Microwave-Assisted Extraction of Polyphenols from Blackcurrant By-Products and Possible Uses of the Extracts in Active Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Process
2.1.1. Raw Materials
2.1.2. Optimization of Microwave-Assisted Extraction (MAE) of Polyphenols from Blackcurrant Residuals (Experimental Design)
- Extraction time (from 60 to 180 min).
- Power of microwave (from 500 to 700 W).
- Solvent/solid sample ratio (from 10 to 15 mL/g).
2.1.3. Determination of TPC and Antioxidant Activity of the Extract
2.1.4. Extraction with Organic Solvent
2.2. Active Pad Production
2.2.1. Active Pad Production and Activation with Phenol Extract
- Pad F0: The electrospinning solution included maltodextrin and water at a 1:1 ratio (wt%) and citric acid as a cross-linker (16% wt of the amount of Glucidex). Active pads were created by impregnation with the phenol extract of the insoluble microfibers obtained as explained before. For the impregnation process, 3 mL of polyphenol extract was used.
- Pad F1: The phenol extract was added to the electrospinning solution that included maltodextrin, water, and extract at weight ratios of 1:0.6:0.3 (wt%), respectively, as well as citric acid (16 wt% of the amount of Glucidex).
2.2.2. Morphological Characterization (Scanning Electron Microscope, SEM)
2.3. Application Test of Active Pads on Fruit Packaging
2.3.1. Fruit
2.3.2. Application Test
- Control: Clamshell containing raspberries cv Clarita without the active pad (CP).
