A Bioactive Chitosan−Based Film Enriched with Benzyl Isothiocyanate/α−Cyclodextrin Inclusion Complex and Its Application for Beef Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
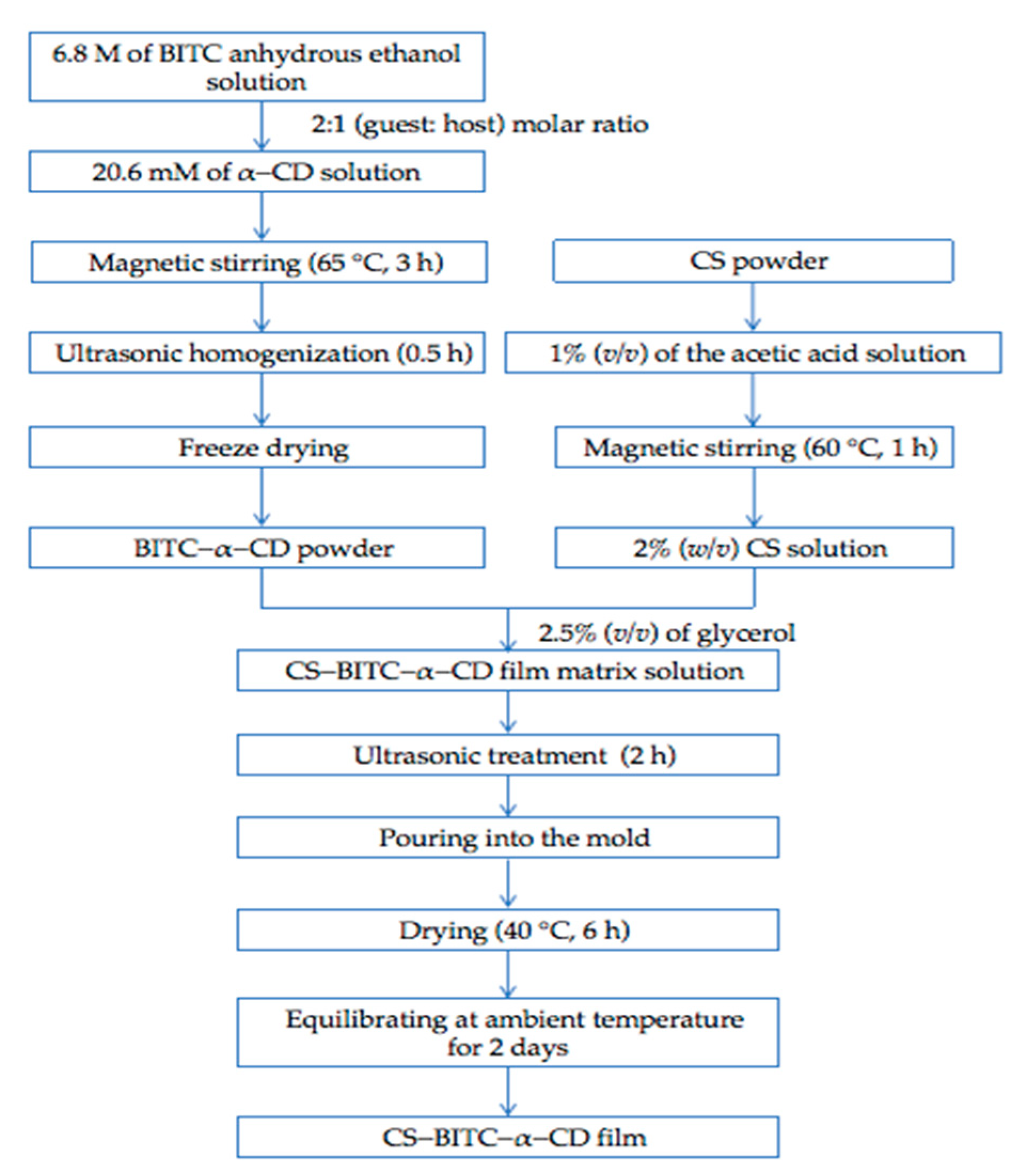
2.2. Fabrication of Inclusion Complex
2.3. Fabrication of the CS−Based Composite Film
2.4. Characterization of Inclusion Complex
2.4.1. FTIR Analysis
2.4.2. XRD Analysis
2.5. Characterization of the CS−Based Composite Film
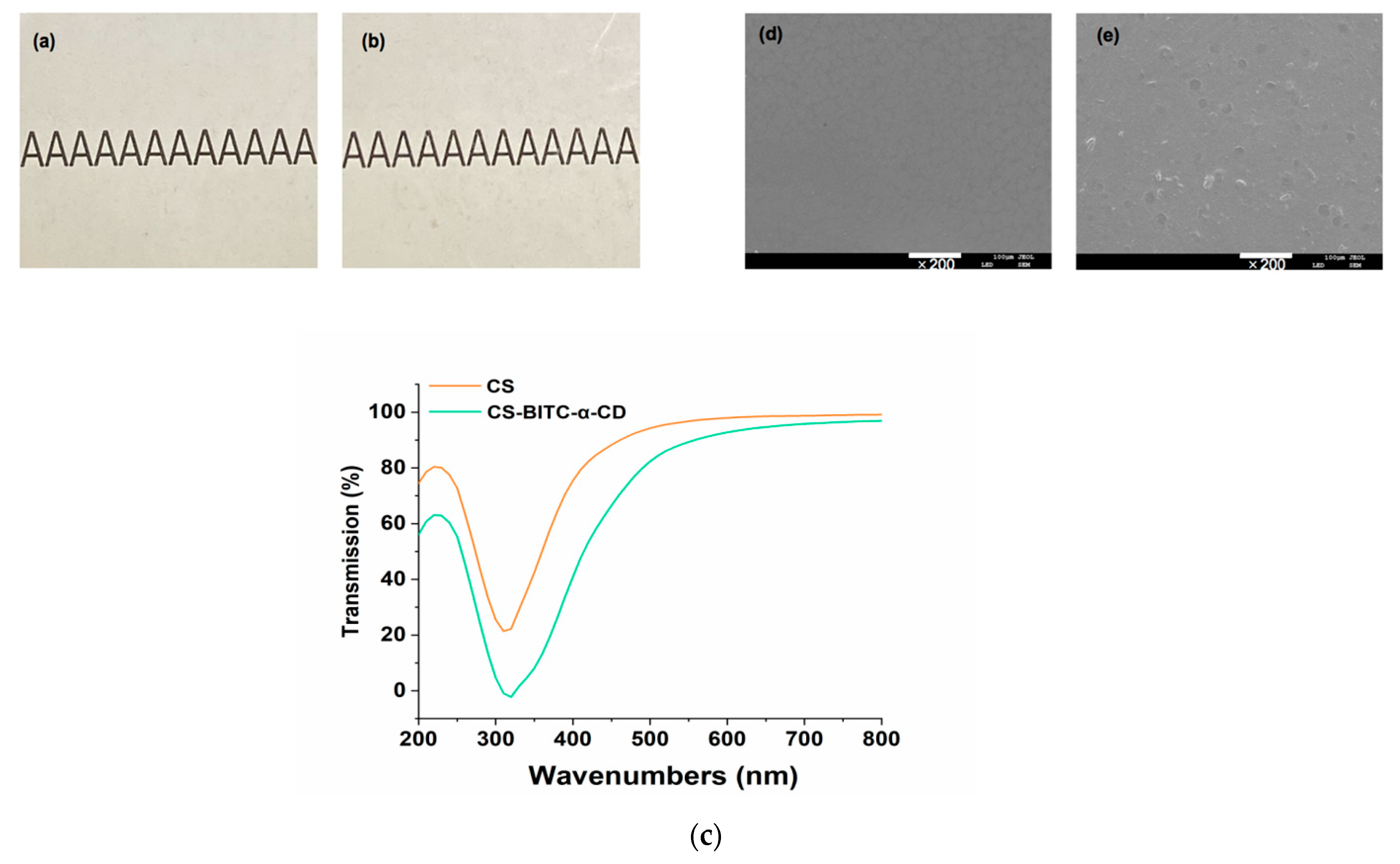
2.6. Appearance, Light Transmittance and Surface Morphology of the CS−Based Composite Film
2.6.1. Appearance
2.6.2. Light Transmittance
2.6.3. Surface Morphology
2.7. Moisture Content (MC), Water Solubility (WS), and Water Vapor Permeability (WVP) of the CS−Based Composite Film
2.8. Antibacterial Activity of the CS−Based Film
2.9. Antioxidant Activity of the CS−Based Film
2.10. Preservative Effects of the CS−Based Composite Film on Fresh Beef
2.10.1. Preparation of Beef Treated by the CS−Based Composite Film
2.10.2. Beef Quality Analysis
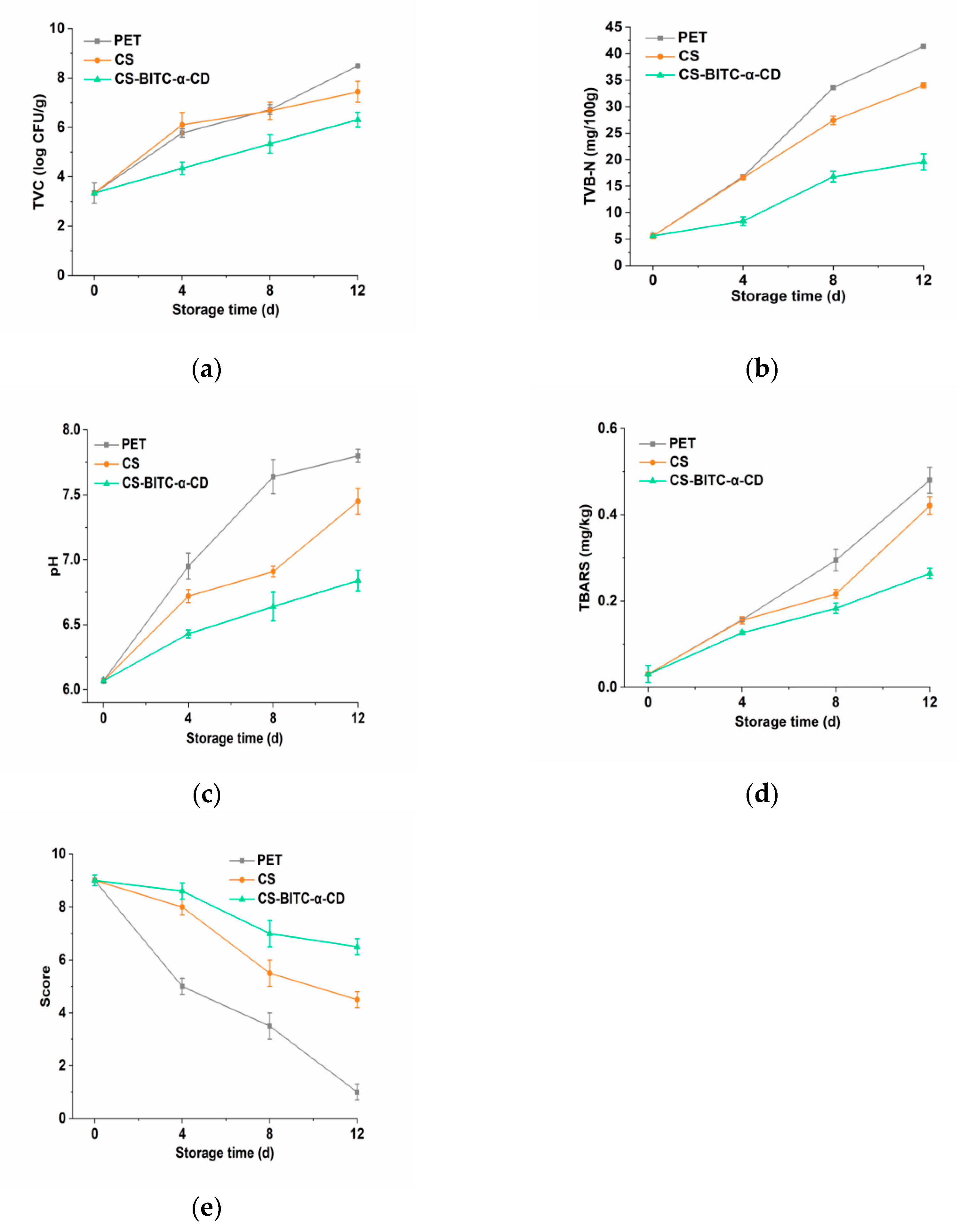
Total Viable Counts (TVC) Determination
Total Volatile Base Nitrogen (TVB−N) Determination
pH Determination
Thiobarbituric Acid−Reactive Substances (TBARS) Determination
Sensory Evaluation
3. Results and Discussion
3.1. Characterization of CS−Based Composite Film
3.2. Appearance, Light Transmittance and Surface Morphology of the CS−Based Composite Film
3.3. MC, WS, and WVP of the CS−Based Composite Film
3.4. Antibacterial and Antioxidant Properties of the CS−Based Composite Film
3.5. Preservative Efficacy of the CS−Based Composite Film on the Beef
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.J.; Kumari, S.; Selamat, J.; Shameli, K.; Sazili, A.Q. Reducing meat perishability through pullulan active packaging. J. Food Qual. 2020, 2020, 8880977. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Sarfraz, J.; Hansen, A.Å.; Haugen, J.E.; Le, T.A.; Nilsen, J.; Skaret, J.; Huynh, T.P.; Pettersen, M.K. Biodegradable active packaging as an alternative to conventional packaging: A case study with chicken fillets. Foods 2021, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liao, B.; Tang, R.Y. Functional application of sulfur-containing spice compounds. J. Agric. Food Chem. 2020, 68, 12505–12526. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Pałasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Zhang, W.; Lu, R.; Bian, J.S.; Nie, X. Therapeutic potential of sulfur-containing natural products in inflammatory diseases. Pharmacol. Ther. 2020, 216, 107687. [Google Scholar] [CrossRef]
