Production of Xylooligosaccharides from Jiuzao by Autohydrolysis Coupled with Enzymatic Hydrolysis Using a Thermostable Xylanase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Jiuzao Composition
2.3. Autohydrolysis Processing
2.4. XynAR Production and Enzyme Assay
2.5. Enzymatic Hydrolysis
2.6. Analytical Methods
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Scanning Electron Microscopy (SEM)
2.9. Data Analysis
3. Results and Discussion
3.1. Composition of Jiuzao
3.2. Optimum Conditions for Autohydrolysis
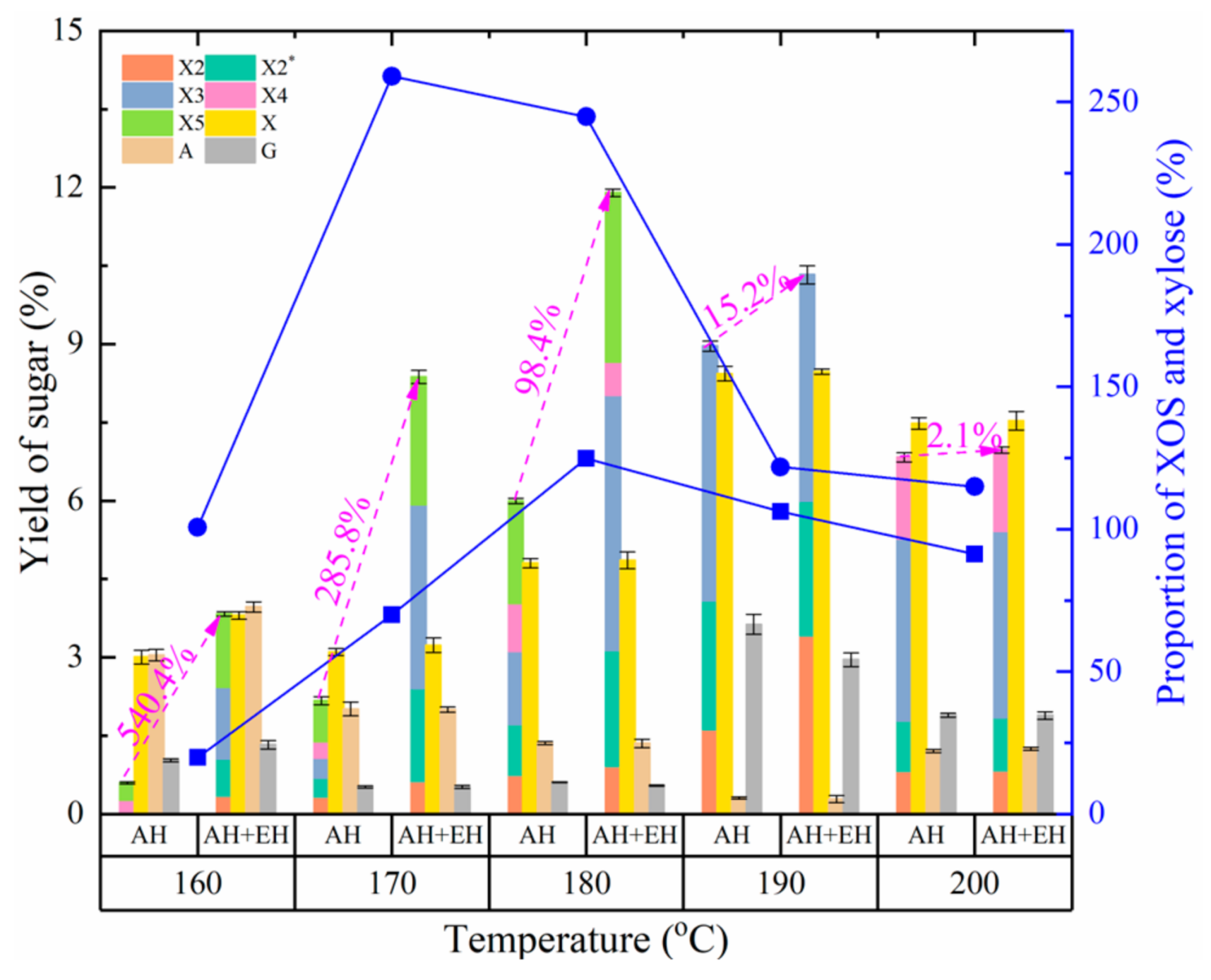
3.2.1. Effect of Autohydrolysis Temperature on the Production of XOS and Monosaccharides
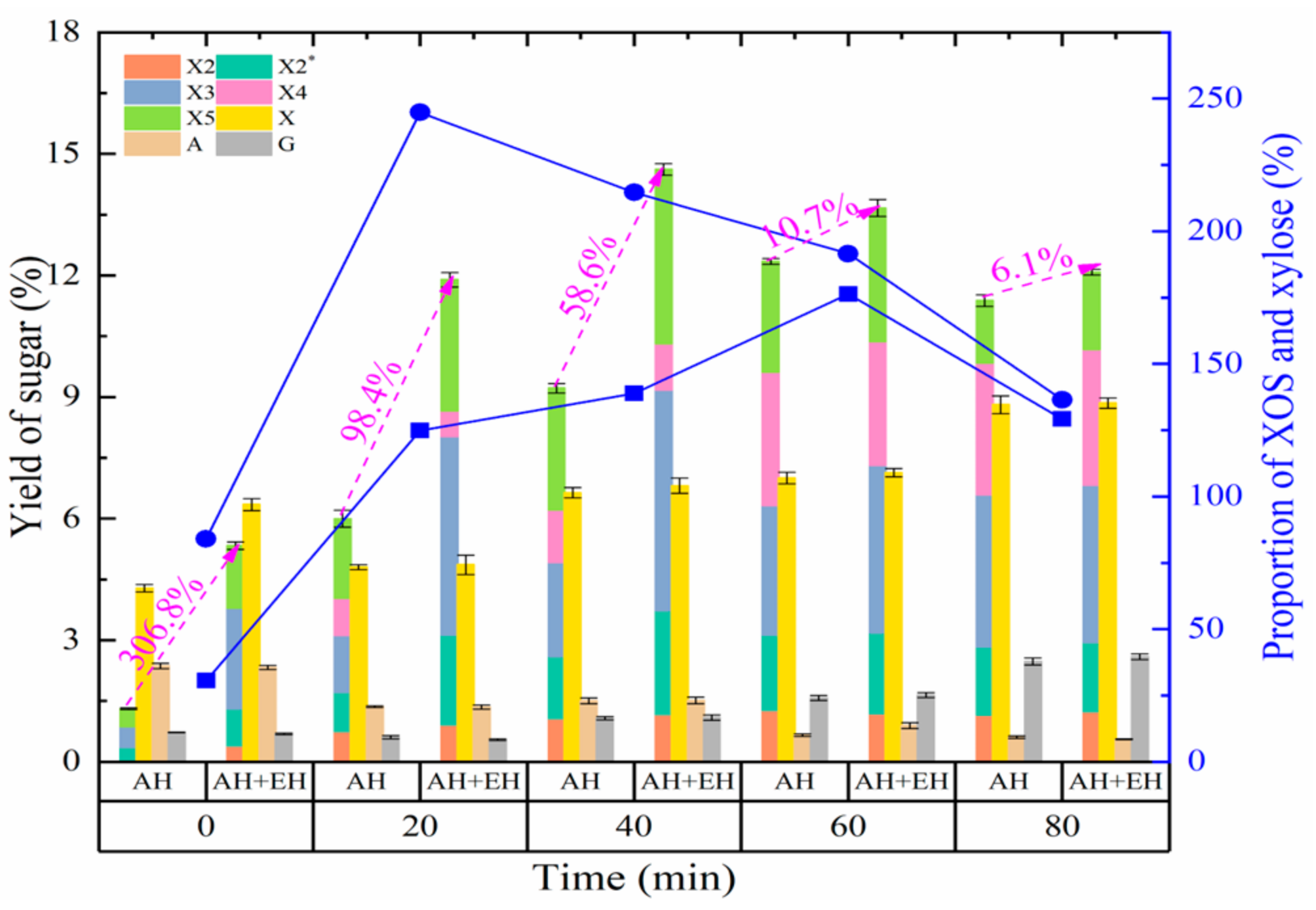
3.2.2. Effect of Autohydrolysis Time on the Production of XOS and Monosaccharides
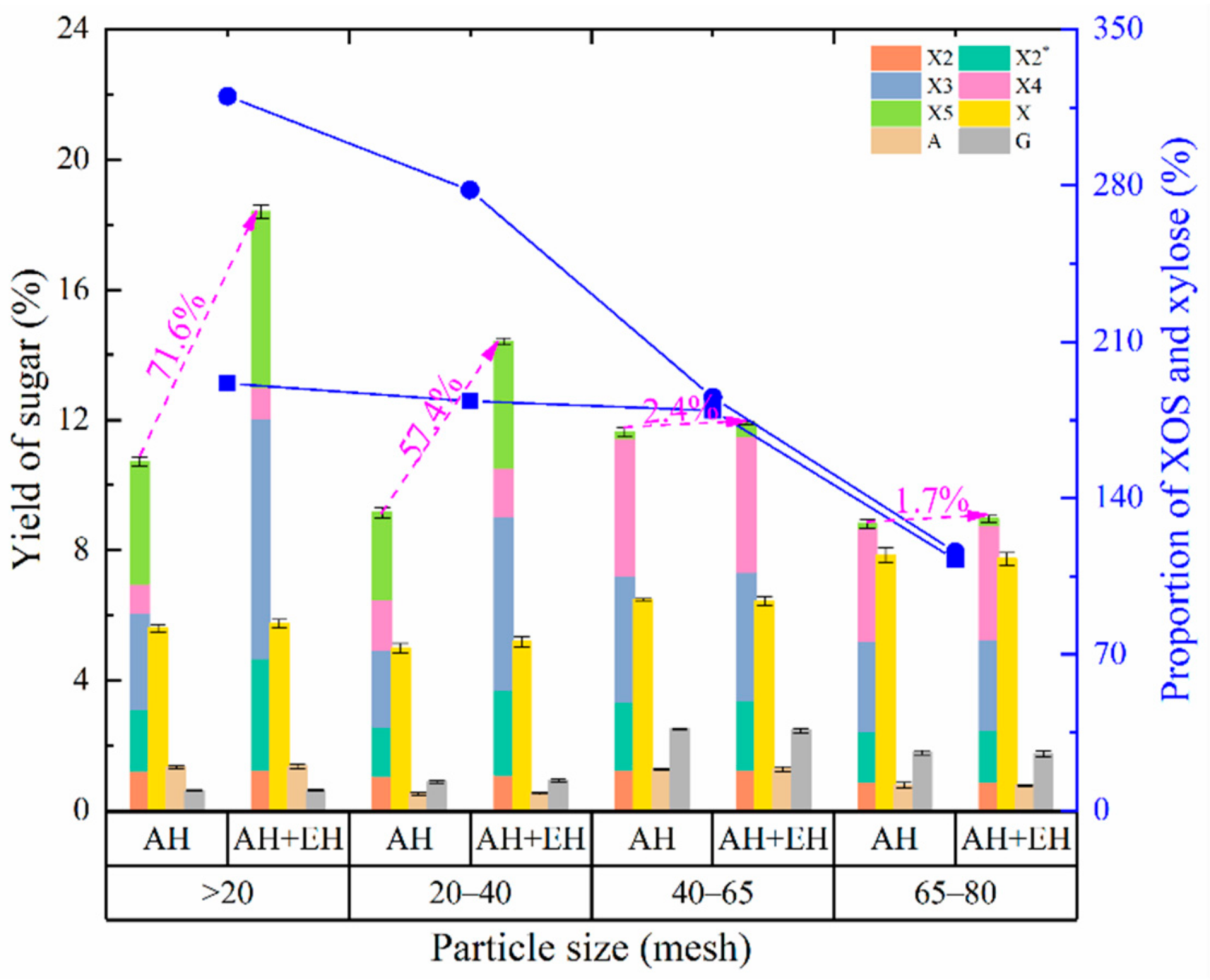
3.2.3. Effect of Particle Size on the Production of XOS and Monosaccharides
3.2.4. Effect of Solid-Liquid Ratio on the Production of XOS and Monosaccharides
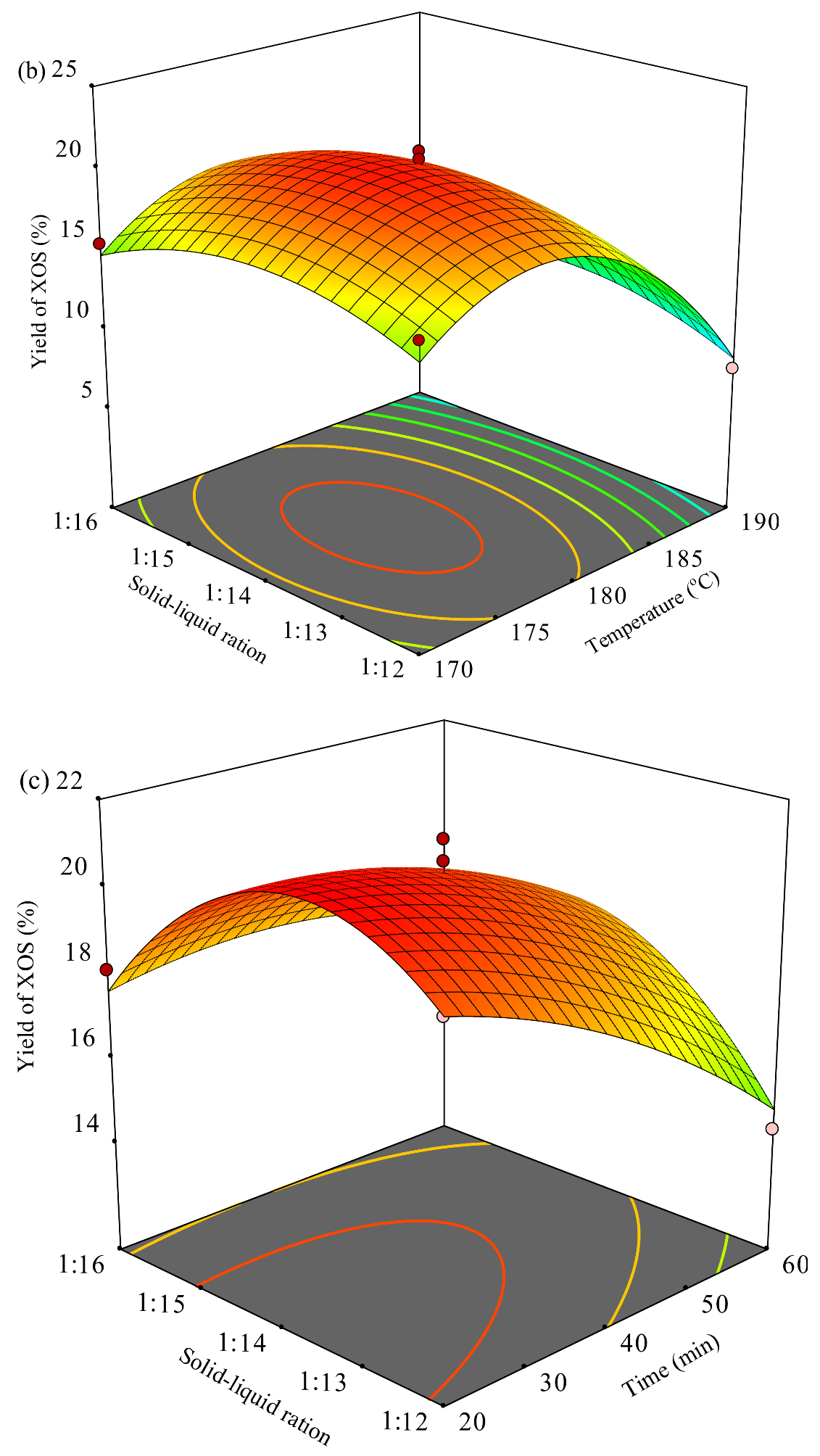
3.2.5. Response Surface Experimental (RSE) Optimized Autohydrolysis Conditions on XOS Yield
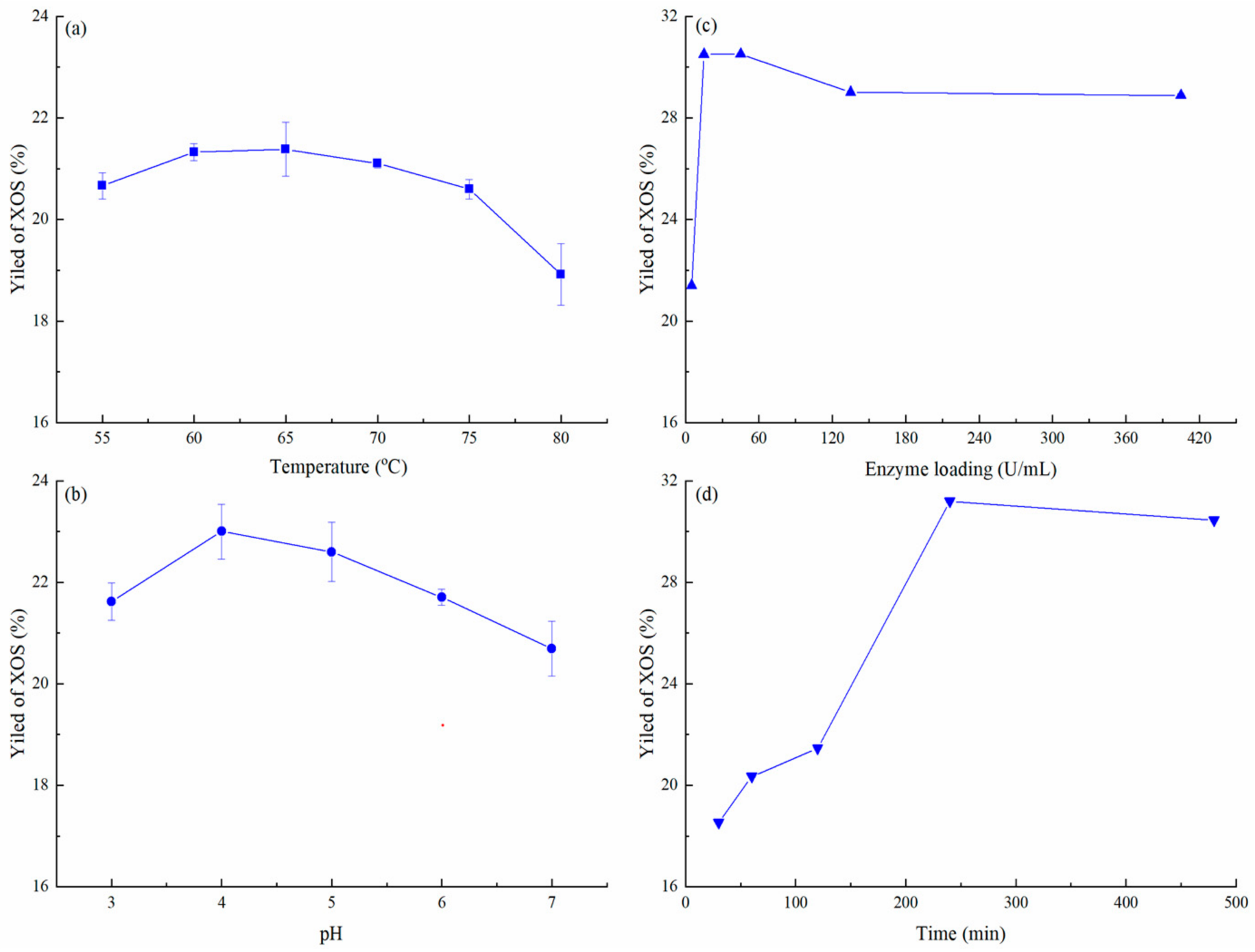
3.3. Optimum Conditions of Enzymatic Hydrolysis
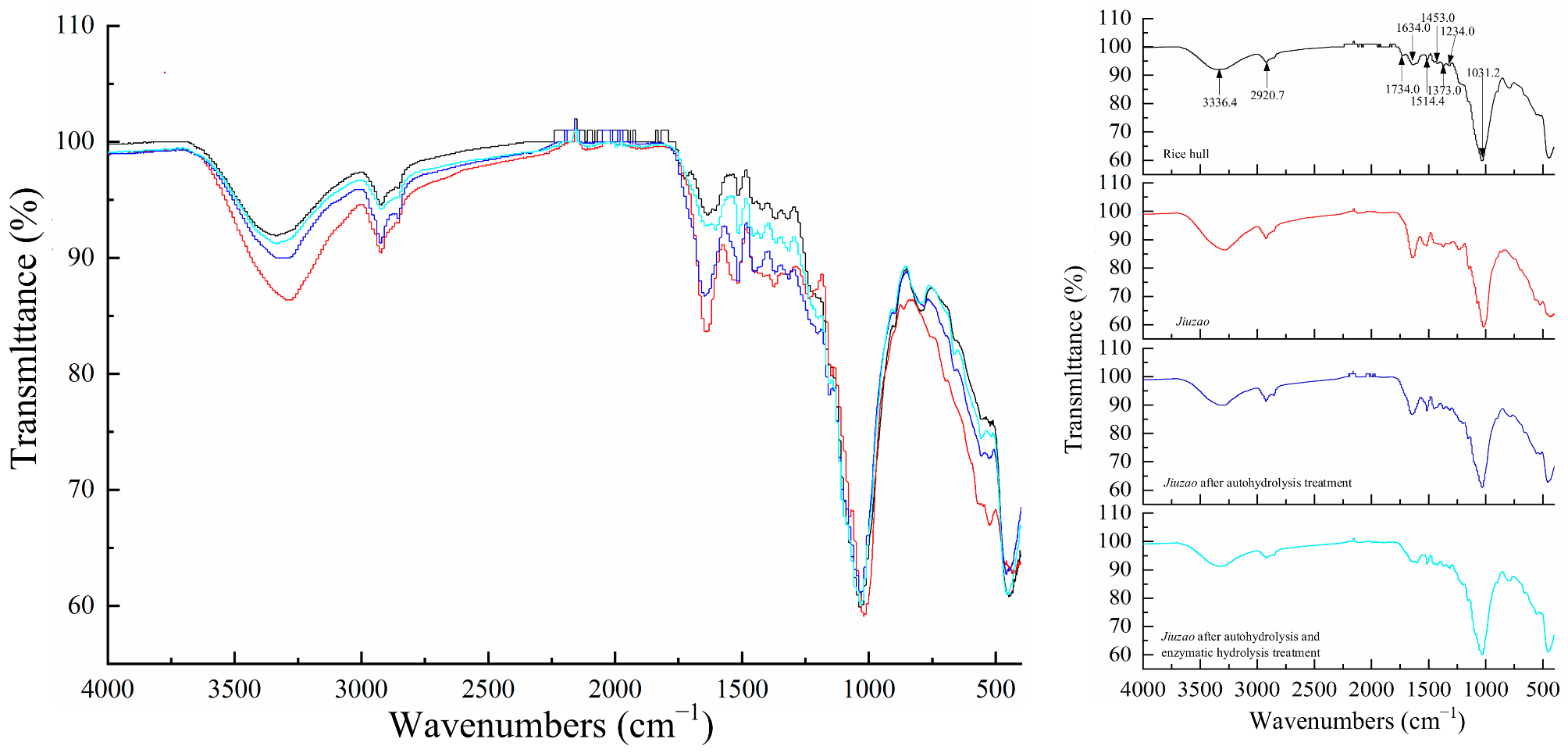
3.4. FTIR Spectroscopy Analysis
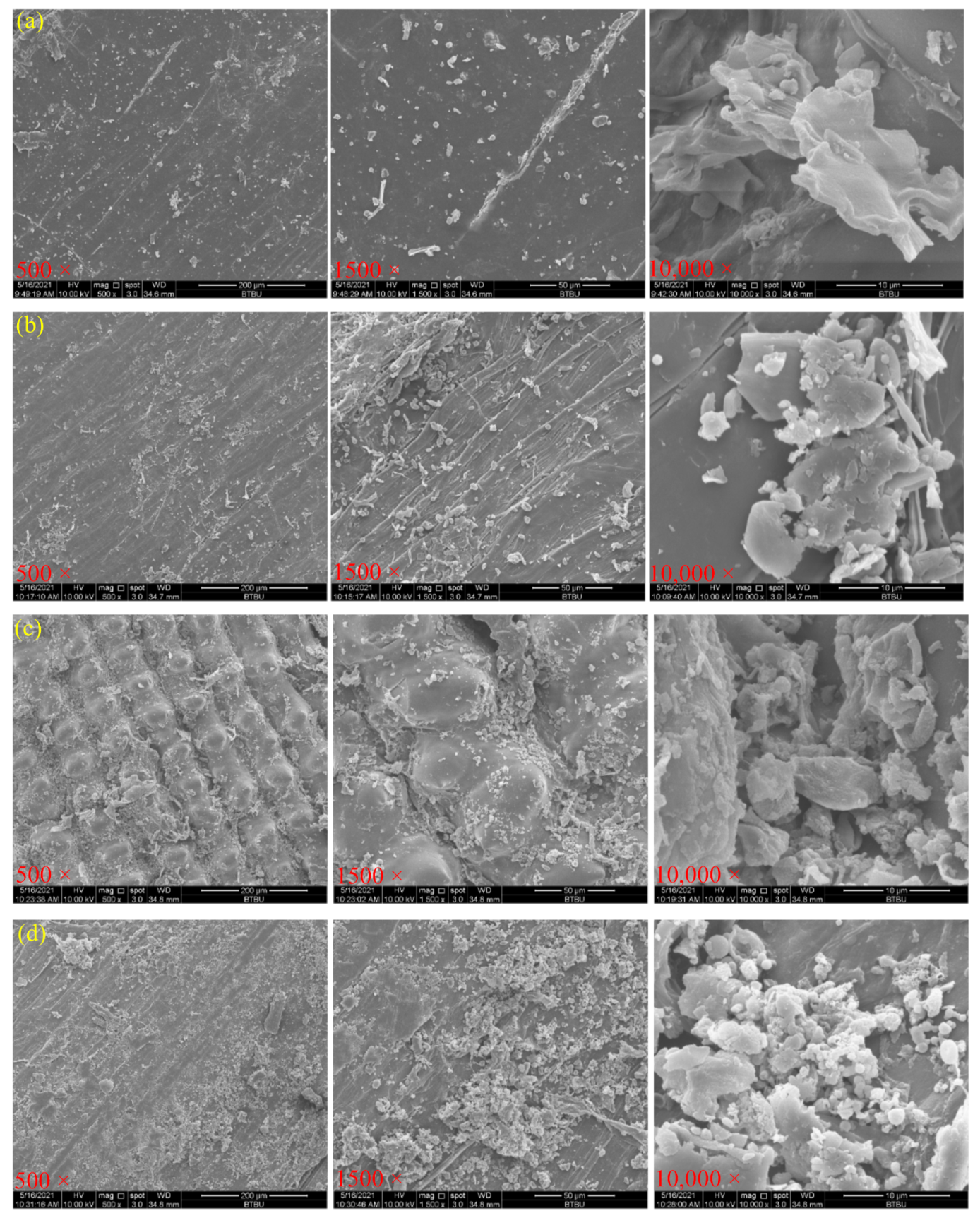
3.5. Morphological Structure of Rice Hull by Different Pretreatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.B.; Wang, G.N.; Zhu, L.; Zheng, F.P.; Ji, J.; Sun, J.Y.; Li, H.H.; Huang, M.Q.; Zhao, Q.Z.; Zhao, M.M.; et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT-Food Sci. Technol. 2021, 146, 111427. [Google Scholar] [CrossRef]
- Ke, Q.M.; Zeng, W.; Shuai, Y.T.; Jin, J. Study on ultrasonic-assisted enzymatic hydrolysis of distiller’s grains cellulose. Sci. Technol. Food Ind. 2021, 43, 196–203. [Google Scholar] [CrossRef]
- Fan, G.S.; Liu, P.X.; Chang, X.; Yin, H.; Cheng, L.J.; Teng, C.; Gong, Y.; Li, X.T. Isolation and identification of a high-yield ethyl caproate-producing yeast from Daqu and optimization of its fermentation. Front. Microbiol. 2021, 12, 663744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, X.B.; Pan, F.; Tuersuntuoheti, T.; Zheng, F.P.; Li, Q.; Hu, S.Q.; Zhao, F.F.; Sun, J.Y.; Sun, B.G. Interaction mechanism of kafirin with ferulic acid and tetramethyl pyrazine: Multiple spectroscopic and molecular modeling studies. Food Chem. 2021, 363, 130298. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Ayyappan, P.; Abirami, A.; Anbuvahini, N.A.; Kumaran, P.S.T.; Naresh, M.; Malathi, D.; Antony, U. Physicochemical properties of cookies enriched with xylooligosaccharides. Food Sci. Technol. Int. 2016, 22, 420–428. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; A Rastall, R.A. Prebiotics in foods. Curr. Opin. Biotechnol. 2012, 23, 187–191. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Qing, Q.; Li, H.J.; Kumar, R.; Wyman, C.E. Xylooligosaccharides production, quantification, and characterization in context of lignocellulosic biomass pretreatment. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Stevens, C.V., Wyman, C.E., Eds.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 391–415. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass- Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Zhang, W.W.; You, Y.Z.; Lei, F.H.; Li, P.F.; Jiang, J.X. Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour. Technol. 2018, 265, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood. J. Chem Technol. Biot. 1999, 74, 1101–1109. [Google Scholar] [CrossRef]
- El Hage, R.; Chrusciel, L.; Desharnais, L.; Brosse, N. Effect of autohydrolysis of Miscanthus × giganteus on lignin structure and organosolv delignification. Bioresour. Technol. 2010, 101, 9321–9329. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Characterization of lignins isolated with alkaline ethanol from the hydrothermal pretreated Tamarix ramosissima. BioEnergy Res. 2013, 6, 519–532. [Google Scholar] [CrossRef]
- Sonkar, R.M.; Gade, P.S.; Bokade, V.; Mudliar, S.N.; Bhatt, P. Ozone assisted autohydrolysis of wheat bran enhances xylooligosaccharide production with low generation of inhibitor compounds: A comparative study. Bioresour. Technol. 2021, 338, 125559. [Google Scholar] [CrossRef]
- Xiao, X.; Bian, J.; Peng, X.P.; Xu, H.; Xiao, B.; Sun, R.C. Autohydrolysis of bamboo (Dendrocalamus giganteus Munro) culm for the production of xylo-oligosaccharides. Bioresour. Technol. 2013, 138, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Muñoz, M.J.G.; Parajó, J.C. Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Martinez-Jimenez, F.; Rezende, C.A.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Subcritical water hydrolysis of sugarcane bagasse: An approach on solid residues characterization. J. Supercrit. Fluids 2016, 108, 69–78. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal treatment and enzymatic hydrolysis of Tamarix ramosissima: Evaluation of the process as a conversion method in a biorefinery concept. Bioresour. Technol. 2013, 135, 73–81. [Google Scholar] [CrossRef]