- F0: Clamshell containing raspberries cv Clarita with active pad F0.
- Control: Clamshell containing raspberries cv Grandeur without the active pad (CP).
- F0: Clamshell containing raspberries cv Grandeur with active pad F0.
- F1: Clamshell containing raspberries cv Grandeur with active pad F1.
2.3.3. Fruit Analysis
2.4. Statistical Analysis of the Experimental Results
3. Results and Discussion
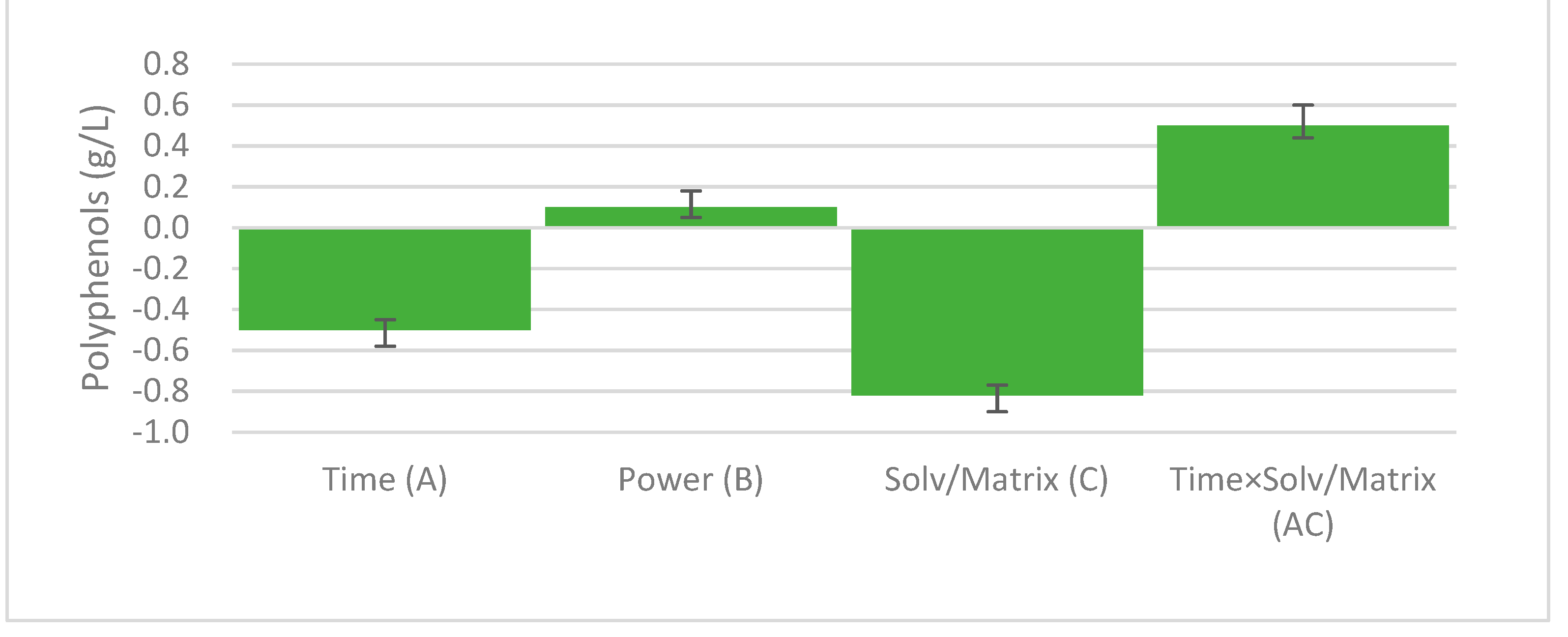
3.1. Model Fitting and Refinement
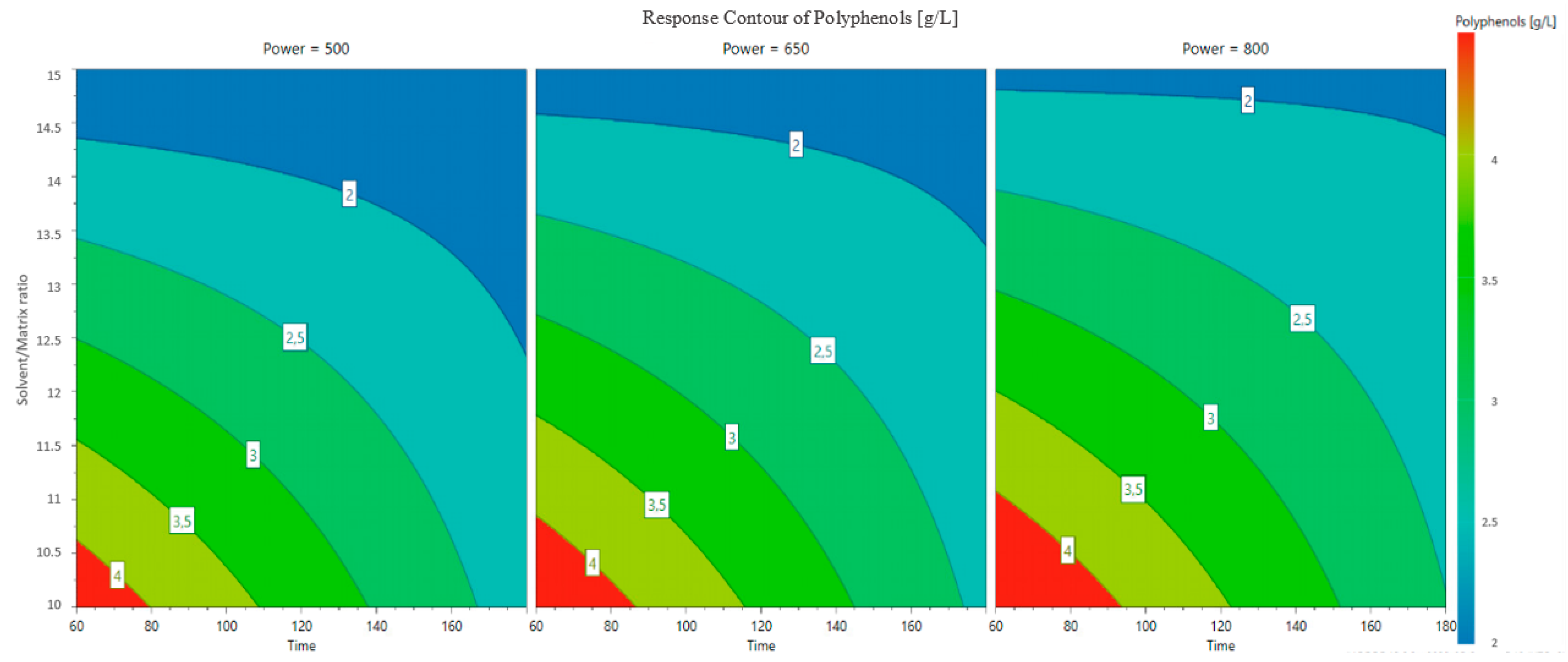
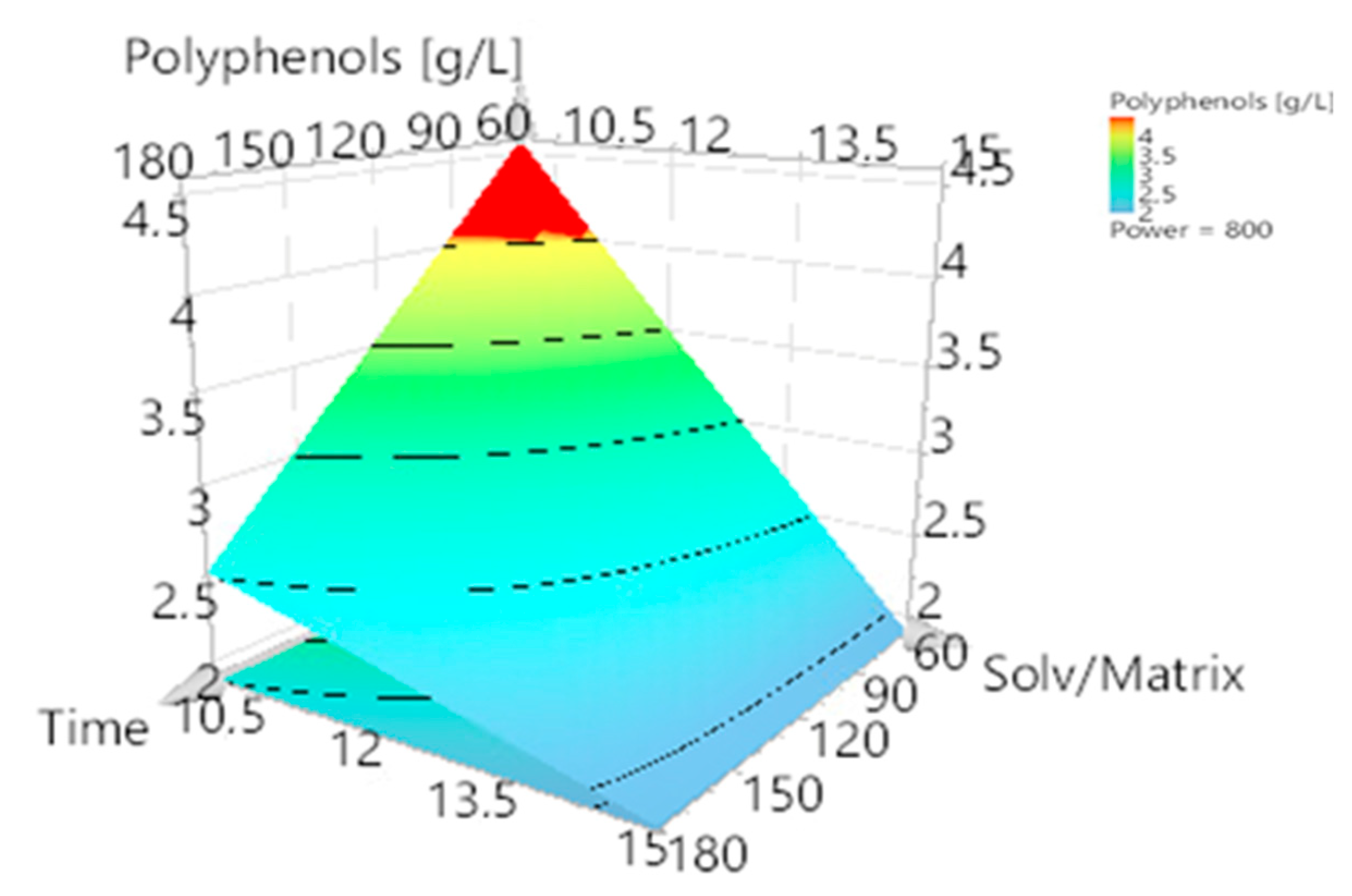
3.2. Optimization of Polyphenol Extraction and Model Verification
3.3. Antioxidant Capability: FRAP Method
3.4. Solvent Extraction Efficiency: Comparison of Microwave-Assisted Polyphenol Extraction with Conventional Method Extraction
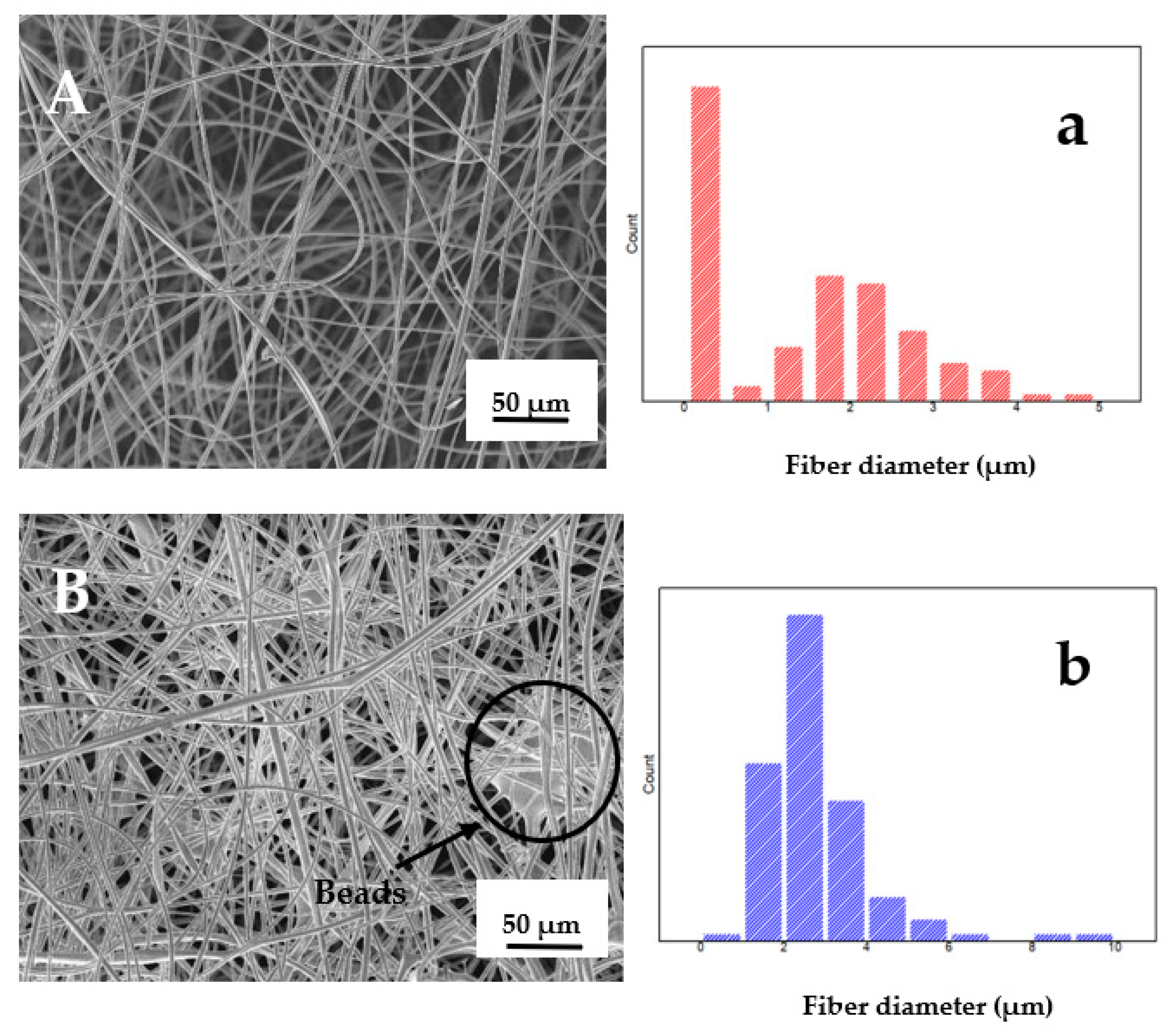
3.5. Characterization of Microfibers and Active Pad
Scanning Electron Microscopy (SEM)
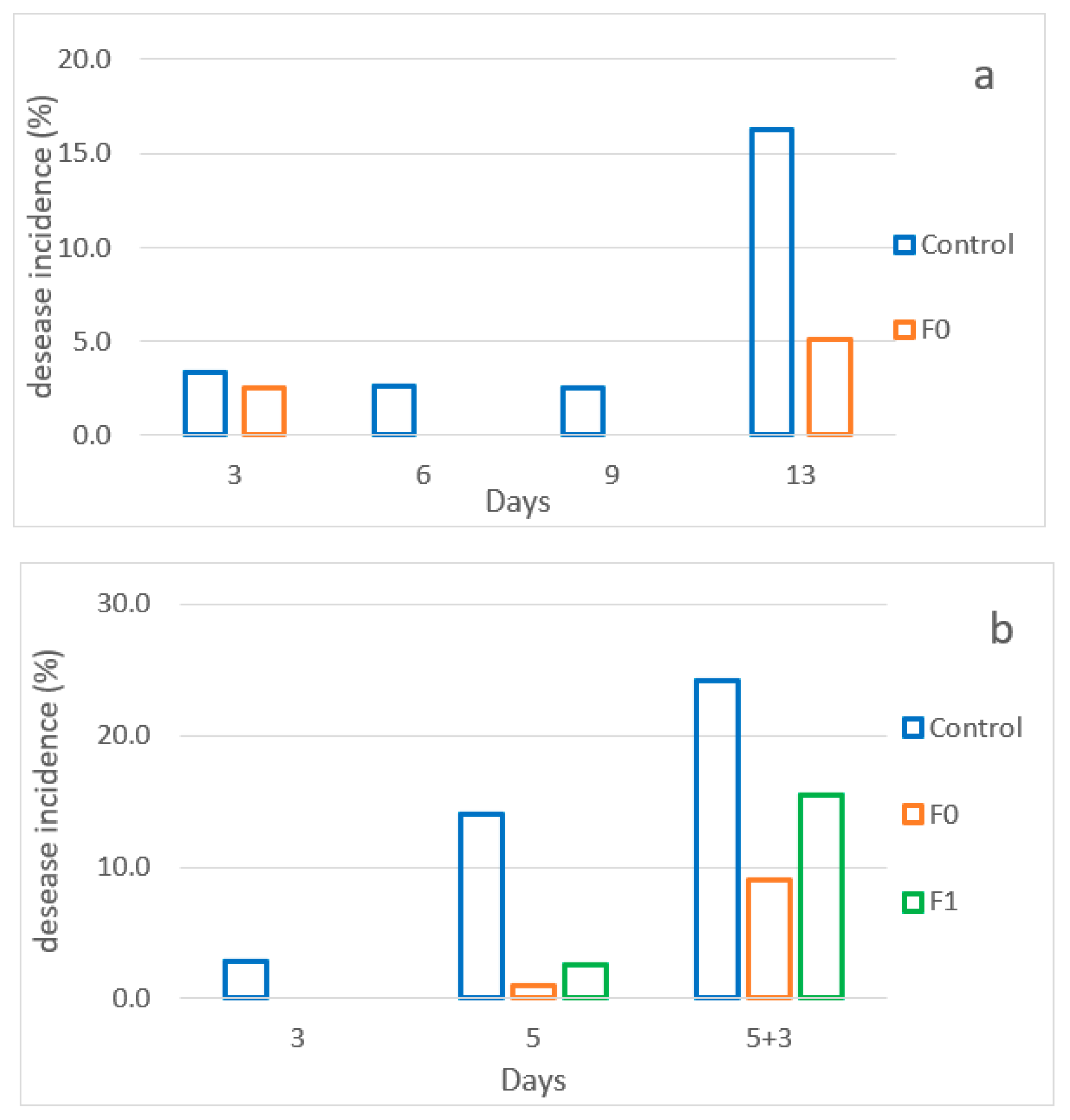
3.6. Evaluation of the Effects of the Active Pad on Raspberries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Singhnia, R.R.; Patel, A.K.; Juneja, A.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.I.; Goodrich-Schneider, R.M.; Barringer, S.A.; Landeros-Urbina, M.A. Proceeding of Fruit and Vegetable Beverages. In Food processing, 2nd ed.; Clark, S., Junk, S., Lamsal, B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 339–362. