Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AAP
2.2. Animal Experiment Design
2.3. Collection of Blood and Faeces Samples
2.4. Fecal Bacteria 16S rRNA Sequencing Analysis
2.5. Extraction and Detection of Serum Metabolites
3. Results
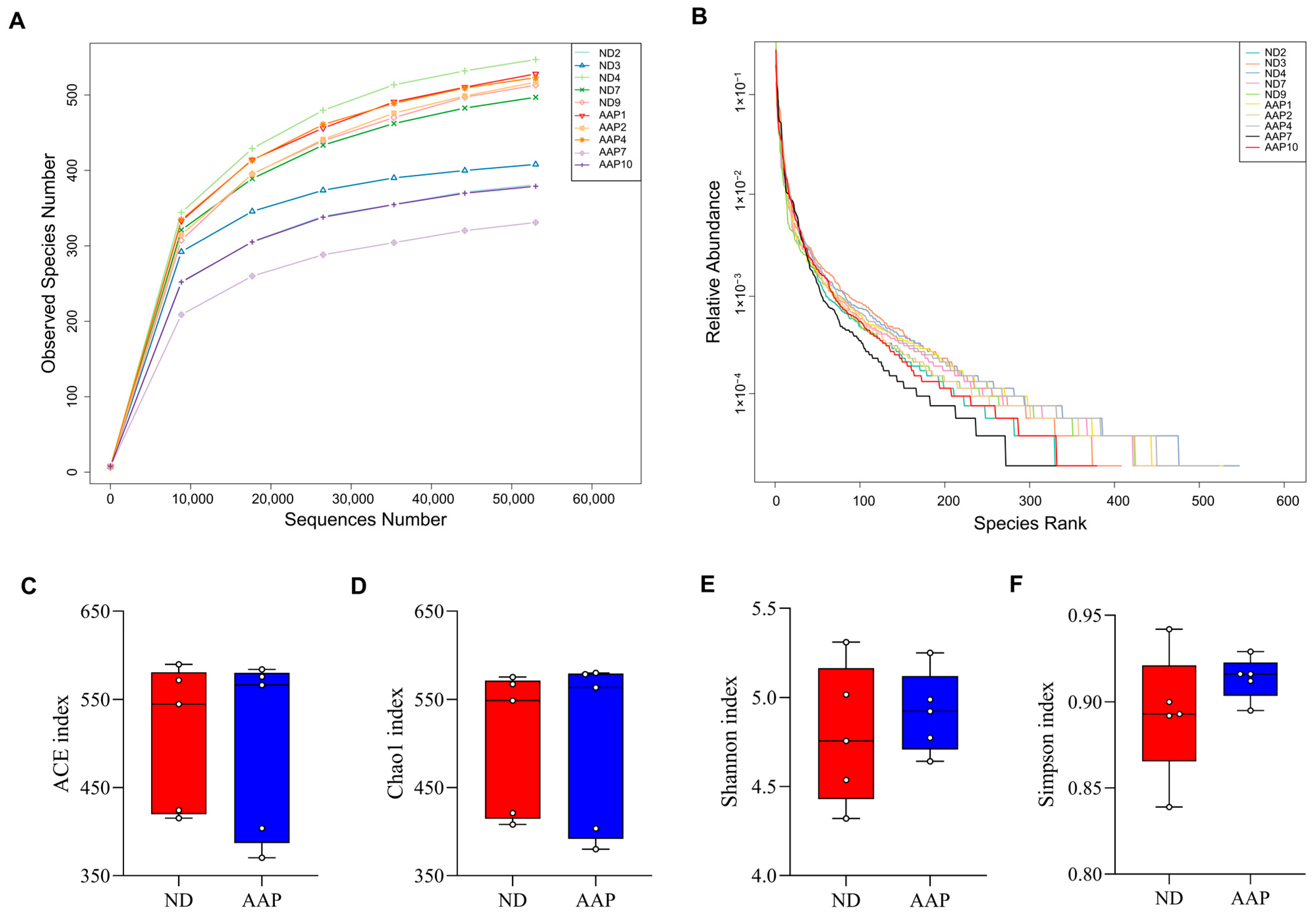
3.1. The Effects of AAP on α Diversity of Intestinal Flora in Mice
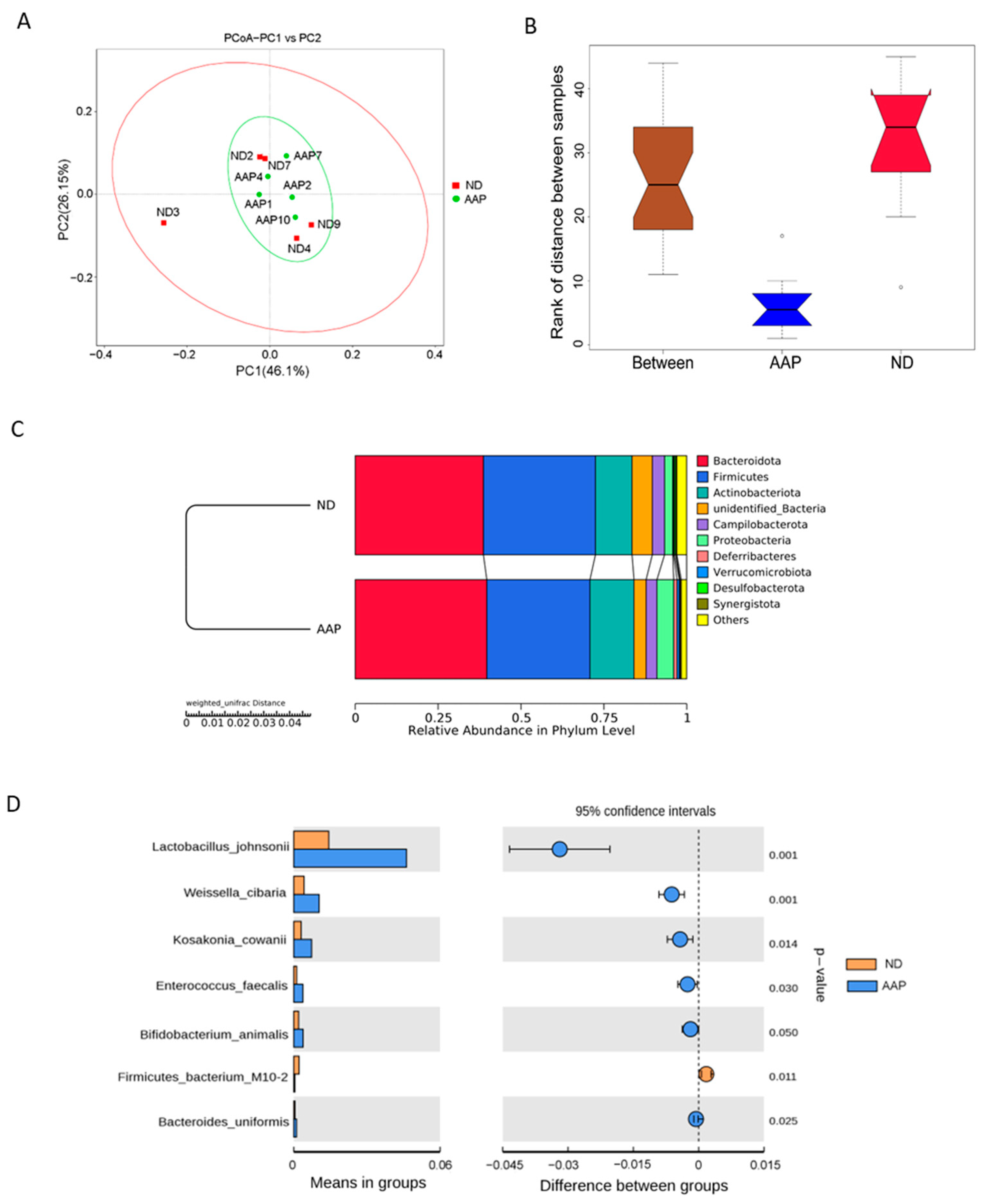
3.2. The Effects of AAP on β Diversity of Intestinal Flora in Mice
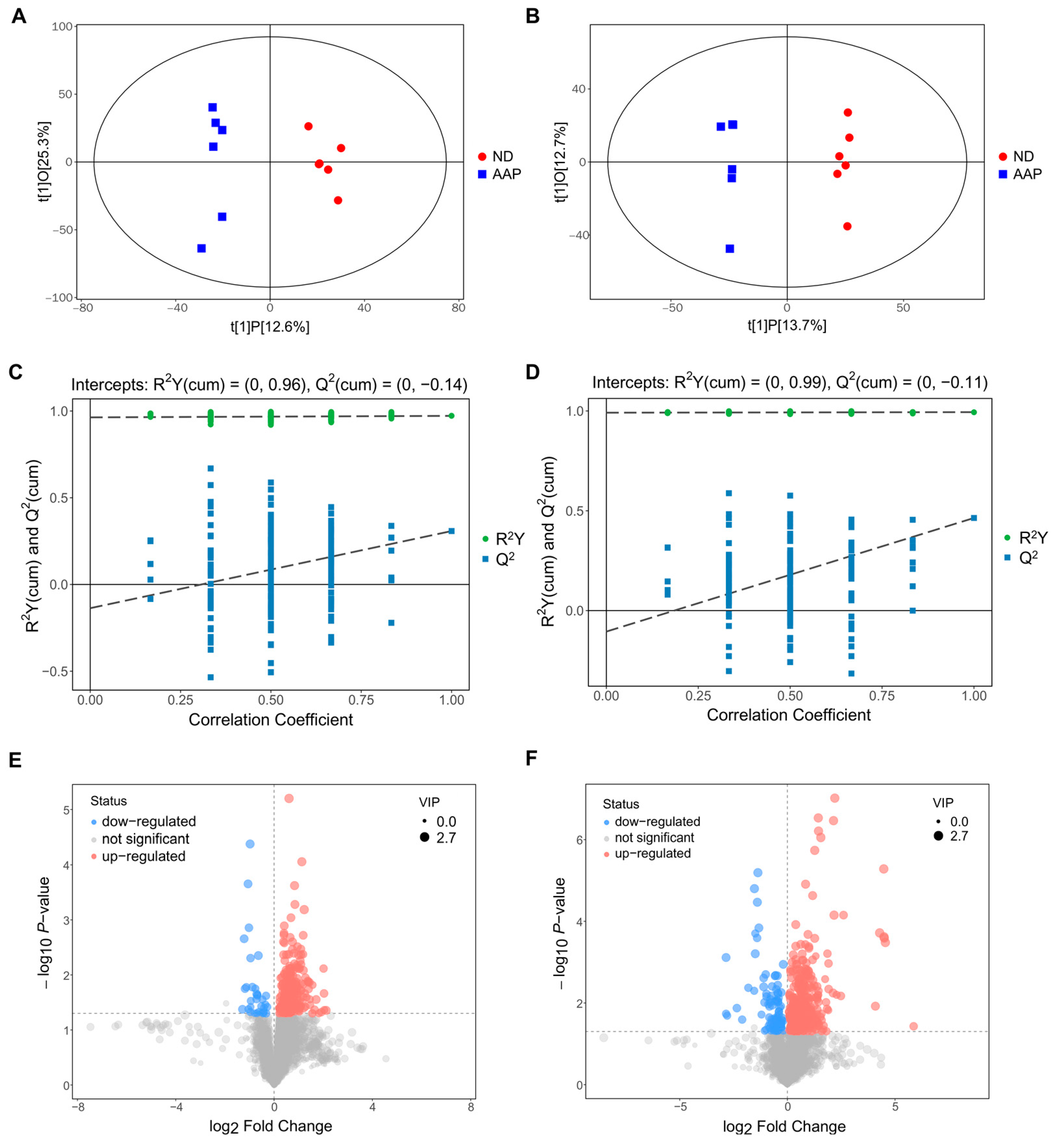
3.3. Screening of Mouse Serum Biomarkers after AAP Consumption
3.4. Changes in Serum Biomarkers in Mice after Auricularia auricula Polysaccharides Intake
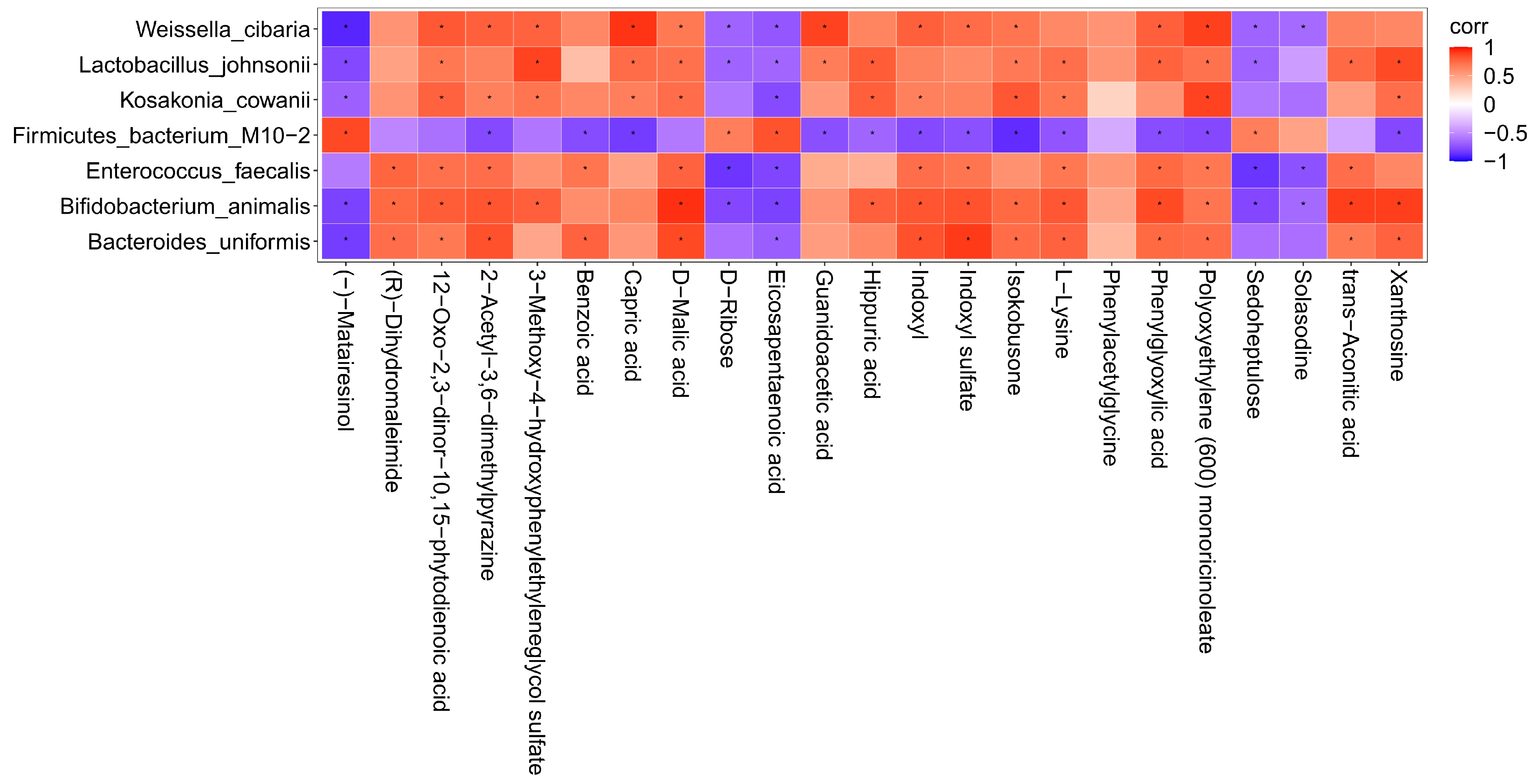
3.5. Correlation Analysis of Intestinal Flora and Metabolites in Mice after Intake of Auricularia auricula Polysaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew’s ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar]
- Liu, E.; Ji, Y.; Zhang, F.; Liu, B.; Meng, X. Review on Auricularia auricula-judae as a functional food: Growth, chemical composition, and biological activities. J. Agric. Food Chem. 2021, 69, 1739–1750. [Google Scholar]
- Li, L.; Zhong, C.H.; Bian, Y.B. The molecular diversity analysis of Auricularia auricula-judae in China by nuclear ribosomal DNA intergenic spacer. Electron. J. Biotechn. 2014, 17, 27–33. [Google Scholar]
- Liu, Q.; Ma, R.; Li, S.; Fei, Y.; Lei, J.; Li, R.; Wang, L. Dietary supplementation of auricularia auricula-judae polysaccharides alleviate nutritional obesity in mice via regulating inflammatory response and lipid metabolism. Foods 2022, 11, 942. [Google Scholar] [PubMed]
- Bian, C.; Wang, Z.; Shi, J. Extraction optimization, structural characterization, and anticoagulant activity of acidic polysaccharides from Auricularia auricula-judae. Molecules 2020, 25, 710. [Google Scholar] [CrossRef]
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr. Opin. Biotech. 2018, 51, 57–63. [Google Scholar]
