Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
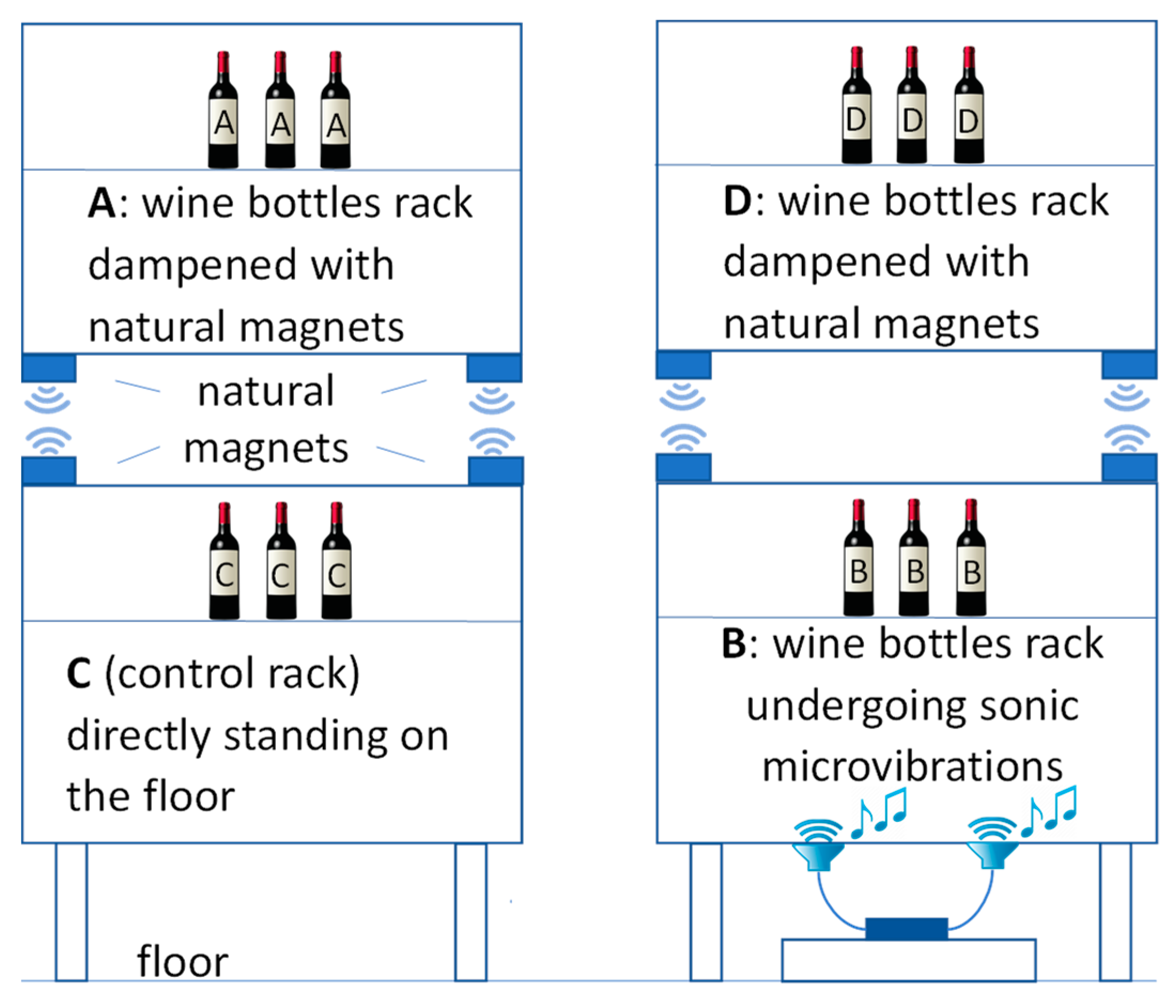
2.2. Wine Samples and Vibration Damping Devices
2.3. Phenolic Profile by HPLC-DAD/FLD and LC-QqQ-MS
2.4. Profile of Volatile Compounds with Headspace Microextraction Bidimensional Gas-Chromatography Mass Spectrometry
2.5. Sensory Profile
2.6. Statistical Analysis and Data Processing
3. Results
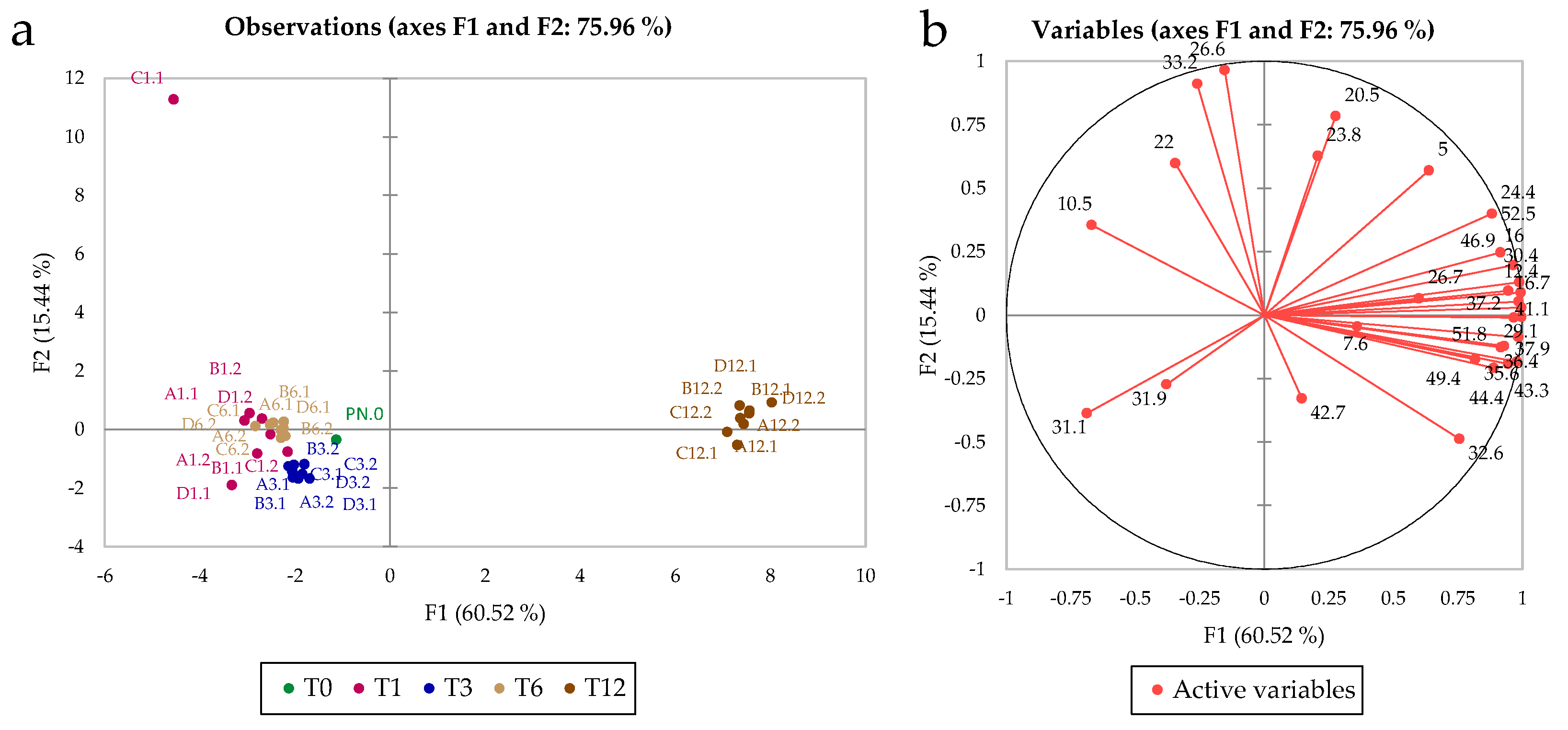
3.1. Phenolic Compounds
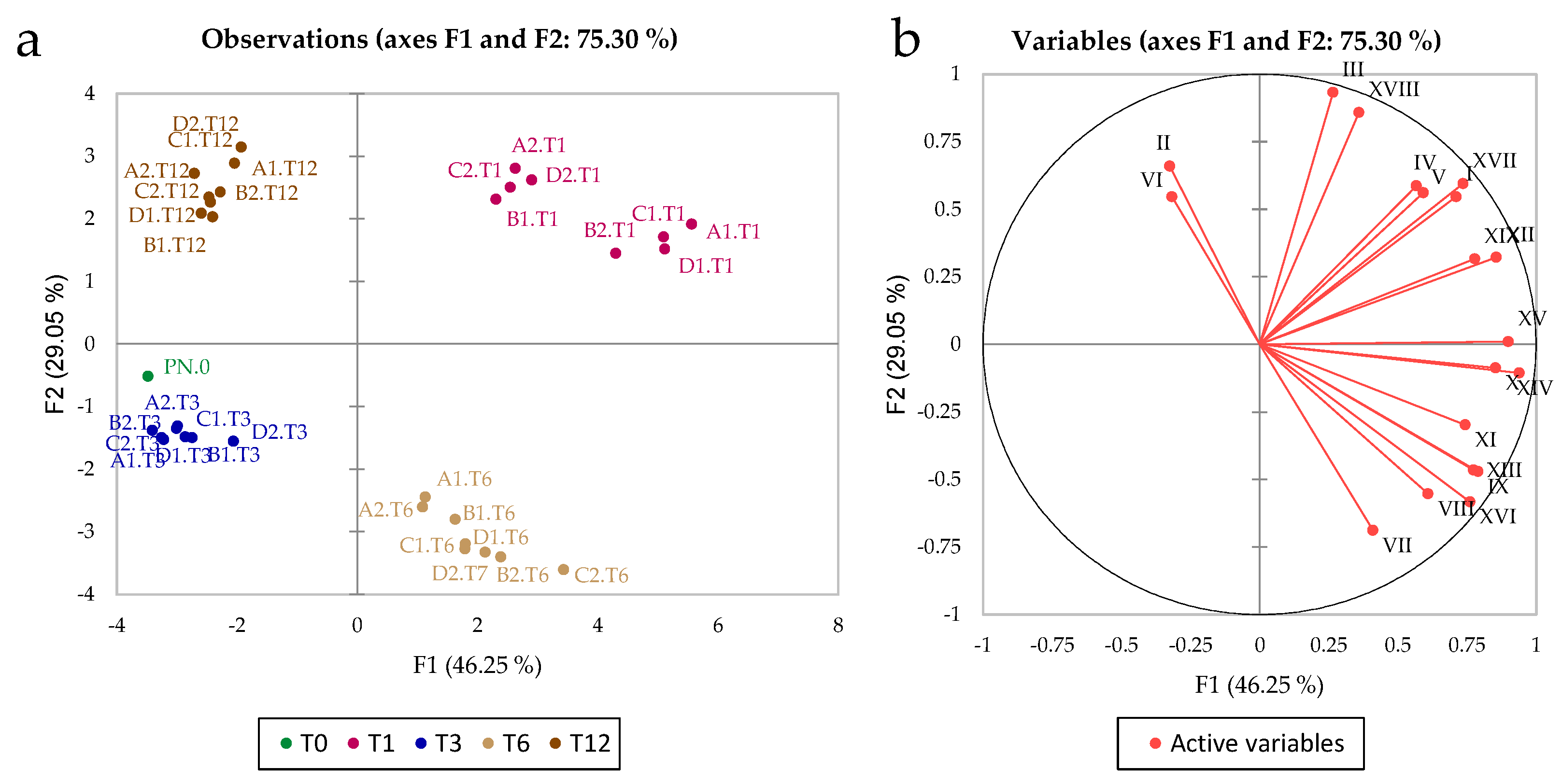
3.2. Volatile Compounds
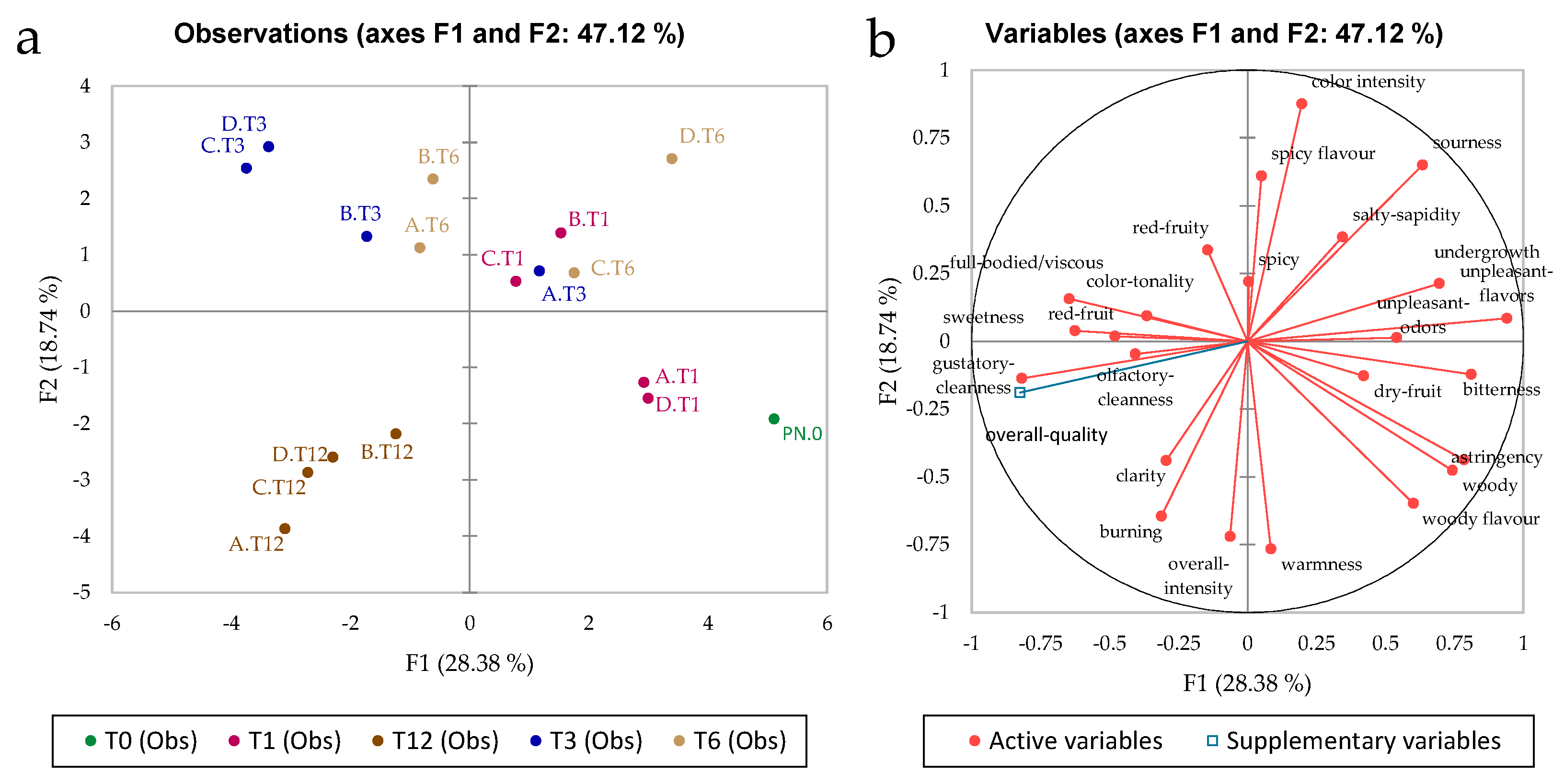
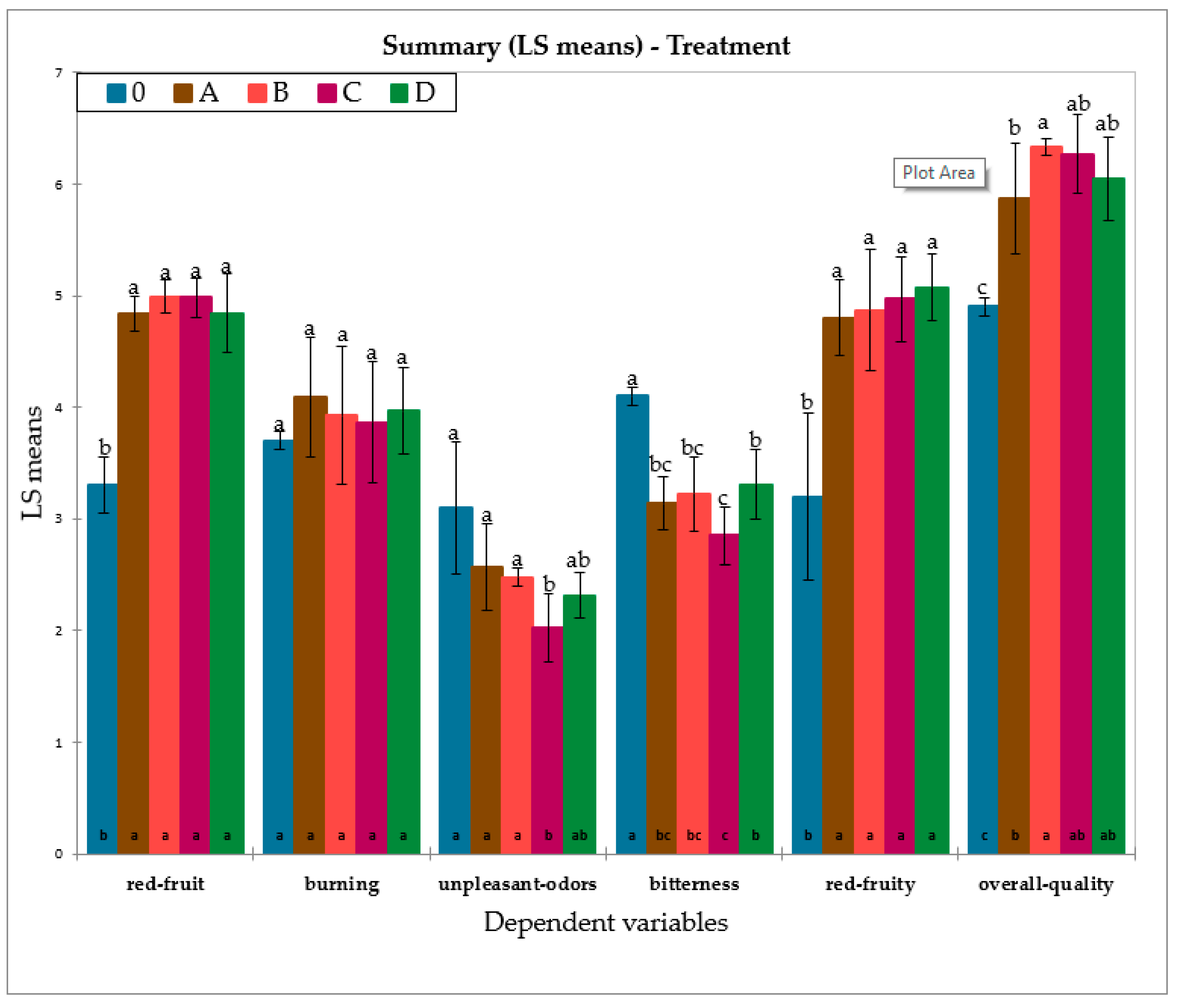
3.3. Sensory Analysis
3.4. Multifactor Analysis of the Effects of the Treatments after 12 Months of Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Arapitsas, P.; Dalledonne, S.; Scholz, M.; Catapano, A.; Carlin, S.; Mattivi, F. White wine light- strike fault: A comparison between flint and green glass bottles under the typical supermarket conditions. Food Packag. Shelf Life 2020, 24, 100492. [Google Scholar] [CrossRef]