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Chaillou, L.L.; Nazareno, M.A. New method to determine antioxidant activity of polyphenols. J. Agric. Food Chem. 2006, 54, 8397–8402. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Agourram, A.; Ghirardello, D.; Rantsiou, K.; Zeppa, G.; Belviso, S.; Romane, A.; Oufdou, K.; Giordano, M. Phenolic Content, Antioxidant Potential, and Antimicrobial Activities of Fruit and Vegetable By-Product Extracts. Int. J. Food Prop. 2013, 16, 1092–1104. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Zeković, Z.; Vladić, J.; Vidović, S.; Adamović, D.; Pavlić, B. Optimization of microwave-assisted extraction (MAE) of coriander phenolic antioxidants—Response surface methodology approach. J. Sci. Food Agric. 2016, 96, 4613–4622. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of functional extracts from berries and their high quality processing: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: Evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021, 9, 105330. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.-T. Influence of Extraction Conditions on Ultrasound-Assisted Recovery of Bioactive Phenolics from Blueberry Pomace and Their Antioxidant Activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surfaces. B: Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.B.M.; Nor, M.S.M. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Cecone, C.; Costamagna, G.; Ginepro, M.; Trotta, F. One-step sustainable synthesis of cationic high-swelling polymers obtained from starch-derived maltodextrins. RSC Adv. 2021, 11, 7653–7662. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, M. Response surface methodology and product optimization. Food Technol. 1983, 37, 41–83. [Google Scholar]
- Vernon, L.O.; Rudolf, S.; Lamuela-Ravents, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Nascimento, N.D. Correlations between subjective quality and physicochemical attributes of fresh fruits and vegetables. Postharvest Biol. Technol. 2015, 107, 43–54. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response surface evaluation of microwave-assisted extraction conditions for Lycium barbarum bioactive compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. omparación de los contenidos de fenoles totales, antocianos totales y la actividad antioxidante de frutos ricos en polifenoles que crecen en Chile. Cienc. e Investig. Agrar. 2014, 41, 49–59. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Czyżowska, A.; Pogorzelski, E. Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part I. Total polyphenols and phenolic acids. Eur. Food Res. Technol. 2002, 214, 148–154. [Google Scholar] [CrossRef]
- Sójka, M.; Król, B. Composition of industrial seedless black currant pomace. Eur. Food Res. Technol. 2008, 228, 597–605. [Google Scholar] [CrossRef]