- Wu, J.; Gao, Y. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev. Proteom. 2015, 12, 623–636. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ibanez, C.; Simo, C.; Bartolomé, B.; Moreno-Arribas, M.V. An ultrahigh-performance liquid chromatography-time-of-flight mass spectrometry metabolomic approach to studying the impact of moderate red-wine consumption on urinary metabolome. J. Proteome Res. 2018, 17, 1624–1635. [Google Scholar]
- Liu, H.; Garrett, T.J.; Su, Z.; Khoo, C.; Zhao, S.; Gu, L. Modifications of the urinary metabolome in young women after cranberry juice consumption were revealed using the UHPLC-Q-orbitrap-HRMS-based metabolomics approach. Food Funct. 2020, 11, 2466–2476. [Google Scholar]
- Liu, H.; Tayyari, F.; Edison, A.S.; Su, Z.; Gu, L. NMR-based metabolomics reveals urinary metabolome modifications in female Sprague-Dawley rats by cranberry procyanidins. J. Nutr. Biochem. 2016, 34, 136–145. [Google Scholar]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [PubMed]
- Zhang, L.; Zhang, Z.; Xu, L.; Zhang, X. Maintaining the balance of intestinal flora through the diet: Effective prevention of illness. Foods 2021, 10, 2312. [Google Scholar] [PubMed]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [PubMed]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar]
- Xu, N.; Zhou, Y.; Lu, X.; Chang, Y. Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota. J. Food Sci. 2021, 86, 5479–5494. [Google Scholar] [PubMed]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [PubMed]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2022, 123, 144. [Google Scholar]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar]
- Hao, Y.; Wang, X.; Yuan, S.; Wang, Y.; Liao, X.; Zhong, M.; He, Q.; Shen, H.; Liao, W.; Shen, J. Flammulina velutipes polysaccharide improves C57BL/6 mice gut health through regulation of intestine microbial metabolic activity. Int. J. Biol. Macromol. 2021, 167, 1308–1318. [Google Scholar] [PubMed]
