Multi-Scale Structures and Functional Properties of Quinoa Starch Extracted by Alkali, Wet-Milling, and Enzymatic Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Starch Using the Alkali, Wet-Milling, and Enzymatic Methods
2.3. Chemical Composition Analysis
2.4. Multi-Scale Structure Analyses
2.4.1. Particle Size Analysis
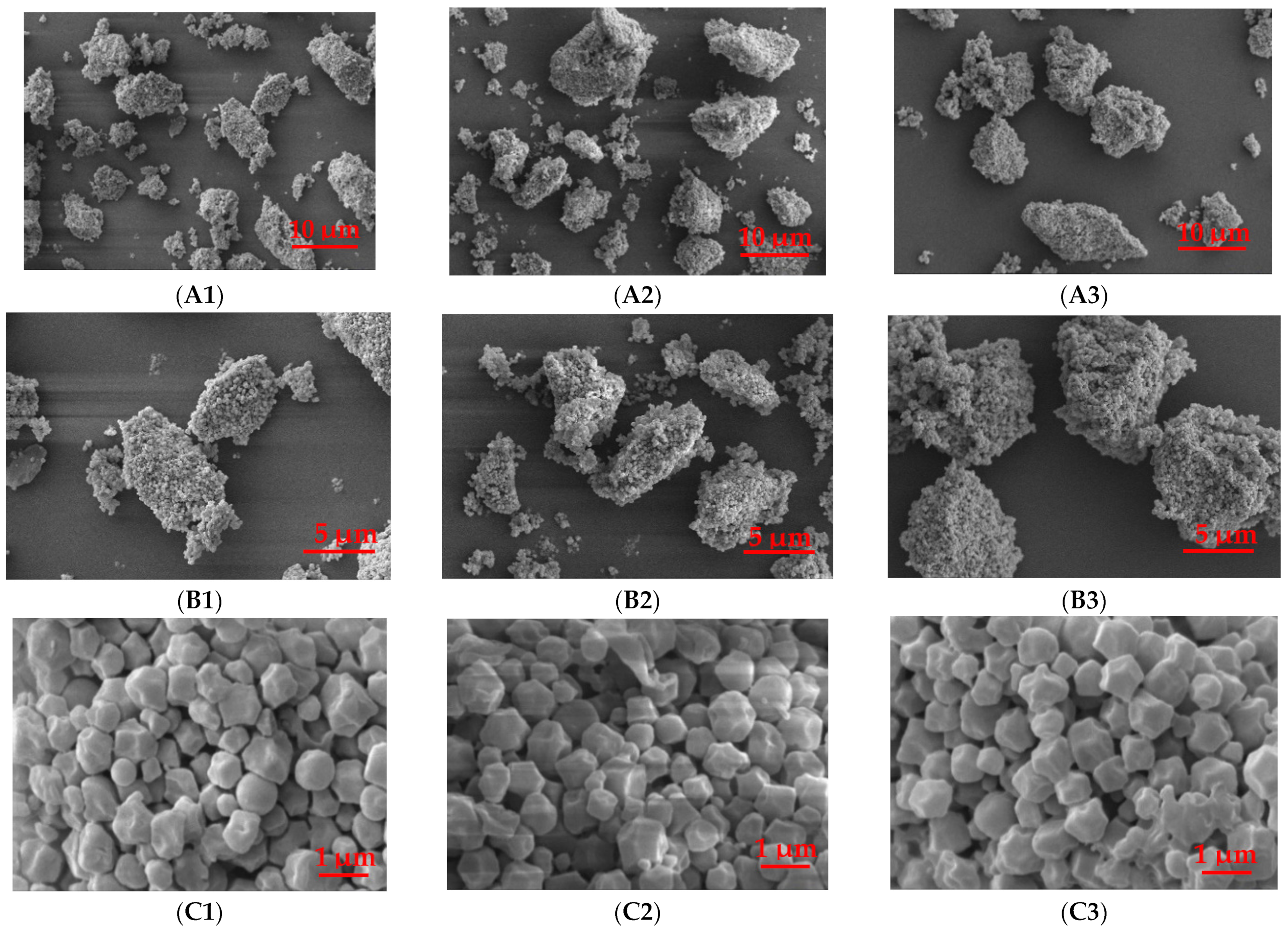
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. X-ray Diffraction (XRD)
2.4.4. Small-Angle X-ray Scattering (SAXS) Analysis
2.4.5. Attenuated Total Reflectance-Fourier Transformed Infrared (ATR-FTIR) Spectroscopy
2.4.6. Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy
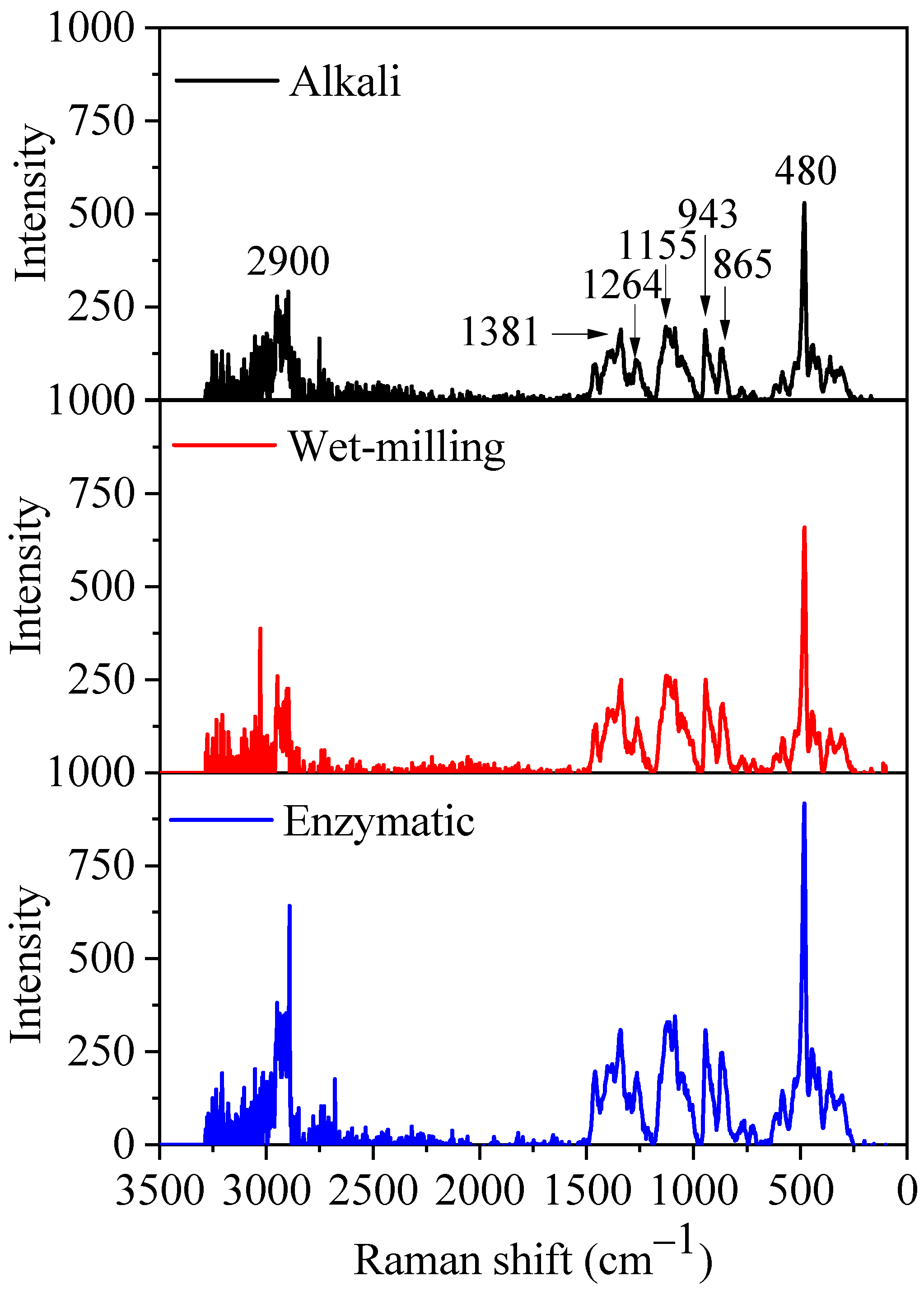
2.4.7. Laser Confocal Micro-Raman (LCM-Raman) Analysis
2.4.8. Gel Permeation Chromatography Coupled with Multi-Angle Light Scattering (GPC-MALS)
2.5. Functional Properties
2.5.1. Differential Scanning Calorimetry (DSC)
2.5.2. Textural Analysis
2.5.3. In Vitro Digestion of Starch
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition
3.2. Multi-Scale Structure Characteristics
3.2.1. Starch Granule Morphology
3.2.2. Crystalline Structure
3.2.3. Lamellar Structure
3.2.4. Ordered Short-Range Structure
3.2.5. Solid-State NMR
3.2.6. LCM-Raman Spectra
3.2.7. Molecular Weight and Molar Mass Ratio
3.3. Functional Properties
3.3.1. Thermal Properties
3.3.2. Textural Properties
3.3.3. In Vitro Digestion Properties
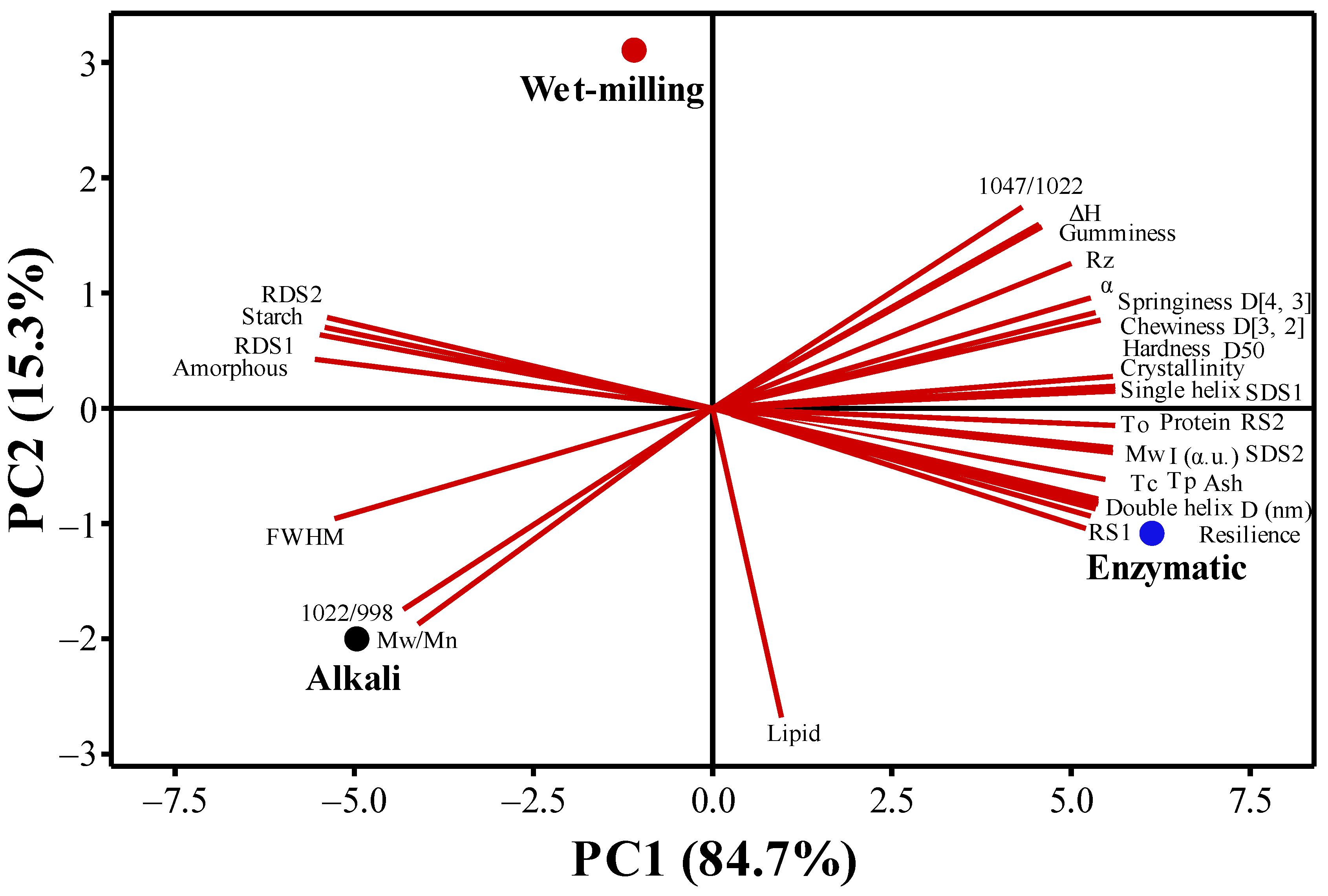
3.3.4. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jan, K.N.; Panesar, P.; Rana, J.; Singh, S. Structural, thermal and rheological properties of starches isolated from Indian quinoa varieties. Int. J. Biol. Macromol. 2017, 102, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Amylopectin molecular structure in relation to physicochemical properties of quinoa starch. Carbohydr. Polym. 2017, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chem. 2018, 241, 380–386. [Google Scholar] [CrossRef]