- Fu, Z.L.; Zhu, Y.T.; Teng, C.; Fan, G.S.; Li, X.T. Biochemical characterization of a novel feruloyl esterase from Burkholderia pyrrocinia B1213 and its application for hydrolyzing wheat bran. 3 Biotech 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A.; Hartley, B.S.; Broda, P.M.A.; Senior, P.J. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Fan, G.S.; Wu, Q.H.; Li, Q.; Sun, B.G.; Ma, Y.L.; Wu, K.L.; Wang, C.Y.; Teng, C.; Yang, R.; Li, X.T. Impact of the disulfide bond on hydrolytic characteristics of a xylanase from Talaromyces thermophiles F1208. Int. J. Biol. Macromol. 2020, 164, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Fan, G.S.; Yu, T.F.; Sun, B.G.; Tang, H.H.; Teng, C.; Yang, R.; Li, X.T. Biochemical characteristics of the mutant xylanase T-XynC(122)C(166) and production of xylooligosaccharides from corncobs. Ind. Crop. Prod. 2019, 142, 111848. [Google Scholar] [CrossRef]
- Wang, D.D.; Wang, L.Y.; Wei, Y.X.; Cui, Q.; Sun, Z.J. Effects of solid-state fermentation of Nongxiang Baijiu on the recalcitrance of rice husk. Liquor-Making Sci. Technol. 2017, 25–29. [Google Scholar] [CrossRef]
- Fan, E.D.; Feng, M.X.; Li, C.Y.; Wu, D.G.; Chen, Y.F.; Xiao, D.G.; Guo, X.W. Study on improving the quality of distiller’s grains feed by steam explosion combined with various microorganisms. J. Agri. Biotechnol. 2021, 1, 194–206. [Google Scholar] [CrossRef]
- Seesuriyachan, P.; Kawee-Ai, A.; Chaiyaso, T. Green and chemical-free process of enzymatic xylooligosaccharide production from corncob: Enhancement of the yields using a strategy of lignocellulosic destructuration by ultra-high pressure pretreatment. Bioresour. Technol. 2017, 241, 537–544. [Google Scholar] [CrossRef]
- Singh, R.D.; Banerjee, J.; Sasmal, S.; Muir, J.; Arora, A. High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerisation. Bioresour. Technol. 2018, 256, 110–117. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Shanmugavelu, K.; Yang, K.L. Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohydr. Polym. 2017, 167, 158–166. [Google Scholar] [CrossRef]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Surek, E.; Buyukkileci, A.O. Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohydr. Polym. 2017, 174, 565–571. [Google Scholar] [CrossRef]
- Chen, M.H.; Bowman, M.J.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Autohydrolysis of Miscanthus × giganteus for the production of xylooligosaccharides (XOS): Kinetics, characterization and recovery. Bioresour. Technol. 2014, 155, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.C.; Garrote, G.; Cruz, J.M.; Dominguez, H. Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends Food Sci. Technol. 2004, 15, 115–120. [Google Scholar] [CrossRef]