- Khan, I.; Huang, G.; Li, X.; Leong, W.; Xia, W.; Hsiao, W. Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 2018, 41, 191–201. [Google Scholar]
- Costa, M.C.; Weese, J.S. Understanding the intestinal microbiome in health and disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar]
- Panchal, S.K.; Brown, L. Cholesterol versus inflammation as cause of chronic diseases. Nutrients 2019, 11, 2332. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar]
- Xin, J.; Zeng, D.; Wang, H.; Sun, N.; Zhao, Y.; Dan, Y.; Pan, K.; Jing, B.; Ni, X. Probiotic Lactobacillus johnsonii BS15 promotes growth performance, intestinal immunity, and gut microbiota in piglets. Probiotics Antimicrob. Proteins 2020, 12, 184–193. [Google Scholar]
- Ekmekciu, I.; Von Klitzing, E.; Neumann, C.; Bacher, P.; Scheffold, A.; Bereswill, S.; Heimesaat, M.M. Fecal microbiota transplantation, commensal Escherichia coli and Lactobacillus johnsonii strains differentially restore intestinal and systemic adaptive immune cell populations following broad-spectrum antibiotic treatment. Front. Microbiol. 2017, 8, 2430. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.R.; Gurung, M.; Li, Z.; García-Jaramillo, M.; Greer, R.; Gaulke, C.; Bauchinger, F.; You, H.; Pederson, J.W.; Vasquez-Perez, S.; et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat. Commun. 2021, 12, 101. [Google Scholar] [PubMed]
- Yu, H.S.; Jang, H.J.; Lee, N.K.; Paik, H.D. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT 2019, 112, 108229. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Singh, A.; Singh, P.; Kaur, R.; Khare, P.; Purama, R.K.; Boparai, R.K.; Rishi, P.; Ambalam, P.; et al. Probiotic attributes and prevention of LPS-induced pro-inflammatory stress in RAW264. 7 macrophages and human intestinal epithelial cell line (Caco-2) by newly isolated Weissella cibaria strains. Food Funct. 2018, 9, 1254–1264. [Google Scholar] [CrossRef]