- Niu, T.X.; Wang, X.N.; Wu, H.Y.; Bi, J.R.; Hao, H.S.; Hou, H.M.; Zhang, G.L. Transcriptomic analysis, motility and biofilm formation characteristics of Salmonella typhimurium exposed to benzyl isothiocyanate treatment. Int. J. Mol. Sci. 2020, 21, 1025. [Google Scholar] [CrossRef]
- Wu, H.Y.; Niu, T.X.; Bi, J.R.; Hou, H.M.; Hao, H.S.; Zhang, G.L. Exploration of the antimicrobial activity of benzyl isothiocyanate against Salmonella enterica serovar Typhimurium. J. Food Meas. Charact. 2022, 16, 1768–1775. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, V.S.; Trifan, A.; Miron, A. Antigenotoxic potential of some dietary non-phenolic phytochemicals. Stud. Nat. Prod. Chem. 2019, 60, 223–297. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S.K. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Alizadeh, N.; Nazari, F. Thymol essential oil/β-cyclodextrin inclusion complex into chitosan nanoparticles: Improvement of thymol properties in vitro studies. J. Mol. Liq. 2022, 346, 118250. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Zaripour, M.; Zare-Shahabadi, V.; Jahromi, H.J. Application of ultrasonic-assisted inclusion complex formation with α–cyclodextrin for simultaneous spectrophotometric determination of gallic acid and vanillic acids in fruit samples. Spectrochim. Acta A Mol. Biomol. 2019, 222, 117197. [Google Scholar] [CrossRef]
- Giacoppo, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Moringa isothiocyanate complexed with α-cyclodextrin: A new perspective in neuroblastoma treatment. BMC Complement Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Romeo, L.; Lanza Cariccio, V.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-cyclodextrin/moringin complex: A new promising antimicrobial agent against Staphylococcus aureus. Molecules 2018, 23, 2097. [Google Scholar] [CrossRef]