- Akpinar, O.; Gunay, K.; Yilmaz, Y.; Levent, O.; Bostanci, S. Enzymatic processing and antioxidant activity of agricultural waste autohydrolysis liquors. Bioresources 2010, 5, 699–711. [Google Scholar]
- Surek, E.; Buyukkileci, A.O.; Yegin, S. Processing of hazelnut (Corylus avellana L.) shell autohydrolysis liquor for production of low molecular weight xylooligosaccharides by Aureobasidium pullulans NRRL Y–2311–1 xylanase. Ind. Crop. Prod. 2021, 161, 113212. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Negro, M.J.; Ballesteros, I.; Oliva, J.M.; Sáez, F. Optimized use of hemicellulose within a biorefinery for processing high value-added xylooligosaccharides. Ind. Crop. Prod. 2017, 99, 41–48. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, Y.K.; Zheng, S.H.; Mo, Y.E.; Sun, S.L.; Ren, J.L.; Li, Y.H.; Wu, A.M.; Li, H.L. Upgrade the torrefaction process of bamboo based on autohydrolysis pretreatment. Ind. Crop. Prod. 2021, 166, 113470. [Google Scholar] [CrossRef]
- Jiang, Y.S.; Xing, M.Z.; Kang, Q.; Sun, J.Y.; Zeng, X.A.; Gao, W.H.; Li, H.H.; Gao, Y.H.; Li, A.J. Pulse electric field assisted process for extraction of Jiuzao glutelin extract and its physicochemical properties and biological activities investigation. Food Chem. 2022, 383, 132304. [Google Scholar] [CrossRef]
- Gütsch, J.S.; Nousiainen, T.; Sixta, H. Comparative evaluation of autohydrolysis and acid-catalyzed hydrolysis of Eucalyptus globulus wood. Bioresour. Technol. 2012, 109, 77–85. [Google Scholar] [CrossRef]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.S.; Sun, J.Y.; Yin, Z.T.; Li, H.H.; Sun, X.T.; Zheng, F.P. Evaluation of antioxidant peptides generated from Jiuzao (residue after Baijiu distillation) protein hydrolysates and their effect of enhancing healthy value of Chinese Baijiu. J. Sci. Food Agric. 2020, 100, 59–73. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhu, X.; Jin, L.Q.; Zheng, Y.; Liao, C.J.; Zheng, Y.G. Post-hydrolysis of the prehydrolysate from eucalyptus pulping with xylanase. J. Clean. Prod. 2017, 142, 2865–2871. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Enhancing the potential of oligosaccharides from corncob autohydrolysis as prebiotic food ingredients. Ind. Crop. Prod. 2006, 24, 152–159. [Google Scholar] [CrossRef]
- Dias, L.M.; Neto, F.S.P.P.; Brienzo, M.; de Oliveira, S.C.; Masarin, F. Experimental design, modeling, and optimization of production of xylooligosaccharides by hydrothermal pretreatment of sugarcane bagasse and straw. Biomass-Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Lian, Z.N.; Wang, Y.N.; Luo, J.; Lai, C.H.; Yong, Q.; Yu, S.Y. An integrated process to produce prebiotic xylooligosaccharides by autohydrolysis, nanofiltration and endo-xylanase from alkali-extracted xylan. Bioresour. Technol. 2020, 314, 123685. [Google Scholar] [CrossRef]