- Adigwe, O.P.; Egharevba, H.O.; Emeje, M.O. Starch: A Veritable Natural Polymer for Economic Revolution. In Starch—Evolution and Recent Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The need for improved quality or quantity--an overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef]
- Correia, P.; Cruz-Lopes, L.; Beirão-Da-Costa, L. Morphology and structure of chestnut starch isolated by alkali and enzymatic methods. Food Hydrocoll. 2012, 28, 313–319. [Google Scholar] [CrossRef]
- Correia, P.R.; Beirão-Da-Costa, M.L. Chestnut and acorn starch properties affected by isolation methods. Starch Stärke 2010, 62, 421–428. [Google Scholar] [CrossRef]
- Syahariza, Z.; Li, E.; Hasjim, J. Extraction and dissolution of starch from rice and sorghum grains for accurate structural analysis. Carbohydr. Polym. 2010, 82, 14–20. [Google Scholar] [CrossRef]
- Tan, I.; Wee, C.; Sopade, P.; Halley, P. Investigation of the starch gelatinisation phenomena in water–glycerol systems: Application of modulated temperature differential scanning calorimetry. Carbohydr. Polym. 2004, 58, 191–204. [Google Scholar] [CrossRef]
- Nadiha, M.N.; Fazilah, A.; Bhat, R.; Karim, A.A. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chem. 2010, 121, 1053–1059. [Google Scholar] [CrossRef]
- Singh, N.; Sandhu, K.S.; Kaur, M. Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J. Food Eng. 2004, 63, 441–449. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Liu, M.; Zhang, Y.; Cao, S. Nutrient composition and starch characteristics of Quercus glandulifera Bl. seeds from China. Food Chem. 2015, 185, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B.; Tan, C.P.; Li, C.; Fu, X.; Huang, Q. Octenylsuccinate quinoa starch granule-stabilized Pickering emulsion gels: Preparation, microstructure and gelling mechanism. Food Hydrocoll. 2019, 91, 40–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, X.; Wu, G.; Gilbert, R.G. Using molecular fine structure to identify optimal methods of extracting starch. Starch Stärke 2020, 72, 1900214. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Zhang, B.; Zhao, Y.; Li, X.; Li, L.; Xie, F.; Chen, L. Supramolecular structural changes of waxy and high-amylose cornstarches heated in abundant water. Food Hydrocoll. 2014, 35, 700–709. [Google Scholar] [CrossRef]
