Characterization of Two Zymomonas mobilis Wild Strains and Analysis of Populations Dynamics during Their Leavening of Bread-like Doughs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Maintenance
2.2. Z. mobilis Growth Kinetics
2.3. Z. mobilis Biomass Production
2.4. Z. mobilis Sensitivity to NaCl: MIC and MBC
2.5. Doughs Production
- -
- 250 g, inserted into a 1 L graduate cylinder to evaluate the dough volume increase every 30 min for up to 6 h of leavening.
- -
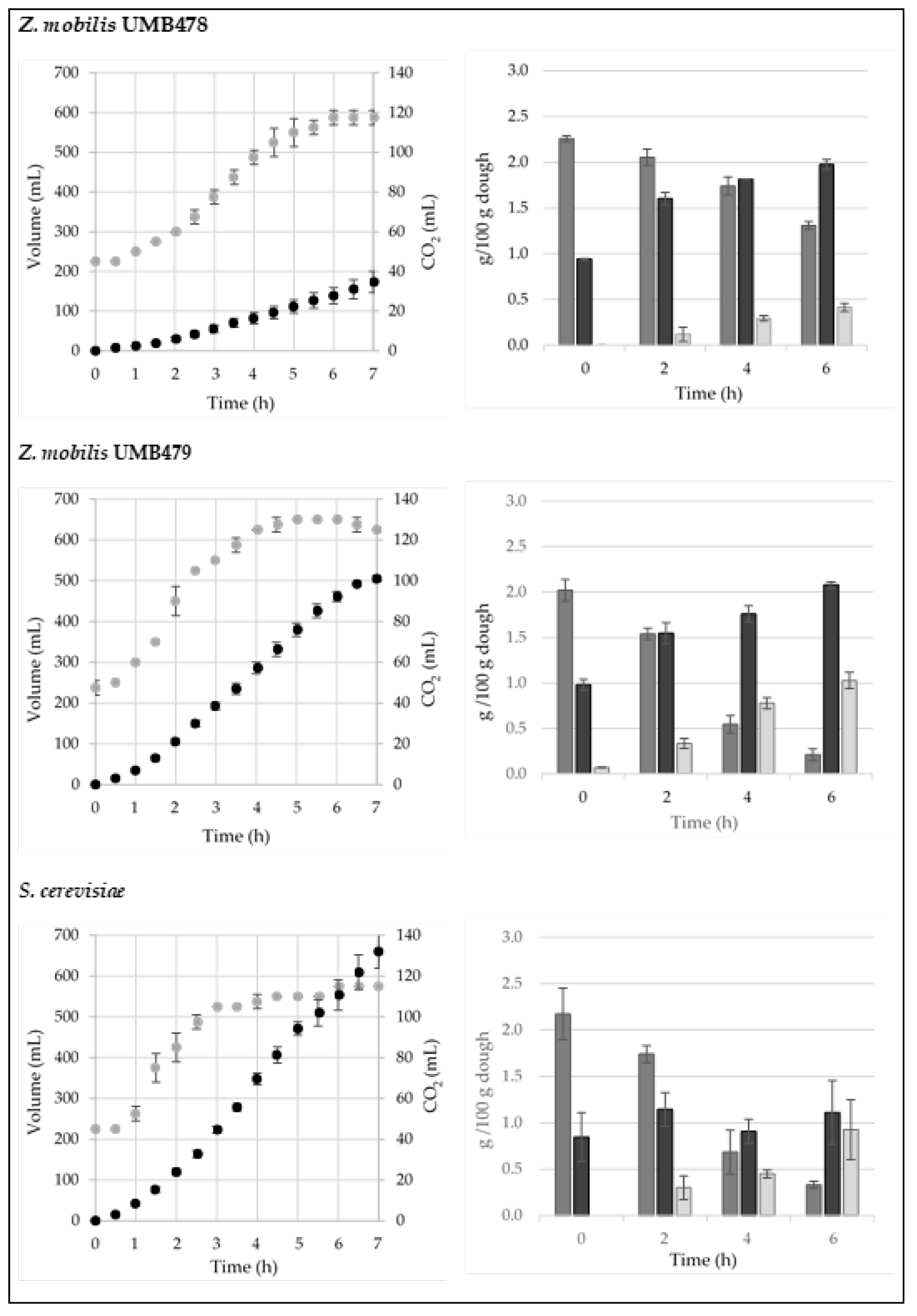
- 25 g, inserted into a double chamber flask connected to a graduate burette filled with 0.1 M NaCl, to evaluate the total amount of CO2 produced every 30 min for up to 6 h of leavening. Two other unconventional indices were extrapolated from the dough development curves: the lag leavening time (LLT, h), defined as the time before an increase in dough height was noticed, the leavening rate (LR, mm/h), as the slope of the first linear part of the curve after LLT, and maximum dough volume (DV max, mL) [12].