- Menezes, B.D.S.; Rossi, D.M.; Squina, F.; Ayub, M.A.Z. Xylooligosaccharides production by fungi cultivations in rice husk and their application as substrate for lactic acid bacteria growth. Bioresour. Technol. Rep. 2018, 2, 100–106. [Google Scholar] [CrossRef]
- da Silva Menezes, B.; Rossi, D.M.; Ayub, M.A.Z. Screening of filamentous fungi to produce xylanase and xylooligosaccharides in submerged and solid-state cultivations on rice husk, soybean hull, and spent malt as substrates. World J. Microbiol. Biotechnol. 2017, 33, 58. [Google Scholar] [CrossRef]
- Liu, M.Q.; Huo, W.K.; Xu, X.; Weng, X.Y. Recombinant Bacillus amyloliquefaciens xylanase A expressed in Pichia pastoris and generation of xylooligosaccharides from xylans and wheat bran. Int. J. Biol. Macromol. 2017, 105, 656–663. [Google Scholar] [CrossRef]
- Faryar, R.; Linares-Pastén, J.A.; Immerzeel, P.; Mamo, G.; Andersson, M.; Stålbrand, H.; Mattiasson, B.; Karlsson, E.N. Production of prebiotic xylooligosaccharides from alkaline extracted wheat straw using the K80R-variant of a thermostable alkali-tolerant xylanase. Food Bioprod. Process. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Falck, P.; Aronsson, A.; Grey, C.; Stålbrand, H.; Karlsson, E.N.; Adlercreutz, P. Production of arabinoxylan-oligosaccharide mixtures of varying composition from rye bran by a combination of process conditions and type of xylanase. Bioresour. Technol. 2014, 174, 118–125. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, Y.; Jiang, Z.Q.; Liu, H.J.; Yang, S.Q.; Yan, Q.J. Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem. 2018, 264, 310–318. [Google Scholar] [CrossRef]
- Purohit, A.; Rai, S.K.; Chownk, M.; Sangwan, R.S.; Yadav, S.K. Xylanase from Acinetobacter pittii MASK 25 and developed magnetic cross-linked xylanase aggregate produce predominantly xylopentose and xylohexose from agro biomass. Bioresour. Technol. 2017, 244, 793–799. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Production of prebiotic xylooligosaccharide from aqueous ammonia-pretreated rice straw by β-xylosidase of Weissella cibaria. J. Appl. Microbiol. 2019, 126, 1861–1868. [Google Scholar] [CrossRef]