- Lila, Z.A.; Mohammed, N.; Kanda, S.; Kamada, T.; Itabashi, H. Effect of α-cyclodextrin-allyl isothiocyanate on ruminal microbial methane production in vitro. Anim. Sci. J. 2003, 74, 321–326. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.Y.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation-A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Yi, G.; Yin, C.; Lao, Y.; Shi, Z.; He, X.; Wu, J.; Jiang, Y.; Gong, L. Antibacterial and antitumor activities of chitosan/polyvinyl alcohol films containing microemulsion of papaya seed essential oil. Mater. Today Commun. 2022, 31, 103475. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Fang, Y.; Holley, R.A. Inhibition of Campylobacter jejuni on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized oriental mustard extract. Int. J. Food Microbiol. 2014, 187, 77–82. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.O.; Jin, T.; Fan, X.; Olanya, M.; Juneja, V. Inactivation of Salmonella in grape tomato stem scars by organic acid wash and chitosan-allyl isothiocyanate coating. Int. J. Food Microbiol. 2018, 266, 234–240. [Google Scholar] [CrossRef]
- Chen, H.; Shu, J.; Li, P.; Chen, B.; Li, N.; Li, L. Application of coating chitosan film-forming solution combined β-CD-citral inclusion complex on beef fillet. J. Food Nutr. Res. 2014, 2, 692–697. [Google Scholar] [CrossRef]
- Adjali, A.; Pontillo, A.R.N.; Kavetsou, E.; Katopodi, A.; Tzani, A.; Grigorakis, S.; Loupassaki, S.; Detsi, A. Clove essential oil–hydroxypropyl-β-cyclodextrin inclusion complexes: Preparation, characterization and incorporation in biodegradable chitosan films. Micro 2022, 2, 212–224. [Google Scholar] [CrossRef]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.; Goff, H.D.; Zhong, F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, Q.; Yu, D.; Dong, J.; Xu, Y.; Xia, W. Physical, antioxidant, and preservation properties of chitosan film doped with proanthocyanidins-loaded nanoparticles. Food Hydrocoll. 2022, 130, 107686. [Google Scholar] [CrossRef]
- Song, X.C.; Canellas, E.; Wrona, M.; Becerril, R.; Nerin, C. Comparison of two antioxidant packaging based on rosemary oleoresin and green tea extract coated on polyethylene terephthalate for extending the shelf life of minced pork meat. Food Packag. 2020, 26, 100588. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Chen, X.; Liu, Y.; Wang, J.; Wang, X.; Wang, C.; Song, H. Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 2020, 162, 838–844. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Meenatchi, V.; Lin, L. Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: Solubility, characterization, DPPH and antibacterial assay. Ultrason. Sonochem. 2020, 64, 104997. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Toghan, A.; Zaki, M.E.; Elamary, R.B. Design, synthesis and evaluation of novel antimicrobial polymers based on the inclusion of polyethylene glycol/tio2 nanocomposites in cyclodextrin as drug carriers for sulfaguanidine. Polymers 2022, 14, 227. [Google Scholar] [CrossRef]
- Durante, M.; Milano, F.; De Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato oil encapsulation by α-, β-, and γ-Cyclodextrins: A comparative study on the formation of supramolecular structures, antioxidant activity, and carotenoid stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Ao, X.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Characterization of the inclusion complexes of isothiocyanates with γ-cyclodextrin for improvement of antibacterial activities against Staphylococcus aureus. Foods 2021, 11, 60. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Kaur, K.; Kumar, R.; Kahlon, N.K.; Singh, R.; Mehta, S.K. Encompassment of Benzyl Isothiocyanate in cyclodextrin using ultrasonication methodology to enhance its stability for biological applications. Ultrason. Sonochem. 2017, 39, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, G. Investigation of non-covalent complex formation between 2-(4-hydroxyphenylazo) benzoic acid and α-cyclodextrin in solid and solution forms. J. Mol. Liq. 2021, 335, 116278. [Google Scholar] [CrossRef]
- Baggetto, L.; Charvillat, C.; Thébault, Y.; Esvan, J.; Lafont, M.C.; Scheid, E.; Veith, G.M.; Vahlas, C. Amorphous alumina thin films deposited on titanium: Interfacial chemistry and thermal oxidation barrier properties. Phys. Status Solidi 2016, 213, 470–480. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Sollogoub, C.; Gaucher, V.; Delpouve, N.; Marais, S. Structure and barrier properties of multinanolayered biodegradable PLA/PBSA films: Confinement effect via forced assembly coextrusion. ACS Appl. Mater. 2017, 9, 29101–29112. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Zhang, G.L.; Hao, H.; Hou, H.M.; Bi, J. Preparation of chitosan-cellulose-benzyl isothiocyanate nanocomposite film for food packaging applications. Carbohydr. Polym. 2022, 285, 119234. [Google Scholar] [CrossRef]