- Lukáš, D.; Sarkar, A.; Martinová, L.; Vodsed’álková, K.; Lubasová, D.; Chaloupek, J.; Pokorný, P.; Mikeš, P.; Chvojka, J.; Komárek, M. Physical principles of electrospinning (Electrospinning as a nano-scale technology of the twenty-first century). Text. Prog. 2009, 41, 59–140. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375–1381. [Google Scholar] [CrossRef]
- Puerta-Gomez, A.F.; Cisneros-Zevallos, L. Postharvest studies beyond fresh market eating quality: Phytochemical antioxidant changes in peach and plum fruit during ripening and advanced senescence. Postharvest Biol. Technol. 2011, 60, 220–224. [Google Scholar] [CrossRef]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Reyes, L.F.; Villarreal, J.E.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Nonnecke, G. Physiological Changes during Ripening of Raspberry Fruit. HortScience 1992, 27, 331–333. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V.; Lefsrud, M. Effect of Pulsed Ultraviolet Light on the Total Phenol Content of Elderberry (Sambucus nigra) Fruit. Food Nutr. Sci. 2012, 03, 774–783. [Google Scholar] [CrossRef] [Green Version]

| Runs | A Time (Min) | B Microwave Power (W) | C Solvent/Matrix (mL/g) | Response TPC 1 (g/L) |
|---|---|---|---|---|
| N1 | 60 | 500 | 10 | 4.28 ± 0.06 |
| N2 | 60 | 800 | 10 | 4.64 ± 0.07 |
| N3 | 180 | 500 | 10 | 2.24 ± 0.1 |
| N4 | 180 | 800 | 10 | 2.58 ± 0.03 |
| N5 | 60 | 500 | 15 | 2.52 ± 0.08 |
| N6 | 60 | 800 | 15 | 1.85 ± 0.25 |
| N7 | 180 | 500 | 15 | 2.22 ± 0.07 |
| N8 | 180 | 800 | 15 | 1.9 ± 0.39 |