- Jung, R.; Leyh, B.; Patz, C.; Rothermel, A.; Schuessler, C. Potential wine ageing during transportation. BIO Web Conf. 2014, 3, 02004. [Google Scholar] [CrossRef]
- Crandles, M.; Wicks-Müller, M.; Schuessler, C.; Jung, R. The Effect of Simulated Transportation Conditions on the Chemical, Physical and Sensory Profiles of Müller-Thurgau and Scheurebe Wines. J. Food Sci. Eng. 2016, 6, 177–196. [Google Scholar] [CrossRef]
- Chung, H.J.; Son, J.H.; Park, E.Y.; Kim, E.J.; Lim, S.T. Effect of vibration and storage on some physico-chemical properties of a commercial red wine. J. Food Compos. Anal. 2008, 21, 655–659. [Google Scholar] [CrossRef]
- Renner, H.; Richling, E.; Durner, D. Influence of Vibration on the Consumption of Oxygen and Sulfur Dioxide in Wine Bottles Considering Bottle Position and Headspace Volume. Am. J. Enol. Vitic. 2022, 73, 48–55. [Google Scholar] [CrossRef]
- Renner, H.; Richling, E.; Durner, D. Influence of Vibration on Volatile Compounds, Color, SO2 and CO2 of Riesling Sparkling Wine and White Wine. Am. J. Enol. Vitic. 2022, 73, ajev.2022.22007. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Li, H. Characterization of the effect of short-term high temperature and vibration on wine by quantitative descriptive analysis and solid phase microextraction-gas chromatography-mass spectrometry. Acta Aliment. 2018, 47, 236–244. [Google Scholar] [CrossRef]
- Ooi, B.T.; Banakar, M.H. Passive and active damper winding for the repulsive magnetic levitation system. IEEE Trans. Magn. 1977, 3, 5. [Google Scholar] [CrossRef]
- Sung, T.H.; Han, Y.H.; Lee, J.S.; Han, S.C.; Jeong, N.H.; Oh, K.S.; Park, B.S.; Oh, J.M. Effect of a passive magnetic damper in a flywheel system with a hybrid superconductor bearing set. IEEE Trans. Appl. Supercond. 2003, 13, 2165–2168. [Google Scholar] [CrossRef]
- Elbuken, C.; Khamesee, M.B.; Yavuz, M. Damping control in magnetic levitation of micro-objects. In Proceedings of the IECON Proceedings (Industrial Electronics Conference), Paris, France, 6–10 November 2006; pp. 4170–4175. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot blanc: Impact of the winemaking variables on the evolution of the phenolic, volatile and sensory profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Kim, C.M.; Lee, S.H.; Kim, W.S.; Jeon, T.I.; Park, K.H.; Moon, J.H. Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia Nakai) fruit. Food Sci. Biotechnol. 2013, 22, 803–810. [Google Scholar] [CrossRef]
- Kuhnert, N.; Jaiswal, R.; Matei, M.F.; Sovdat, T.; Deshpande, S. How to distinguish between feruloyl quinic acids and isoferuloyl quinic acids by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Petropulos, V.; Naceva, Z.; Sándor, V.; Makszin, L.; Deutsch-Nagy, L.; Berkics, B.; Stafilov, T.; Kilár, F. Fast determination of lactic, succinic, malic, tartaric, shikimic, and citric acids in red Vranec wines by CZE-ESI-QTOF-MS. Electrophoresis 2018, 39, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, J.D.; Nagel, C.W. Isolation and identification of the hydroxycinnamic acid derivatives in white Riesling wine. Am. J. Enol. Vitic. 1981, 32, 5–13. [Google Scholar]
- Fayad, S.; Le Scanff, M.; Waffo-Teguo, P.; Marchal, A. Understanding sweetness of dry wines: First evidence of astilbin isomers in red wines and quantitation in a one-century range of vintages. Food Chem. 2021, 352, 129293. [Google Scholar] [CrossRef] [PubMed]
- Tchouakeu Betnga, P.F.; Longo, E.; Poggesi, S.; Boselli, E. Effects of transport conditions on the stability and sensory quality of wines. OENO One 2021, 55, 197–208. [Google Scholar] [CrossRef]

| Descriptors | Definition |
|---|---|
| Visual evaluation | |
| Clarity | Absence of veiling and suspension in the wine |
| Colour tonality | Tonality of the colour red |
| Colour intensity | Intensity of the colour red |
| Olfactory evaluation | |
| Overall intensity | Total intensity of odour perceived through the nose |
| Red fruit | Strawberry, blueberries, raspberry, blackcurrant |
| Dried fruit | Strawberry jam, raisin, prune, fig |
| Undergrowth | Mushroom, wet wood, musk, fern |
| Spicy | Clove, black pepper, star anise, liquorice |
| Woody | Vanilla, oak, coffee |
| Cleanness | Absence of faults/taints odours |
| Off-odours | Presence of faults/taints odours |
| Gustatory evaluation | |
| Warmness | The sensation of alcohol (hot) in-mouth |
| Sweetness | Taste of sucrose |
| Sourness | Taste of acid solution |
| Saltiness | Taste of sodium chloride solution |
| Bitterness | Taste of caffeine solution |
| Astringency | Tactile sensation related to the dryness in the mouth |
| Cleanness | Absence of faults/taints flavours |
| Unpleasant flavours | Presence of faults/taints flavours |
| Overall quality judgment | An evaluation of the global quality of wine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggesi, S.; Merkytė, V.; Longo, E.; Boselli, E. Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage. Foods 2022, 11, 2761. https://doi.org/10.3390/foods11182761
Poggesi S, Merkytė V, Longo E, Boselli E. Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage. Foods. 2022; 11(18):2761. https://doi.org/10.3390/foods11182761
Chicago/Turabian StylePoggesi, Simone, Vakarė Merkytė, Edoardo Longo, and Emanuele Boselli. 2022. "Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage" Foods 11, no. 18: 2761. https://doi.org/10.3390/foods11182761
APA StylePoggesi, S., Merkytė, V., Longo, E., & Boselli, E. (2022). Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage. Foods, 11(18), 2761. https://doi.org/10.3390/foods11182761