- Goldbeck, R.; Gonçalves, T.A.; Damasio, A.R.L.; Brenelli, L.B.; Wolf, L.D.; Paixão, D.A.A.; Rocha, G.J.M.; Squina, F.M. Effect of hemicellulolytic enzymes to improve sugarcane bagasse saccharification and xylooligosaccharides production. J. Mol. Catal. B Enzym. 2016, 131, 36–46. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhang, X.; Xu, Y. An eco-friendly biorefinery strategy for xylooligosaccharides production from sugarcane bagasse using cellulosic derived gluconic acid as efficient catalyst. Bioresour. Technol. 2019, 289, 121755. [Google Scholar] [CrossRef]
- Reddy, S.S.; Krishnan, C. Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT—Food Sci. Technol. 2016, 65, 237–245. [Google Scholar] [CrossRef]
- Bragatto, J.; Segato, F.; Squina, F.M. Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind. Crop. Prod. 2013, 51, 123–129. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Martínez-Abad, A.; Tan, H.T.; Bulone, V.; Vilaplana, F. Sequential fractionation of feruloylated hemicelluloses and oligosaccharides from wheat bran using subcritical water and xylanolytic enzymes. Green Chem. 2017, 19, 1919–1931. [Google Scholar] [CrossRef]
- Mathew, S.; Aronsson, A.; Karlsson, E.N.; Adlercreutz, P. Xylo- and arabinoxylooligosaccharides from wheat bran by endoxylanases, utilisation by probiotic bacteria, and structural studies of the enzymes. Appl. Microbiol. Biotechnol. 2018, 102, 3105–3120. [Google Scholar] [CrossRef]
- Liu, X.Q.; Yang, S.Q.; Ma, J.W.; Yu, J.; Yan, Q.J.; Jiang, Z.Q. Efficient production of acetylated xylooligosaccharides from Hawthorn kernels by a xylanase from Paecilomyces aerugineus. Ind. Crop. Prod. 2020, 158, 112962. [Google Scholar] [CrossRef]
- Huang, C.X.; Wang, X.C.; Liang, C.; Jiang, X.; Yang, G.; Xu, J.; Yong, Q. A sustainable process for procuring biologically active fractions of high-purity xylooligosaccharides and water-soluble lignin from Moso bamboo prehydrolyzate. Biotechnol. Biofuels 2019, 12, 189. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef]
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50. [Google Scholar] [CrossRef]
- Akpinar, O.; Erdogan, K.; Bakir, U.; Yilmaz, L. Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT—Food Sci. Technol. 2010, 43, 119–125. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Kolte, A.P.; Senani, S.; Sridhar, M.; Suresh, K.P.; Sampath, K.T. Enzymatic production of xylooligosaccharides from alkali solubilized xylan of natural grass (Sehima nervosum). Bioresour. Technol. 2012, 112, 199–205. [Google Scholar] [CrossRef]
- Hao, X.X.; Wen, P.Y.; Wang, J.; Wang, J.Y.; You, J.X.; Zhang, J.H. Production of xylooligosaccharides and monosaccharides from hydrogen peroxide-acetic acid-pretreated poplar by two-step enzymatic hydrolysis. Bioresour. Technol. 2019, 297, 122349. [Google Scholar] [CrossRef]