- Mutungi, C.; Onyango, C.; Doert, T.; Paasch, S.; Thiele, S.; Machill, S.; Jaros, D.; Rohm, H. Long- and short-range structural changes of recrystallised cassava starch subjected to in vitro digestion. Food Hydrocoll. 2011, 25, 477–485. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A method for estimating the nature and relative proportions of amorphous, single, and double-helical components in starch granules by 13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, X.; Huang, Q.; Zhang, B. Effects of wet-media milling on multi-scale structures and in vitro digestion of tapioca starch and the structure-digestion relationship. Carbohydr. Polym. 2022, 284, 119176. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, B.; Baumgartner, B.; Özkaya, H. Effects of concentrated and dephytinized wheat bran and rice bran addition on bread properties. J. Texture Stud. 2018, 49, 84–93. [Google Scholar] [CrossRef]
- Yuan, T.; Ye, F.; Chen, T.; Li, M.; Zhao, G. Structural characteristics and physicochemical properties of starches from winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch. ex Poir.). Food Hydrocoll. 2022, 122, 107115. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- El Halal, S.L.M.; Kringel, D.H.; Zavareze, E.D.R.; Dias, A.R.G. Methods for extracting cereal starches from different sources: A Review. Starch Stärke 2019, 71, 1900128. [Google Scholar] [CrossRef]
- Nyakabau, T.; Wokadala, O.C.; Emmambux, M.N. Effect of steeping additives on tef starch extraction and its quality. Starch Stärke 2013, 65, 738–746. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Jiang, S.-W.; Zheng, Z.; Cao, X.-M.; Hou, Z.-G.; Xu, J.-J.; Wang, H.-L.; Jiang, S.-T.; Pan, L.-J. Preparation and properties of OSA-modified taro starches and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2019, 137, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Estrada-León, R.J.; Moo-Huchin, V.M.; Ríos-Soberanis, C.; Betancur-Ancona, D.; May, L.; Carrillo-Sánchez, F.; Cervantes-Uc, J.; Pérez-Pacheco, E. The effect of isolation method on properties of parota (Enterolobium cyclocarpum) starch. Food Hydrocoll. 2016, 57, 1–9. [Google Scholar] [CrossRef]
