions from Sacha inchi seeds after a simulated gastrointestinal digestion. LWT Food Sci. Technol. 2021, 145, 111356. [Google Scholar] [CrossRef]




| Amount (g/100 g) Dry Basis | ||||||
|---|---|---|---|---|---|---|
| <3 kDa | 3–5 kDa | 5–10 kDa | 10–30 kDa | >30 kDa | TPSPH | |
| Asp | 9.46 ± 1.25 ab | 7.23 ± 0.85 b | 9.48 ± 2.29 ab | 10.09 ± 1.76 a | 8.32 ± 0.24 ab | 8.08 ± 0.17 ab |
| Thr | 2.93 ± 0.24 ab | 1.79 ± 0.26 d | 2.30 ± 0.35 cd | 2.71 ± 0.47 ab | 3.26 ± 0.13 a | 2.50 ± 0.21 bc |
| Ser | 3.74 ± 0.37 b | 0.79 ± 0.08 d | 2.98 ± 0.25 c | 3.79 ± 0.55 b | 5.58 ± 0.12 a | 3.58 ± 0.36 b |
| Glu | 22.91 ± 0.89 a | 11.77 ± 0.63 b | 22.99 ± 1.37 a | 23.10 ± 2.75 a | 20.09 ± 1.82 a | 20.88 ± 1.32 a |
| Gly | 3.94 ± 0.33 a | 4.02 ± 0.27 a | 3.28 ± 0.22 c | 3.47 ± 0.34 ab | 4.19 ± 0.22 a | 3.67 ± 0.18 ab |
| Ala | 3.51 ± 0.25 b | 5.86 ± 0.73 a | 2.71 ± 0.62 b | 3.06 ± 0.48 b | 4.91 ± 0.66 a | 3.13 ± 0.44 b |
| Cys | 2.15 ± 0.62 b | 0.90 ± 0.28 c | 1.10 ± 0.26 c | 0.34 ± 0.07 d | 0.20 ± 0.05 d | 2.91 ± 0.19 a |
| Val | 3.66 ± 0.17 bc | 4.27 ± 0.58 b | 3.41 ± 0.29 c | 3.66 ± 0.36 bc | 5.34 ± 0.16 a | 5.12 ± 0.37 a |
| Met | 0.93 ± 0.25 b | 1.16 ± 0.15 ab | 0.79 ± 0.07 b | 0.81 ± 0.25 b | 1.12 ± 0.11 ab | 1.35 ± 0.29 a |
| Ile | 3.51 ± 0.21 a | 3.78 ± 0.05 a | 2.91 ± 0.18 b | 2.69 ± 0.26 b | 3.43 ± 0.17 a | 3.67 ± 0.31 a |
| Leu | 5.73 ± 0.54 b | 5.73 ± 0.24 b | 4.38 ± 0.12 c | 5.09 ± 0.75 bc | 7.57 ± 1.05 a | 5.75 ± 0.56 b |
| Tyr | 1.58 ± 0.15 cd | 1.59 ± 0.25 cd | 1.21 ± 0.23 d | 1.68 ± 0.33 c | 3.10 ± 0.13 b | 3.83 ± 0.25 a |
| Phe | 3.22 ± 0.09 c | 3.14 ± 0.05 c | 3.51 ± 0.05 b | 2.56 ± 0.28 d | 3.88 ± 0.15 a | 3.62 ± 0.12 ab |
| Lys | 2.20 ± 0.24 a | 1.92 ± 0.12 ab | 1.59 ± 0.25 bc | 1.06 ± 0.15 d | 1.65 ± 0.22 bc | 1.49 ± 0.16 c |
| His | 1.82 ± 0.08 a | 1.35 ± 0.05 c | 1.38 ± 0.28 c | 1.42 ± 0.17 bc | 1.83 ± 0.14 a | 1.73 ± 0.23 ab |
| Arg | 7.13 ± 0.46 a | 6.86 ± 0.15 ab | 4.93 ± 0.86 c | 4.04 ± 0.25 d | 6.52 ± 0.23 ab | 6.12 ± 0.38 b |
| Trp | 1.39 ± 0.21 a | 0.84 ± 0.23 bc | 1.10 ± 0.11 ab | 0.48 ± 0.12 c | 1.33 ± 0.25 a | 1.30 ± 0.33 a |