- You, L.; Gong, Y.; Li, L.; Hu, X.; Brennan, C.; Kulikouskaya, V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 2020, 55, 1199–1206. [Google Scholar] [CrossRef]
- Deng, J.; Zhong, J.; Long, J.; Zou, X.; Wang, D.; Song, Y.; Zhou, K.; Liang, Y.; Huang, R.; Wei, X.; et al. Hypoglycemic effects and mechanism of different molecular weights of konjac glucomannans in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 165 Pt B, 2231–2243. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.; Zijlstra, R.; Mosenthin, R.; Ganzle, M. Dietary calcium phosphate content and oat beta-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol. Ecol. 2011, 75, 402–413. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef]
- Dodd, D.; Mackie, R.I.; Cann, I.K. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol. Microbiol. 2011, 79, 292–304. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hu, J.; Yao, H.; Geng, F.; Nie, S. Interaction between four galactans with different structural characteristics and gut microbiota. Crit. Rev. Food Sci. 2021, 61, 1–11. [Google Scholar]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastro. Hepat. 2019, 16, 35–56. [Google Scholar]
- Martí i Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [PubMed]
- Zou, S.; Wang, X.; Liu, P.; Ke, C.; Xu, S. Arginine metabolism and deprivation in cancer therapy. Biomed. Pharm. 2019, 118, 109210. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E. Creatine metabolism and the urea cycle. Mol. Genet. Metab. 2010, 100, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Halloran, K.M.; Stenhouse, C.; Wu, G.; Bazer, F.W. Arginine, agmatine, and polyamines: Key regulators of conceptus development in mammals. Adv. Exp. Med. Biol. 2021, 1332, 85–105. [Google Scholar]
- Newsholme, E.A.; Beis, I.S.; Leech, A.R.; Zammit, V.A. The role of creatine kinase and arginine kinase in muscle. Biochem. J. 1978, 172, 533–537. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]