- Singh, S.; Thakur, S.; Singh, M.; Kumar, A.; Kumar, A.; Kumar, A.; Punia, R.; Kushwaha, J.; Kumar, R.; Singh, H. Influence of different isolation methods on physicochemical and rheological properties of native and heat-moisture-treated chickpea starch. J. Food Process. Preserv. 2018, 42, e13523. [Google Scholar] [CrossRef]
- Han, J. Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydr. Polym. 2004, 55, 193–199. [Google Scholar] [CrossRef]
- Peng, M.; Yin, L.; Dong, J.; Shen, R.; Zhu, Y. Physicochemical characteristics and in vitro digestibility of starches from colored quinoa (Chenopodium quinoa) varieties. J. Food Sci. 2022, 87, 2147–2158. [Google Scholar] [CrossRef]
- Palacios-Fonseca, A.; Castro-Rosas, J.; Gómez-Aldapa, C.; Tovar-Benítez, T.; Millán-Malo, B.; Del Real, A.; Rodriguez-Garcia, M.E.; Gomez-Aldapa, C. Effect of the alkaline and acid treatments on the physicochemical properties of corn starch. CyTA-J. Food 2013, 11, 67–74. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, B.; Chen, L.; Li, X.; Li, L.; Xie, F. Effect of planetary ball-milling on multi-scale structures and pasting properties of waxy and high-amylose cornstarches. Innov. Food Sci. Emerg. Technol. 2015, 30, 198–207. [Google Scholar] [CrossRef]
- Koroteeva, D.A.; Kiseleva, V.; Krivandin, A.V.; Shatalova, O.V.; Blaszczak, W.; Bertoft, E.; Piyachomkwan, K.; Yuryev, V.P. Structural and thermodynamic properties of rice starches with different genetic background: Part 2. Defectiveness of different supramolecular structures in starch granules. Int. J. Biol. Macromol. 2007, 41, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, Y.; Wang, J.; Sun, B. Relations between chain-length distribution, molecular size, and amylose content of rice starches. Int. J. Biol. Macromol. 