Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
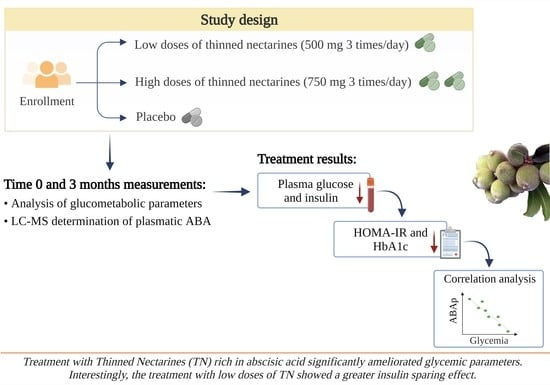
2.1. Study Population and Design
2.2. Assessments
2.3. Quantification of Plasmatic Abscisic Acid by LC-MS/MS Analysis
2.3.1. Chemicals and Reagents
2.3.2. Stock Solution Preparation
2.3.3. Sample Preparation
2.3.4. LC-MS/MS Analysis
2.3.5. Method Validation
2.4. Statistics
3. Results
3.1. Study Sample
3.2. Glucometabolic Parameters
3.3. Correlation Analysis of ABA Plasmatic Levels and Glycemia in Groups Treated with Low and High Doses of Thinned Nectarines
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Doumas, M.; Imprialos, K.; Stavropoulos, K.; Athyros, V.G. Pharmacological Management of Type 2 Diabetes Complications. Curr. Vasc. Pharmacol. 2020, 18, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Krass, I.; Schieback, P.; Dhippayom, T. Adherence to diabetes medication: A systematic review. Diabet. Med. 2015, 32, 725–737. [Google Scholar] [CrossRef]
- Marium, R.; Khan, M.; Jia, Z.; Chua, Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. medicina From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Rasool, S.; Geetha, T.; Babu, J.R. Review Article Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 8165707. [Google Scholar] [CrossRef]
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184. [Google Scholar] [CrossRef]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic acid: A conserved hormone in plants and humans and a promising aid to combat prediabetes and the metabolic syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts; The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. Res. Commun. 2012, 26, 1251–1260. [Google Scholar] [CrossRef]
- Bruzzone, S.; Bodrato, N.; Usai, C.; Guida, L.; Moreschi, I.; Nano, R.; Antonioli, B.; Fruscione, F.; Magnone, M.; Scarfì, S.; et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J. Biol. Chem. 2008, 283, 32188–32197. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Mannino, E.; Scarfì, S.; Bruzzone, S.; Magnone, M.; Sociali, G.; Booz, V.; Guida, L.; Vigliarolo, T.; Fresia, C.; et al. Abscisic acid enhances glucose disposal and induces brown fat activity in adipocytes in vitro and in vivo. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1454. [Google Scholar] [CrossRef]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfi, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y.; Chen, P. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, C.; Yin, X.; Ye, T.; Cai, B.; Song, W.; Feng, Y. Screening and Identi fi cation of Potential Abscisic Acid Catabolites by Chemical Labeling-Assisted Ultrahigh-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2022, 70, 8808–8818. [Google Scholar] [CrossRef]
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and product-related causes for food waste and implications for the food supply chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef]
- Mengyuan, W.; Haoli, W.; Tingting, M.; Qian, G.; Yulin, F.; Xiangyu, S. Comprehensive Utilization of Thinned Unripe Fruits from Horticultural Crops. Foods 2021, 10, 2043. [Google Scholar]
- Ameri, P.; Bruzzone, S.; Mannino, E.; Sociali, G.; Andraghetti, G.; Salis, A.; Ponta, M.L.; Briatore, L.; Adami, G.F.; Ferraiolo, A.; et al. Impaired increase of plasma abscisic acid in response to oral glucose load in type 2 diabetes and in gestational diabetes. PLoS ONE 2015, 10, e0115992. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; D’avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A pilot screening of agro-food waste products as sources of nutraceutical formulations to improve simulated postprandial glycaemia and insulinaemia in healthy subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Gavin, J.R.; Alberti, K.G.M.M.; Davidson, M.B.; DeFronzo, R.A.; Drash, A.; Gabbe, S.G.; Genuth, S.; Harris, M.I.; Kahn, R.; Keen, H.; et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1998, 21, S5–S19. [Google Scholar] [CrossRef]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013, 309, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; Raffaelli, A.; et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002, 25, 1135–1141. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal aba in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Sanchez-Perez, A. Abscisic acid, a promising therapeutic molecule to prevent Alzheimer’s and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 1035–1036. [Google Scholar] [CrossRef]
- Baliño, P.; Gómez-Cadenas, A.; López-Malo, D.; Romero, F.J.; Muriach, M. Is There A Role for Abscisic Acid, A Proven Anti-Inflammatory Agent, in the Treatment of Ischemic Retinopathies? Antioxidants 2019, 8, 104. [Google Scholar] [CrossRef]
- Sakthivel, P.; Sharma, N.; Klahn, P.; Gereke, M.; Bruder, D. Abscisic Acid: A Phytohormone and Mammalian Cytokine as Novel Pharmacon with Potential for Future Development into Clinical Applications. Curr. Med. Chem. 2016, 23, 1549–1570. [Google Scholar] [CrossRef]
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 193–205. [Google Scholar] [CrossRef]
- Leick, L.; Fentz, J.; Biensø, R.S.; Knudsen, J.G.; Jeppesen, J.; Kiens, B.; Wojtaszewski, J.F.P.; Pilegaard, H. PGC-1α is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, 456–465. [Google Scholar] [CrossRef]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of Glucose Transport by AMP-activated Protein Kinase via Activation of p38 Mitogen-activated Protein Kinase. J. Biol. Chem. 2001, 276, 41029–41034. [Google Scholar] [CrossRef]
- Armoni, M.; Kritz, N.; Harel, C.; Bar-Yoseph, F.; Chen, H.; Quon, M.J.; Karnieli, E. Peroxisome proliferator-activated receptor-γ represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J. Biol. Chem. 2003, 278, 30614–30623. [Google Scholar] [CrossRef]
- Magnone, M.; Spinelli, S.; Begani, G.; Guida, L.; Sturla, L.; Emionite, L.; Zocchi, E. Abscisic Acid Improves Insulin Action on Glycemia in Insulin-Deficient Mouse Models of Type 1 Diabetes. Metabolites 2022, 12, 523. [Google Scholar] [PubMed]
- Tenore, G.C.; Carotenuto, A.; Caruso, D.; Buonomo, G.; D’Avino, M.; Brancaccio, D.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Novellino, E. A nutraceutical formulation based on Annurca apple polyphenolic extract is effective on intestinal cholesterol absorption: A randomised, placebo-controlled, crossover study. PharmaNutrition 2018, 6, 85–94. [Google Scholar] [CrossRef]
- Schiano, E.; Annunziata, G.; Ciampaglia, R.; Iannuzzo, F.; Maisto, M.; Tenore, G.C.; Novellino, E. Bioactive Compounds for the Management of Hypertriglyceridemia: Evidence From Clinical Trials and Putative Action Targets. Front. Nutr. 2020, 7, 586178. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.; Margolis, S.; Bachorik, P.; Kwiterovich, P.O. Effect of improved glycemic control on the response of plasma triglycerides to ingestion of a saturated fat load in normotriglyceridemic and hypertriglyceridemic diabetic subjects. Metabolism. 1988, 37, 866–871. [Google Scholar]
- Maisto, M.; Schiano, E.; Novellino, E.; Piccolo, V.; Iannuzzo, F.; Salviati, E.; Summa, V.; Annunziata, G.; Tenore, G.C. Application of a Rapid and Simple Technological Process to Increase Levels and Bioccessibility of Free Phenolic Compounds in Annurca Apple Nutraceutical Product. Foods 2022, 11, 1453. [Google Scholar]
- Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells 2021, 10, 1441. [Google Scholar] [CrossRef]
- Ježek, P.; Hlavatá, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Lorinczova, H.T.; Begum, G.; Temouri, L.; Renshaw, D.; Zariwala, M.G. Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients 2022, 14, 712. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Low Doses of TN | High Doses of TN | Placebo | |||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | Baseline | 12 Weeks | |
| Patients (n) | 20 | 20 | 21 | 21 | 20 | 20 |
| M/F | 11/9 | - | 12/9 | - | 13/7 | - |
| Age (years) | 63 ± 6.5 | - | 64 ± 7.8 | - | 64 ± 5.9 | - |
| Height (m) | 1.69 ± 0.08 | - | 1.66 ± 0.09 | - | 1.68 ± 0.07 | - |
| Weight (kg) | 83.4 ± 7.9 | 83.2 ± 6.8 | 86.1 ± 6.2 | 85.9 ± 4.2 | 85.4 ± 4.5 | 85.3 ± 4.4 |
| BMI (kg/m2) | 29.2 ± 4.1 | 29.1 ± 4.1 | 31.6 ± 4.5 | 31.6 ± 4.5 | 30.5 ± 4.3 | 30.5 ± 4.2 |
| WC (cm) | 99.0 ± 8.5 | 98.9 ± 8.6 | 102.8 ± 6.8 | 102.7 ± 6.9 | 99.5 ± 7.8 | 99.5 ± 7.9 |
| Parameter | Low Doses of TN (n = 20) | High Doses of TN (n = 21) | Placebo (n = 20) | |||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | Baseline | 3 Months | |
| FPG (mg/dL) | 151.4 ± 28.6 | 129.9 ± 12.7 ** | 147.2 ± 36.8 | 137.1 ± 26.9 * | 155.1 ± 31.5 | 154.6 ± 30.6 |
| FPI (µU/mL) | 12.0 ± 9.1 | 8.5 ± 4.0 * | 15.22 ± 7.6 | 12.77 ± 6.6 * | 15.9 ± 6.7 | 15.4 ± 6.9 |
| HbA1c (%) | 8.0 ± 0.9 | 6.9 ± 0.7 *** | 7.8 ± 0.6 | 6.9 ± 0.4 *** | 7.7 ± 0.5 | 7.7 ± 0.3 |
| HOMA-IR | 4.7 ± 4.0 | 2.7 ± 1.2 * | 5.6 ± 3.3 | 4.4 ± 2.6 * | 5.8 ± 3.2 | 5.8 ± 3.2 |
| TC (mg/dL) | 146.4 ± 36.1 | 152.8 ± 30.9 | 164.2 ± 27.0 | 167.5 ± 26.5 | 150.5 ± 28.9 | 151.6 ± 29.8 |
| LDL-C (mg/dL) | 83.4 ± 28.8 | 83.7 ± 26.5 | 109.5 ± 19.8 | 108.8 ± 31 | 84.5 ± 35.9 | 84.6 ± 34.8 |
| HDL-C (mg/dL) | 36.2 ± 8.1 | 39.0 ± 8.3 * | 34.2 ± 8.7 | 39.0 ± 7.9 * | 36.7 ± 7.5 | 36.6 ± 7.2 |
| TG (mg/dL) | 134.3 ± 42.1 | 100.6 ± 22.1 ** | 102.7 ± 27.5 | 98.5 ± 28.5 | 130.5 ± 32.1 | 133.6 ± 33.2 |
| AST (UI/L) | 25.7 ± 8.2 | 21.7 ± 6.2 | 21.5 ± 6.3 | 21.1 ± 5.6 | 24.5 ± 4.6 | 24.3 ± 4.8 |
| ALT (UI/L) | 18.9 ± 6.3 | 20.0 ± 6.3 | 19.3 ± 4.7 | 18.4 ± 3.6 | 19.8 ± 5.6 | 19.9 ± 5.5 |
| Cre (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.1 |
| Fasting Plasma Glucose | Fasting Plasma Insulin | HOMA-IR | ||||
|---|---|---|---|---|---|---|
| HD Treatment Group | Baseline | 3 Months | Baseline | 3 Months | Baseline | 3 Months |
| Mean ± SD | 147.2 ± 36.8 | 137.1 ± 26.9 | 15.2 ± 7.6 | 12.7 ± 6.6 | 5.6 ± 3.3 | 4.4 ± 2.6 |
| % Variation | −6.9% | −16.5% | −22.2% | |||
| p-value | 0.0472 | 0.0345 | 0.0482 | |||
| LD Treatment Group | ||||||
| Mean ± SD | 151.4 ± 28.6 | 129.9 ± 12.7 | 12.0 ± 9.1 | 8.5 ± 4.0 | 4.7 ± 4.0 | 2.5 ± 1.2 |
| % Variation | −14.8% | −29.2% | −41.6% | |||
| p-value | 0.0035 | 0.0404 | 0.0230 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiano, E.; Maisto, M.; Piccolo, V.; Novellino, E.; Annunziata, G.; Ciampaglia, R.; Montesano, C.; Croce, M.; Caruso, G.; Iannuzzo, F.; et al. Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial. Foods 2022, 11, 2637. https://doi.org/10.3390/foods11172637
Schiano E, Maisto M, Piccolo V, Novellino E, Annunziata G, Ciampaglia R, Montesano C, Croce M, Caruso G, Iannuzzo F, et al. Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial. Foods. 2022; 11(17):2637. https://doi.org/10.3390/foods11172637
Chicago/Turabian StyleSchiano, Elisabetta, Maria Maisto, Vincenzo Piccolo, Ettore Novellino, Giuseppe Annunziata, Roberto Ciampaglia, Camilla Montesano, Martina Croce, Giuseppe Caruso, Fortuna Iannuzzo, and et al. 2022. "Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial" Foods 11, no. 17: 2637. https://doi.org/10.3390/foods11172637