| No. | Metabolites | Sub Class | VIP Value | p Value | Experimental m/z | Molecular Formula | HMDB ID | KEGG ID | AAP vs. ND |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Polyoxyethylene (600) monoricinoleate | Fatty alcohols | 2.286 | 0.001 | 341.3039 | C21H40O3 | HMDB0032476 | --- | ↑ |
| 2 | Solasodine | Steroidal alkaloids | 2.286 | 0.004 | 414.3364 | C27H43NO2 | HMDB0035282 | C10822 | ↓ |
| 3 | Guanidoacetic acid | Amino acids, peptides, and analogues | 2.240 | 0.001 | 118.0612 | C3H7N3O2 | HMDB0000128 | C00581 | ↑ |
| 4 | Epidermin | Carbohydrates and carbohydrate conjugates | 2.194 | 0.020 | 262.1273 | C11H19NO6 | HMDB0034777 | ↑ | |
| 5 | (R)-Dihydromaleimide | Pyrrolines (Class) | 2.094 | 0.005 | 100.0397 | C4H5NO2 | HMDB0030276 | --- | ↑ |
| 6 | (R)-Pelletierine | Piperidines (Class) | 2.053 | 0.041 | 142.1224 | C8H15NO | HMDB0030324 | ↑ | |
| 7 | Indoxyl sulfate | Arylsulfates | 2.031 | 0.006 | 214.0163 | C8H7NO4S | HMDB0000682 | --- | ↑ |
| 8 | 3-(5-Methyl-2-furanyl)butanal | Heteroaromatic compounds (Class) | 2.016 | 0.047 | 153.0907 | C9H12O2 | HMDB0036169 | --- | ↑ |
| 9 | 2-Acetyl-3,6-dimethylpyrazine | Carbonyl compounds | 1.981 | 0.008 | 151.0861 | C8H10N2O | HMDB0039998 | --- | ↑ |
| 10 | Indoxyl | Hydroxyindoles | 1.970 | 0.010 | 134.0598 | C8H7NO | HMDB0004094 | C05658 | ↑ |
| 11 | 4,5-Dihydropiperlonguminine | Benzodioxoles (Class) | 1.957 | 0.025 | 276.1587 | C16H21NO3 | HMDB0041488 | --- | ↑ |
| 12 | Perlolyrine | Harmala alkaloidal (Class) | 1.915 | 0.020 | 265.0966 | C16H12N2O2 | HMDB0030327 | C09231 | ↑ |
| 13 | N-Nitroso-pyrrolidine | Pyrrolidines (Class) | 1.741 | 0.037 | 101.0712 | C4H8N2O | HMDB0031642 | C19285 | ↑ |
| 14 | Citrulline | Amino acids, peptides, and analogues | 1.734 | 0.043 | 176.1025 | C6H13N3O3 | HMDB0000904 | C00327 | ↑ |
| 15 | L-Lysine | Amino acids, peptides, and analogues | 1.667 | 0.040 | 147.1125 | C6H14N2O2 | HMDB0000182 | C00047 | ↑ |