| N9 | 120 | 447.03 | 12.5 | 2.55 ± 0.35 |
| N10 | 120 | 852.97 | 12.5 | 2.08 ±0.24 |
| N11 | 38.81 | 650 | 12.5 | 1.82 ± 0.18 |
| N12 | 201 | 650 | 12.5 | 1.89 ± 0.42 |
| N13 | 120 | 650 | 9 | 2.03 ± 0.09 |
| N14 | 120 | 650 | 15.88 | 1.58 ± 0.27 |
| N15 | 120 | 650 | 12.5 | 2.55 ± 0.53 |
| N16 | 120 | 650 | 12.5 | 2.61 ± 0.05 |
| N17 | 120 | 650 | 12.5 | 2.56 ± 0.07 |
| Polyphenols | F-Value | p-Value |
|---|---|---|
| Coefficient 1 | ||
| A | 958.09 | 0.001 |
| B | 118.55 | 0.008 |
| C | 2913.12 | 0.000 |
| A2 | 14.09 | 0.064 |
| B2 | 6.38 | 0.127 |
| C2 | 2.28 | 0.270 |
| AB | 0.10 | 0.785 |
| AC | 1077.12 | 0.001 |
| Polyphenols | DF a | SS b | MS (Variance) c | F | p | SD d |
|---|---|---|---|---|---|---|
| Total | 12 | 90.99 | 7.58 | |||
| Constant | 1 | 81.28 | 81.28 | |||
| Total corrected | 11 | 9.72 | 0.88 | 0.94 | ||
| Regression | 5 | 9.68 | 1.94 | 350.14 | 0.00 e | 1.39 |
| Residual | 6 | 0.03 | 0.01 | 0.07 | ||
| Lake of fit | 4 | 0.03 | 0.01 | 7.53 | 0.12 e | 0.09 |
| Pure error | 2 | 0.00 | 0.00 | 0.03 |
| Extraction Factor | FRAP 1 (mmol Fe2+/kg) r | p-Value |
|---|---|---|
| Solv/matrix | −0.540 | 0.025 |
| Time | −0.193 | 0.458 |
| Power | 0.014 | 0.958 |
| Extraction Method | TPC 1 (mgGAE/gdry Matrix) |
|---|---|
| Organic Solvent | 31.25 ± 0.41 B |
| Water solvent (MAE 2) | 41.77 ± 0.6 A |
| Day 3 | Day 5 | Day 5 + 3 | |
|---|---|---|---|
| Control | 2.2 | 4.7 | 10.4 |
| F0 | 2.2 | 4.7 | 10.6 |
| F1 | 1.9 | 3.8 | 9.2 |