- Salas-Veizaga, D.M.; Villagomez, R.; Linares-Pastén, J.A.; Carrasco, C.; Álvarez, M.T.; Adlercreutz, P.; Karlsson, E.N. Extraction of glucuronoarabinoxylan from quinoa stalks (Chenopodium quinoa Willd.) and evaluation of xylooligosaccharides produced by GH10 and GH11 xylanases. J. Agric. Food Chem. 2017, 65, 8663–8673. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liao, A.M.; Thakur, K.; Huang, J.H.; Zhang, J.G.; Wei, Z.J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M. Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization. Carbohydr. Res. 2011, 346, 76–85. [Google Scholar] [CrossRef]
- Gil-López, D.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments. Ind. Crop. Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef]


| Test Number | Temperature (°C) | Time (Min) | Solid-Liquid Ratio | Yield of XOS (%) | |||
|---|---|---|---|---|---|---|---|
| A | Code A | B | Code B | C | Code C | Y | |
| 1 | 190 | 1 | 40 | 0 | 1:16 | 1 | 7.8 |
| 2 | 180 | 0 | 20 | −1 | 1:16 | 1 | 18.1 |
| 3 | 180 | 0 | 60 | 1 | 1:12 | −1 | 14.3 |
| 4 | 190 | 1 | 40 | 0 | 1:12 | −1 | 7.5 |
| 5 | 180 | 0 | 40 | 0 | 1:14 | 0 | 19.3 |
| 6 | 180 | 0 | 40 | 0 | 1:14 | 0 | 21.1 |
| 7 | 180 | 0 | 40 | 0 | 1:14 | 0 | 20.6 |
| 8 | 190 | 1 | 20 | −1 | 1:14 | 0 | 17.6 |
| 9 | 180 | 0 | 20 | −1 | 1:12 | −1 | 19.7 |
| 10 | 180 | 0 | 60 | 1 | 1:16 | 1 | 17.1 |
| 11 | 190 | 1 | 60 | 1 | 1:14 | 0 | 5.0 |
| 12 | 170 | −1 | 20 | −1 | 1:14 | 0 | 11.8 |
| 13 | 170 | −1 | 40 | 0 | 1:16 | 1 | 15.4 |
| 14 | 170 | −1 | 40 | 0 | 1:12 | −1 | 16.5 |
| 15 | 170 | −1 | 60 | 1 | 1:14 | 0 | 19.9 |
| Source | Coefficient Estimate | Sum of Squares | DF | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|---|
| Model | 362.47 | 9 | 40.27 | 20.74 | 0.0019 | ** | |
| Intercept | 20.33 | ||||||
| A-Temperature | −3.21 | 82.56 | 1 | 82.56 | 42.52 | 0.0013 | ** |
| B-Time | −1.36 | 14.85 | 1 | 14.85 | 7.65 | 0.0396 | * |
| C-Solid-liquid ratio | 0.0500 | 0.02 | 1 | 0.02 | 0.01 | 0.9231 | |
| AB | −5.17 | 107.12 | 1 | 107.12 | 55.17 | 0.0007 | ** |
| AC | 0.3500 | 0.49 | 1 | 0.49 | 0.25 | 0.6368 | |
| BC | 1.10 | 4.84 | 1 | 4.84 | 2.49 | 0.1752 | |
| A² | −6.13 | 138.71 | 1 | 138.71 | 71.43 | 0.0004 | ** |
| B² | −0.6292 | 1.46 | 1 | 1.46 | 0.75 | 0.4253 | |
| C² | −2.40 | 21.34 | 1 | 21.34 | 10.99 | 0.0211 | * |
| Residual | 9.71 | 5 | 1.94 | ||||
| Lack of Fit | 7.98 | 3 | 2.66 | 3.08 | 0.2545 | not significant | |
| Pure Error | 1.73 | 2 | 0.86 | ||||
| Cor Total | 372.18 | 14 | |||||
| R2 = 0.9739 | R2Adj = 0.9270 | CV = 9.02% |
| Test Number | Factors | Yield of XOS (%) | |||
|---|---|---|---|---|---|