2018, 120, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, Y.; Liu, J.; Li, K.; Li, J. Insights into the multiscale structure and pasting properties of ball-milled waxy maize and waxy rice starches. Int. J. Biol. Macromol. 2021, 168, 205–214. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Zhang, C.; Zhou, X.; Dong, Y.; Zhang, F.; Liu, Q.; Wei, C. Structural changes of high-amylose rice starch residues following in vitro and in vivo digestion. J. Agric. Food Chem. 2012, 60, 9332–9341. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Flanagan, B.M.; Hasjim, J.; Gidley, M.J. Cryo-milling of starch granules leads to differential effects on molecular size and conformation. Carbohydr. Polym. 2011, 84, 1133–1140. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, W.; Li, C.; Yu, J.; Wang, S. Molecular order and functional properties of starches from three waxy wheat varieties grown in China. Food Chem. 2015, 181, 43–50. [Google Scholar] [CrossRef]
- Praznik, W.; Buksa, K.; Ziobro, R.; Gambuś, H.; Nowotna, A. The effect of long-term alkali treatment on the molecular characteristics of native and extruded starches at 35 °C. Starch Stärke 2012, 64, 890–897. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. A review of milling damaged starch: Generation, measurement, functionality and its effect on starch-based food systems. Food Chem. 2020, 315, 126267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, K.; Hasjim, J.; Li, E.; Flanagan, B.M.; Gidley, M.J.; Dhital, S. Freeze-drying changes the structure and digestibility of B-polymorphic starches. J. Agric. Food Chem. 2014, 62, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.; Nunes, M.C.; Beirão-Da-Costa, M.L. The effect of starch isolation method on physical and functional properties of Portuguese nut starches. II. Q. rotundifolia Lam. and Q. suber Lam. acorns starches. Food Hydrocoll. 2013, 30, 448–455. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Campbell, G.M.; Gaisford, S.; Royall, P.G.; Butterworth, P.J.; Ellis, P.R. A study of starch gelatinisation behaviour in hydrothermally-processed plant food tissues and implications for in vitro digestibility. Food Funct. 