| Pro | 5.89 ± 0.15 a | 3.30 ± 0.07 d | 3.86 ± 0.21 c | 3.75 ± 0.14 b | 4.54 ± 0.09 b | 3.56 ± 0.26 cd |
| TAAs | 84.32 a | 66.31 c | 72.91 b | 74.12 b | 86.83 a | 82.28 a |
| AAAs | 6.19 b | 5.57 c | 4.82 cd | 5.07 c | 8.30 a | 8.75 a |
| BCAAs | 12.91 bc | 13.77 b | 10.70 d | 11.43 c | 16.33 a | 14.54 b |
| HAAs | 24.63 b | 24.94 b | 19.15 c | 19.53 c | 28.22 a | 23.37 b |
| PCAAs | 11.15 a | 10.13 ab | 7.90 c | 6.52 d | 9.99 ab | 9.34 b |
| NCAAs | 32.37 b | 19.00 d | 32.48 b | 33.18 a | 28.41 c | 28.96 c |
| EAAs | 27.22 b | 25.02 c | 20.59 d | 19.96 d | 29.79 a | 27.74 ab |
| Hydrolysates | IC50 (mg/mL) | ||
|---|---|---|---|
| DPPH Radical Scavenging Activity | Hydroxyl Radical Scavenging Activity | Fe2+ Chelating Activity | |
| > 30 kDa | 0.04 ± 0.01 c | 0.90 ± 0.10 d | 0.62 ± 0.08 b |
| 10–30 kDa | 0.11 ± 0.02 b | 1.59 ± 0.08 ab | 0.10 ± 0.02 e |
| 5–10 kDa | 0.11 ± 0.01 b | 1.70 ± 0.17 ab | 0.25 ± 0.03 d |
| 3–5 kDa | 0.14 ± 0.04 ab | 1.81 ± 0.20 a | 0.43 ± 0.07 c |
| < 3 kDa | 0.17 ± 0.03 a | 1.86 ± 0.12 a | 0.07 ± 0.01 e |
| TPSPH | 0.12 ± 0.02 b | 1.47 ± 0.31 b | 0.74 ± 0.02 a |
| GSH | 0.14 ± 0.03 ab | 1.23 ± 0.16 c | 0.46 ± 0.04 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Wang, C.; He, J.; Mo, H. Antioxidant Activity, Functional Properties, and Cytoprotective Effects on HepG2 Cells of Tree Peony (Paeonia suffruticosa Andr.) Seed Protein Hydrolysate as Influenced by Molecular Weights Fractionation. Foods 2022, 11, 2592. https://doi.org/10.3390/foods11172592
Wang Y, Li Y, Wang C, He J, Mo H. Antioxidant Activity, Functional Properties, and Cytoprotective Effects on HepG2 Cells of Tree Peony (Paeonia suffruticosa Andr.) Seed Protein Hydrolysate as Influenced by Molecular Weights Fractionation. Foods. 2022; 11(17):2592. https://doi.org/10.3390/foods11172592
Chicago/Turabian StyleWang, Yingying, Yingqiu Li, Chenying Wang, Jinxing He, and Haizhen Mo. 2022. "Antioxidant Activity, Functional Properties, and Cytoprotective Effects on HepG2 Cells of Tree Peony (Paeonia suffruticosa Andr.) Seed Protein Hydrolysate as Influenced by Molecular Weights Fractionation" Foods 11, no. 17: 2592. https://doi.org/10.3390/foods11172592