| A (Temperature, °C) | B (pH) | C (Enzyme Loading, U/mL) | D (Time, Min) | ||
| 1 | 1 (60 °C) | 1 (3) | 1 (5 U/mL) | 1 (120 min) | 28.6 |
| 2 | 2 (65 °C) | 1 | 2 (15 U/mL) | 2 (240 min) | 29.0 |
| 3 | 3 (70 °C) | 1 | 3 (25 U/mL) | 3 (360 min) | 28.0 |
| 4 | 1 | 2 (4) | 2 | 3 | 31.3 |
| 5 | 2 | 2 | 3 | 1 | 31.5 |
| 6 | 3 | 2 | 1 | 2 | 27.9 |
| 7 | 1 | 3 (5) | 3 | 2 | 33.0 |
| 8 | 2 | 3 | 1 | 3 | 30.4 |
| 9 | 3 | 3 | 2 | 1 | 32.3 |
| Average K1 | 31.0 | 28.5 | 29.0 | 30.8 | |
| Average K2 | 30.3 | 30.2 | 30.9 | 30.0 | |
| Average K3 | 29.4 | 31.9 | 30.9 | 29.9 | |
| Range (R) | 1.6 | 3.4 | 1.9 | 0.9 | |
| Optimal level | 1 | 3 | 2 | 1 | |
| Type of Biomass | Hemicellulose (%) | Yield of XOS (%) | References |
|---|---|---|---|
| Rice husk | 11.2 | 69 a | [48] |
| 33.8 b | [48] | ||
| 12.9 | [49] | ||
| Wheat straw | 40 | 40.3 | [50] |
| 20.1 | 21 | [51] | |
| Rye bran | 21 | 60 c | [52] |
| 40 d | [52] | ||
| Corncob | 31.2 | 75 | [53] |
| 22 | 57.6 | [54] | |
| 33.4 | 10.7 | [29] | |
| Rice straw | 24.14 | 54.3 | [55] |
| 17 | 50.5 | [54] | |
| Mahogany sawdust | 24.3 | 36.8 | [31] |
| Mango sawdust | 26.5 | 25.5 | [31] |
| Sugarcane bagasse | 25.7 | 55.4 | [56] |
| 26.5 | 53.2 | [57] | |
| 20.75 | 51.1 | [58] | |
| 32.7 | 36.4 | [59] | |
| Wheat bran | 30 | 55.9 | [60] |
| 17.6 | 22.8 e | [61] | |
| 19 f | [61] | ||
| 18.6 g | [61] | ||
| Hawthorn kernels | 28 | 66.8 | [62] |
| Moso bamboo | 17.3 | 42.7 | [63] |
| Almond shell | 20.2 | 40.6 | [64] |
| Arecanut husk | 24.6 | 35.1 | [30] |
| Jiuzao | 13.08 | 34.2 | This study |
| Brewers’ spent grain | 16.5 | 31.5 | [65] |
| Pineapple peel waste | 31.8 | 25.6 | [66] |
| Tobacco stalk | 22 | 11.4 | [67] |
| Natural grass | 28 | 11 | [68] |
| Poplar | 15.8 | 10.7 | [69] |
| Quinoa stalks | 10.9 | 1.26 | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Ma, J.; Tian, H.; Ma, Y.; Wu, Q.; Cheng, S.; Fan, G. Production of Xylooligosaccharides from Jiuzao by Autohydrolysis Coupled with Enzymatic Hydrolysis Using a Thermostable Xylanase. Foods 2022, 11, 2663. https://doi.org/10.3390/foods11172663
Qin L, Ma J, Tian H, Ma Y, Wu Q, Cheng S, Fan G. Production of Xylooligosaccharides from Jiuzao by Autohydrolysis Coupled with Enzymatic Hydrolysis Using a Thermostable Xylanase. Foods. 2022; 11(17):2663. https://doi.org/10.3390/foods11172663
Chicago/Turabian StyleQin, Liqin, Jinghao Ma, Huafeng Tian, Yanli Ma, Qiuhua Wu, Shuang Cheng, and Guangsen Fan. 2022. "Production of Xylooligosaccharides from Jiuzao by Autohydrolysis Coupled with Enzymatic Hydrolysis Using a Thermostable Xylanase" Foods 11, no. 17: 2663. https://doi.org/10.3390/foods11172663