2015, 6, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Waigh, T.A.; Gidley, M.J.; Komanshek, B.U.; Donald, A.M. The phase transformations in starch during gelatinisation: A liquid crystalline approach. Carbohydr. Res. 2000, 328, 165–176. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Reynaers, H.; Delcour, J.A. Gelatinisation related structural aspects of small and large wheat starch granules. Carbohydr. Polym. 2005, 62, 170–181. [Google Scholar] [CrossRef]
- Liu, J.; Zhaowei, L.; Zhou, L.; Cao, Z.; Shi, C.; Cheng, F. Influence of environmental temperature during grain filling period on granule size distribution of rice starch and its relation to gelatinization properties. J. Cereal Sci. 2017, 76, 42–55. [Google Scholar] [CrossRef]
- Pascua, Y.; Koç, H.; Foegeding, E.A. Food structure: Roles of mechanical properties and oral processing in determining sensory texture of soft materials. Curr. Opin. Colloid Interface Sci. 2013, 18, 324–333. [Google Scholar] [CrossRef]
- Patil, S.S.; Rudra, S.G.; Varghese, E.; Kaur, C. Effect of extruded finger millet (Eleusine coracan L.) on textural properties and sensory acceptability of composite bread. Food Biosci. 2016, 14, 62–69. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Yu, J.; Guo, P.; Wang, S. Multi-scale structures and functional properties of starches from Indica hybrid, Japonica and waxy rice. Int. J. Biol. Macromol. 2017, 102, 136–143. [Google Scholar] [CrossRef]
- Reddy, C.K.; Haripriya, S.; Mohamed, A.N.; Suriya, M. Preparation and characterization of resistant starch III from elephant foot yam (Amorphophallus paeonifolius) starch. Food Chem. 2014, 155, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of starch after extrusion: A Review. Starch Stärke 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Mahasukhonthachat, K.; Sopade, P.; Gidley, M. Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. J. Cereal Sci. 2010, 51, 392–401. [Google Scholar] [CrossRef]