| 16 | (±)-Metalaxyl | Amino acids, peptides, and analogues | 1.648 | 0.037 | 280.1532 | C15H21NO4 | HMDB0031802 | C10947 | ↑ |
| 17 | PC(14:0/14:0) | Glycerophosphocholines | 1.625 | 0.049 | 678.5077 | C36H72NO8P | HMDB0007866 | C00157 | ↓ |
| 18 | Ornithine | Amino acids, peptides, and analogues | 1.614 | 0.047 | 133.0969 | C5H12N2O2 | HMDB0000214 | C00077 | ↑ |
| 19 | Linoleamide | Fatty amides | 1.361 | 0.050 | 280.2626 | C18H33NO | HMDB0062656 | --- | ↑ |
| 20 | Sphingosine | Amines | 1.358 | 0.036 | 300.2883 | C18H37NO2 | HMDB0000252 | C00319 | ↑ |
| 21 | Xanthosine | Purine nucleosides (Class) | 2.569 | 0.000 | 283.0687 | C10H12N4O6 | HMDB0000299 | C01762 | ↑ |
| 22 | 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate | Arylsulfates | 2.301 | 0.000 | 263.0233 | C9H12O7S | HMDB0000559 | --- | ↑ |
| 23 | (-)-Matairesinol | Tetrahydrofuran lignans | 2.274 | 0.000 | 357.1350 | C20H22O6 | HMDB0035698 | C10682 | ↓ |
| 24 | Dimethylglycine | Amino acids, peptides, and analogues | 2.183 | 0.020 | 102.0551 | C4H9NO2 | HMDB0000092 | C01026 | ↑ |
| 25 | Isokobusone | Alcohols and polyols | 2.156 | 0.003 | 221.1548 | C14H22O2 | HMDB0036791 | --- | ↑ |
| 26 | Indoxyl sulfate | Arylsulfates | 2.146 | 0.002 | 212.0020 | C8H7NO4S | HMDB0000682 | --- | ↑ |
| 27 | 12-Oxo-2,3-dinor-10,15-phytodienoic acid | Eicosanoids | 2.134 | 0.000 | 263.1658 | C16H24O3 | HMDB0032090 | --- | ↑ |
| 28 | Eicosapentaenoic acid | Fatty acids and conjugates | 2.078 | 0.006 | 301.2173 | C20H30O2 | HMDB0001999 | C06428 | ↓ |
| 29 | D-Malic acid | Beta hydroxy acids and derivatives | 2.065 | 0.004 | 133.0134 | C4H6O5 | HMDB0031518 | C00497 | ↑ |
| 30 | Phenylacetylglycine | Amino acids, peptides, and analogues | 2.046 | 0.005 | 192.0662 | C10H11NO3 | HMDB0000821 | C05598 | ↑ |
| 31 | trans-Aconitic acid | Tricarboxylic acids and derivatives | 2.019 | 0.007 | 173.0085 | C6H6O6 | HMDB0000958 | C02341 | ↑ |