| TSS 1 (°brix) | |||||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 5 + 3 | ||
| Control | 10.1 ± 0.1 B | 10.5 ± 0.1 A | 10.7 ± 0.1 bA | 8.5 ± 0.1 cC | * |
| F0 | 10.1 ± 0.1 B | 10.5 ± 0.3 B | 11 ± 0.1 aA | 9 ± 0 bC | * |
| F1 | 10.1 ± 0.1 B | 10.8 ± 0.3 A | 11 ± 0.1 aA | 9.6 ± 0.1 aC | * |
| n.s. | * | * | |||
| TPC 1 (gGAE/L) | |||||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 5 + 3 | ||
| Control | 1.39 ± 0.01 C | 1.14 ± 0.0 cD | 1.66 ± 0.03 cB | 2.06 ± 0.01 aA | * |
| F0 | 1.39 ± 0.01 D | 1.64 ± 0.01 aB | 1.67 ± 0.01 bA | 1.59 ± 0.03 cC | * |
| F1 | 1.39 ± 0.01 D | 1.51 ± 0.05 bC | 1.7 ± 0.02 aB | 1.79 ± 0.01 bA | * |
| * | * | * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alchera, F.; Ginepro, M.; Giacalone, G. Microwave-Assisted Extraction of Polyphenols from Blackcurrant By-Products and Possible Uses of the Extracts in Active Packaging. Foods 2022, 11, 2727. https://doi.org/10.3390/foods11182727
Alchera F, Ginepro M, Giacalone G. Microwave-Assisted Extraction of Polyphenols from Blackcurrant By-Products and Possible Uses of the Extracts in Active Packaging. Foods. 2022; 11(18):2727. https://doi.org/10.3390/foods11182727
Chicago/Turabian StyleAlchera, Federica, Marco Ginepro, and Giovanna Giacalone. 2022. "Microwave-Assisted Extraction of Polyphenols from Blackcurrant By-Products and Possible Uses of the Extracts in Active Packaging" Foods 11, no. 18: 2727. https://doi.org/10.3390/foods11182727