- Jiang, L.; Han, Y.; Meng, X.; Xiao, Y.; Zhang, H. Cellulose nanocrystals reinforced Zein/Catechin/β-cyclodextrin inclusion complex nanoparticles nanocomposite film for active food packaging. Polymers 2021, 13, 2759. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.H.; Chiu, F.C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef]
- Bai, M.Y.; Zhou, Q.; Zhang, J.; Li, T.; Cheng, J.; Liu, Q.; Xu, W.R.; Zhang, Y.C. Antioxidant and antibacterial properties of essential oils-loaded β-cyclodextrin-epichlorohydrin oligomer and chitosan composite films. Colloids Surf. 2022, 215, 112504. [Google Scholar] [CrossRef]
- Shin, J.; Kathuria, A.; Lee, Y.S. Effect of hydrophilic and hydrophobic cyclodextrins on the release of encapsulated allyl isothiocyanate (AITC) and their potential application for plastic film extrusion. J. Appl. Polym. Sci. 2019, 136, 48137. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Dixit, S. Influence of boswellic acid on multifunctional properties of chitosan/poly (vinyl alcohol) films for active food packaging. Int. J. Biol. Macromol. 2020, 154, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Liu, Y. Structural, physicochemical, and functional (antioxidant-antimicrobial) properties of 2-O-methyl-β-cyclodextrin inclusion with hexahydro-β-acids in chitosan films. Colloids Surf. B. 2020, 191, 111002. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liang, X.; Zhou, Y.; Bao, K.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Liu, Y. Preparation of polylactic acid/TiO2/GO nano-fibrous films and their preservation effect on green peppers. Int. J. Biol. Macromol. 2021, 177, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable poly (butylene adipate-co-terephthalate) and thermoplastic starch-blended Tio2 nanocomposite blown films as functional active packaging of fresh fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. 2022, 31, 100787. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant activity and physicochemical properties of chitosan films incorporated with Lycium barbarum fruit extract for active food packaging. IJFST 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Lu, Y.; Wu, T.; Pang, J.; Hu, Y. In situ self-assembly chitosan/ε-polylysine bionanocomposite film with enhanced antimicrobial properties for food packaging. Int. J. Biol. Macromol. 