| 32 | Adipic acid | Lipids and lipid-like molecules | 2.005 | 0.013 | 145.0497 | C6H10O4 | HMDB0000448 | C06104 | ↑ |
| 33 | Benzoic acid | Benzoic acids and derivatives | 1.991 | 0.006 | 121.0285 | C7H6O2 | HMDB0001870 | C00539 | ↑ |
| 34 | D-Ribose | Carbohydrates and carbohydrate conjugates | 1.988 | 0.008 | 149.0447 | C5H10O5 | HMDB0000283 | C00121 | ↓ |
| 35 | L-Lysine | Amino acids, peptides, and analogues | 1.951 | 0.007 | 145.0974 | C6H14N2O2 | HMDB0000182 | C00047 | ↑ |
| 36 | Capric acid | Fatty acids and conjugates | 1.941 | 0.007 | 171.1384 | C10H20O2 | HMDB0000511 | C01571 | ↑ |
| 37 | (10E,12Z)-(9S)-9-Hydroperoxyoctadeca-10,12-dienoic acid | Lineolic acids and derivatives | 1.930 | 0.016 | 311.2235 | C18H32O4 | HMDB0062434 | C14827 | ↑ |
| 38 | Sedoheptulose | Carbohydrates and carbohydrate conjugates | 1.921 | 0.009 | 209.0662 | C7H14O7 | HMDB0003219 | C08355 | ↓ |
| 39 | D-Alanine | Amino acids, peptides, and analogues | 1.918 | 0.015 | 88.0396 | C3H7NO2 | HMDB0001310 | C00133 | ↑ |
| 40 | Succinic acid | Dicarboxylic acids and derivatives | 1.917 | 0.020 | 117.0184 | C4H6O4 | HMDB0000254 | C00042 | ↑ |
| 41 | 12-Hydroxydodecanoic acid | Medium-chain hydroxy acids and derivatives | 1.876 | 0.023 | 215.1653 | C12H24O3 | HMDB0002059 | C08317 | ↑ |
| 42 | Citrulline | Amino acids, peptides, and analogues | 1.800 | 0.034 | 174.0877 | C6H13N3O3 | HMDB0000904 | C00327 | ↑ |
| 43 | 2-Hydroxy-3-methylbutyric acid | Fatty acids and conjugates | 1.775 | 0.033 | 117.0547 | C5H10O3 | HMDB0000407 | --- | ↑ |
| 44 | L-Pipecolic acid | Amino acids, peptides, and analogues | 1.731 | 0.029 | 128.0707 | C6H11NO2 | HMDB0000716 | C00408 | ↑ |
| 45 | Glyceraldehyde | Carbohydrates and carbohydrate conjugates | 1.694 | 0.039 | 89.0233 | C3H6O3 | HMDB0001051 | C02154 | ↑ |
| 46 | Deoxyinosine | Purine 2′-deoxyribonucleosides | 1.682 | 0.020 | 251.0791 | C10H12N4O4 | HMDB0000071 | C05512 | ↓ |