- Martens, B.M.J.; Gerrits, W.J.J.; Bruininx, E.M.A.M.; Schols, H.A. Amylopectin structure and crystallinity explains variation in digestion kinetics of starches across botanic sources in an in vitro pig model. J. Anim. Sci. Biotechnol. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lim, H.S.; Lim, S.-T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]

| Variables | Alkali | Wet-milling | Enzymatic |
|---|---|---|---|
| Composition (DB) B | |||
| Total starch content (%) | 98.7 ± 1.5 a | 98.6 ± 1.1 a | 97.1 ± 0.8 a |
| Protein content (%) | 0.6 ± 0.1 c | 0.9 ± 0.1 b | 2.4 ± 0.0 a |
| Lipid content (%) | 0.5 ± 0.0 a | 0.3 ± 0.0 b | 0.5 ± 0.0 a |
| Ash content (%) | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.0 a |
| Granule size distribution C | |||
| D50 (μm) | 27.6 ± 4.5 c | 34.9 ± 0.4 b | 44.1 ± 3.2 a |
| D[4, 3] (μm) | 34.5 ± 3.6 b | 39.9 ± 2.8 b | 47.7 ± 0.3 a |
| D[3, 2] (μm) | 6.2 ± 0.7 c | 7.4 ± 0.2 b | 11.3 ± 0.7 a |
| Structural Characteristics B | Variables C | Alkali | Wet-milling | Enzymatic |
|---|---|---|---|---|
| XRD | Relative crystallinity (%) | 26.8 ± 0.2 b | 27.7 ± 0.5 b | 29.0 ± 0.3 a |
| SAXS | α | 2.3 ± 0.3 b | 2.5 ± 0.2 a | 2.6 ± 0.1 a |
| I (α.u.) | 13.4 ± 0.7 b | 13.6 ± 0.3 b | 17.8 ± 0.5 a | |
| q (1/nm) | 0.6 ± 0.0 a | 0.6 ± 0.0 a | 0.6 ± 0.0 a | |
| D (nm) | 10.1 ± 0.1 a | 10.0 ± 0.1 a | 10.1 ± 0.2 a | |
| ATR-FTIR | Ratio of 1047/1022 cm−1 | 0.8 ± 0.0 b | 0.9 ± 0.0 a | 0.9 ± 0.0 a |
| Ratio of 1022/998 cm−1 | 1.3 ± 0.0 a | 1.2 ± 0.0 b | 1.2 ± 0.0 b | |
| 13C CP/MAS NMR | Single helix (%) | 6.7 ± 0.2 b | 7.3 ± 0.3 b | 8.2 ± 0.0 a |
| Double helix (%) | 21.1 ± 0.2 b | 21.2 ± 0.2 b | 23.1 ± 0.4 a | |
| Amorphous (%) | 72.2 ± 0.3 a | 71.5 ± 0.1 a | 68.7 ± 0.3 a | |
| LCM-Raman | FWHM at 480 cm−1 | 2.1 ± 0.0 a | 1.7 ± 0.0 b | 1.5 ± 0.0 c |
| GPC-MALS | Average Mw (g/moL) | 1.13 × 105 (2%) | 1.18 × 107 (3%) | 1.58 × 107 (3%) |
| Average Rz (nm) | 41.6 (4%) | 101.1 (1%) | 106.8 (1%) | |
| Mw/Mn | 2.7 (10%) | 1.2 (6%) | 1.3 (5%) |
| Variables | Alkali | Wet-milling | Enzymatic |