2019, 132, 385–392. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella typhimurium during refrigerated storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer-Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Kumar, B.; Madras, G.; Bose, S. Chitosan immobilized porous polyolefin as sustainable and efficient antibacterial membranes. ACS Sustain. Chem. Eng. 2016, 4, 862–870. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tong, J.; Zhou, J. Physicochemical properties of chitosan films incorporated with honeysuckle flower extract for active food packaging. J. Food Process Eng. 2017, 40, e12305. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef]
- Soni, B.; Mahmoud, B.; Chang, S.; El-Giar, E.M. Physicochemical, antimicrobial and antioxidant properties of chitosan/TEMPO biocomposite packaging films. Food Packag. 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Valgimigli, L.; Iori, R. Antioxidant and pro-oxidant capacities of ITCs. Environ. Mol. Mutagen. 2009, 50, 222–237. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Li, Y.; Luo, C.; Yang, C.; Shi, W.; Li, L. Covalent immobilization of polypeptides on polylactic acid films and their application to fresh beef preservation. J. Agric. Food Chem. 2020, 68, 10532–10541. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Yang, J.Y.; Lu, H.B.; Wang, S.S.; Yang, J.; Yang, X.C.; Chai, M.; Li, L.; Cao, J.X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Jia, R.; Ge, S.; Ren, S.; Luo, Y.; Xiu, L.; Liu, H.; Cai, D. Antibacterial mechanism of adzuki bean seed coat polyphenols and their potential application in preservation of fresh raw beef. IJFST 2021, 56, 5025–5039. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Afshar Mehrabi, F.; Sharifi, A.; Ahvazi, M. Effect of chitosan coating containing Nepeta pogonosperma extract on shelf life of chicken fillets during chilled storage. Food Sci. Nutr. 2021, 9, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Quek, S.Y.; Gu, M.; Guo, Y.; Liu, Y. Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage. Food Control. 2020, 108, 106870. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Yuan, L.; Feng, W.; Zhang, Z.; Peng, Y.; Xiao, Y.; Chen, J. Effect of potato starch-based antibacterial composite films with thyme oil microemulsion or microcapsule on shelf life of chilled meat. LWT 2021, 139, 110462. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, D.; Sun, Z.; Liu, F.; Zhang, D.; Wang, D. Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydrocoll. 2022, 127, 107546. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadeh, S.; Khaneghah, A.M. Packaging of beef fillet with active chitosan film incorporated with ɛ-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and characterization of fish myofibrillar protein/chitosan/rosemary extract composite edible films and the improvement of lipid oxidation stability during the grass carp fillets storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Effect of chitosan/starch aldehyde-catechin conjugate composite coating on the quality and shelf life of fresh pork loins. J. Sci. Food Agric. 2022, 102, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]

| Samples | MC (%) | WS (%) | WVP ×10−12 (g/m·s·Pa) |
|---|---|---|---|
| CS | 47.7 ± 6.3 a | 41.4 ± 6.3 a | 4.2 ± 0.1 a |
| CS−BITC−α−CD | 44.7 ± 1.5 a | 48.3 ± 6.4 a | 3.9 ± 0.1 a |
| Samples | S. Typhimurium Growth Inhibition (%) | DPPH Scavenging (%) | |
|---|---|---|---|
| 12 h | 24 h | ||
| CS | 4.2 ± 0.3 b | 1.3 ± 0.1 b | 11.4 ± 0.4 b |
| CS−BITC−α−CD | 31.0 ± 0.4 a | 53.3 ± 0.1 a | 19.8 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. A Bioactive Chitosan−Based Film Enriched with Benzyl Isothiocyanate/α−Cyclodextrin Inclusion Complex and Its Application for Beef Preservation. Foods 2022, 11, 2687. https://doi.org/10.3390/foods11172687
Wu H, Ao X, Liu J, Zhu J, Bi J, Hou H, Hao H, Zhang G. A Bioactive Chitosan−Based Film Enriched with Benzyl Isothiocyanate/α−Cyclodextrin Inclusion Complex and Its Application for Beef Preservation. Foods. 2022; 11(17):2687. https://doi.org/10.3390/foods11172687
Chicago/Turabian StyleWu, Hongyan, Xinying Ao, Jianan Liu, Junya Zhu, Jingran Bi, Hongman Hou, Hongshun Hao, and Gongliang Zhang. 2022. "A Bioactive Chitosan−Based Film Enriched with Benzyl Isothiocyanate/α−Cyclodextrin Inclusion Complex and Its Application for Beef Preservation" Foods 11, no. 17: 2687. https://doi.org/10.3390/foods11172687