| 47 | Hippuric acid | Benzoic acids and derivatives | 1.656 | 0.006 | 178.0505 | C9H9NO3 | HMDB0000714 | C01586 | ↑ |
| 48 | Pentadecanoic acid | Fatty acids and conjugates | 1.655 | 0.041 | 241.2176 | C15H30O2 | HMDB0000826 | C16537 | ↑ |
| 49 | Phenylglyoxylic acid | Benzoyl derivatives | 1.644 | 0.005 | 149.0236 | C8H6O3 | HMDB0001587 | C02137 | ↑ |
| 50 | Chenodeoxycholic acid | Lipids and lipid-like molecules | 1.622 | 0.044 | 391.2871 | C24H40O4 | HMDB0000518 | C02528 | ↑ |
| 51 | Threonic acid | Carbohydrates and carbohydrate conjugates | 1.576 | 0.046 | 135.0291 | C4H8O5 | HMDB0000943 | C01620 | ↑ |
| 52 | 1,11-Undecanedicarboxylic acid | Fatty acids and conjugates | 1.463 | 0.049 | 243.1605 | C13H24O4 | HMDB0002327 | --- | ↑ |
| 53 | 2-Hydroxystearic acid | Fatty acids and conjugates | 1.452 | 0.039 | 299.2592 | C18H36O3 | HMDB0062549 | C03045 | ↑ |
| 54 | 2-Hydroxyethanesulfonate | Organosulfonic acids and derivatives | 1.303 | 0.029 | 124.9904 | C2H6O4S | HMDB0003903 | C05123 | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; An, X.; Chen, Y.; Deng, Y.; Niu, H.; Ma, R.; Zhao, H.; Cao, W.; Wang, X.; Wang, M. Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice. Foods 2022, 11, 2700. https://doi.org/10.3390/foods11172700
Liu Q, An X, Chen Y, Deng Y, Niu H, Ma R, Zhao H, Cao W, Wang X, Wang M. Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice. Foods. 2022; 11(17):2700. https://doi.org/10.3390/foods11172700
Chicago/Turabian StyleLiu, Qian, Xin An, Yuan Chen, Yuxuan Deng, Haili Niu, Ruisen Ma, Haoan Zhao, Wei Cao, Xiaoru Wang, and Meng Wang. 2022. "Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice" Foods 11, no. 17: 2700. https://doi.org/10.3390/foods11172700
APA StyleLiu, Q., An, X., Chen, Y., Deng, Y., Niu, H., Ma, R., Zhao, H., Cao, W., Wang, X., & Wang, M. (2022). Effects of Auricularia auricula Polysaccharides on Gut Microbiota and Metabolic Phenotype in Mice. Foods, 11(17), 2700. https://doi.org/10.3390/foods11172700