|---|---|---|---|
| Thermal properties B | |||
| To (°C) | 61.7 ± 0.9 c | 66.3 ± 0.9 b | 82.1 ± 1.2 a |
| Tp (°C) | 67.8 ± 0.5 b | 70.0 ± 0.5 b | 83.8 ± 0.5 a |
| Tc (°C) | 75.9 ± 0.5 b | 76.2 ± 0.9 b | 86.3 ± 0.2 a |
| ΔH (J/g) | 5.4 ± 0.8 b | 6.7 ± 0.5 a | 6.8 ± 0.5 a |
| Textural properties | |||
| Hardness (N) | 160.5 ± 2.1 c | 192.7 ± 2.3 b | 238.8 ± 2.4 a |
| Springiness | 0.3 ± 0.0 c | 0.6 ± 0.0 b | 0.8 ± 0.0 a |
| Gumminess (N) | 41.5 ± 0.6 c | 91.3 ± 0.8 b | 105.6 ± 1.1 a |
| Chewiness (N) | 13.5 ± 0.3 c | 54.9 ± 0.5 b | 80.2 ± 0.9 a |
| Resilience | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.0 a |
| In vitro digestion of raw (uncooked) samples C | |||
| RDS (%) | 86.6 ± 0.6 a | 85.4 ± 0.5 a | 77.1 ± 0.4 b |
| SDS (%) | 5.2 ± 0.6 b | 5.9 ± 0.4 b | 7.5 ± 0.8 a |
| RS (%) | 8.2 ± 0.0 b | 8.6 ± 0.9 b | 15.4 ± 0.4 a |
| In vitro digestion of cooked samples C | |||
| RDS (%) | 88.3 ± 0.5 a | 87.7 ± 0.2 a | 81.9 ± 2.0 b |
| SDS (%) | 3.9 ± 0.4 b | 4.1 ± 0.1 a | 4.8 ± 0.0 b |
| RS (%) | 7.9 ± 0.9 b | 7.6 ± 0.2 b | 13.9 ± 1.8 a |
| Variables | Alkali | Wet-Milling | Enzymatic |
|---|---|---|---|
| y1 | −4.98343 | −1.10765 | 6.09108 |
| y2 | −2.01439 | 3.09893 | 1.05454 |
| y | 6.99782 | 1.99128 | 8.9891 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junejo, S.A.; Wang, J.; Liu, Y.; Jia, R.; Zhou, Y.; Li, S. Multi-Scale Structures and Functional Properties of Quinoa Starch Extracted by Alkali, Wet-Milling, and Enzymatic Methods. Foods 2022, 11, 2625. https://doi.org/10.3390/foods11172625
Junejo SA, Wang J, Liu Y, Jia R, Zhou Y, Li S. Multi-Scale Structures and Functional Properties of Quinoa Starch Extracted by Alkali, Wet-Milling, and Enzymatic Methods. Foods. 2022; 11(17):2625. https://doi.org/10.3390/foods11172625
Chicago/Turabian StyleJunejo, Shahid Ahmed, Jun Wang, Ying Liu, Rui Jia, Yibin Zhou, and Songnan Li. 2022. "Multi-Scale Structures and Functional Properties of Quinoa Starch Extracted by Alkali, Wet-Milling, and Enzymatic Methods" Foods 11, no. 17: 2625. https://doi.org/10.3390/foods11172625