- -
- The remaining sample was left to leaven in 4 closed sterile containers, one for each sampling time. The doughs were incubated at 30 °C and samples were taken at 0, 2, 4 and 6 h to determine dough pH and to perform microbiological and HPLC (High-Performance Liquid Chromatography) analyses.
2.6. Microbiological Analyses
2.7. Analytical Determinations
2.8. Automated Ribosomal Intergenic Spacer Analysis (ARISA)
2.9. Statistical Analysis
3. Results and Discussion
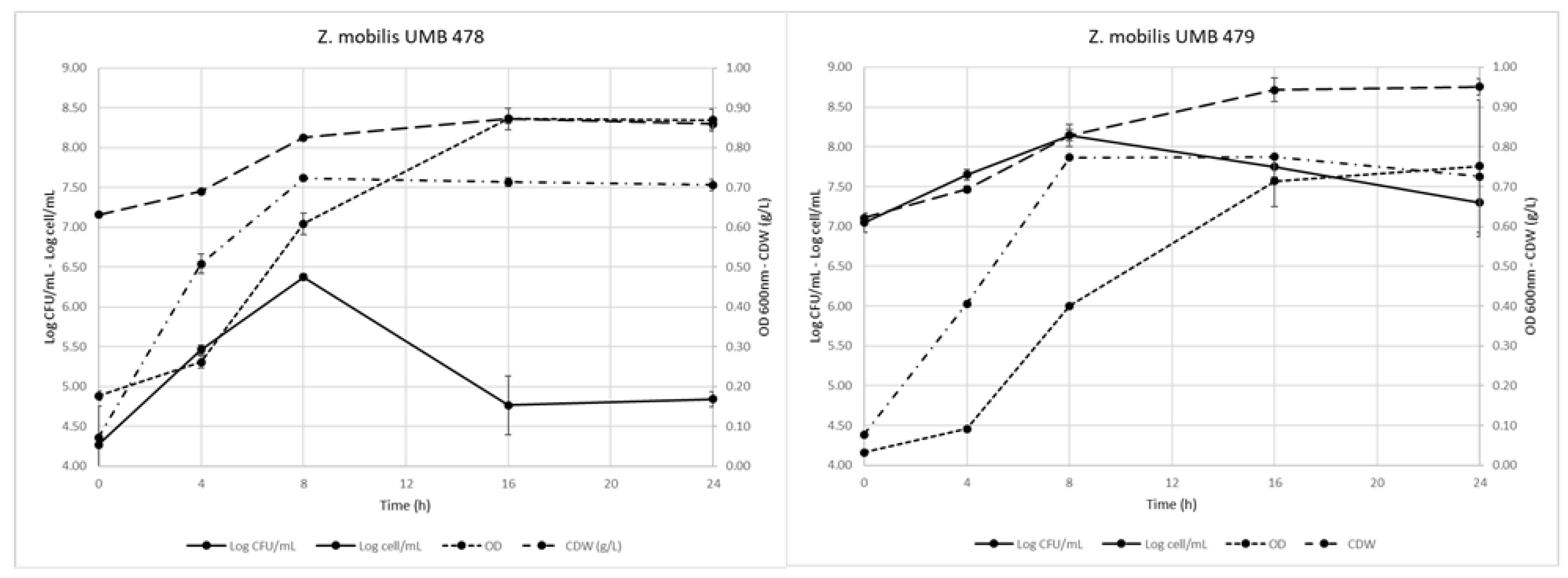
3.1. Z. mobilis Growth and Biomass Production
3.2. Strains Sensitivity to NaCl: MIC and MBC
3.3. Doughs Production
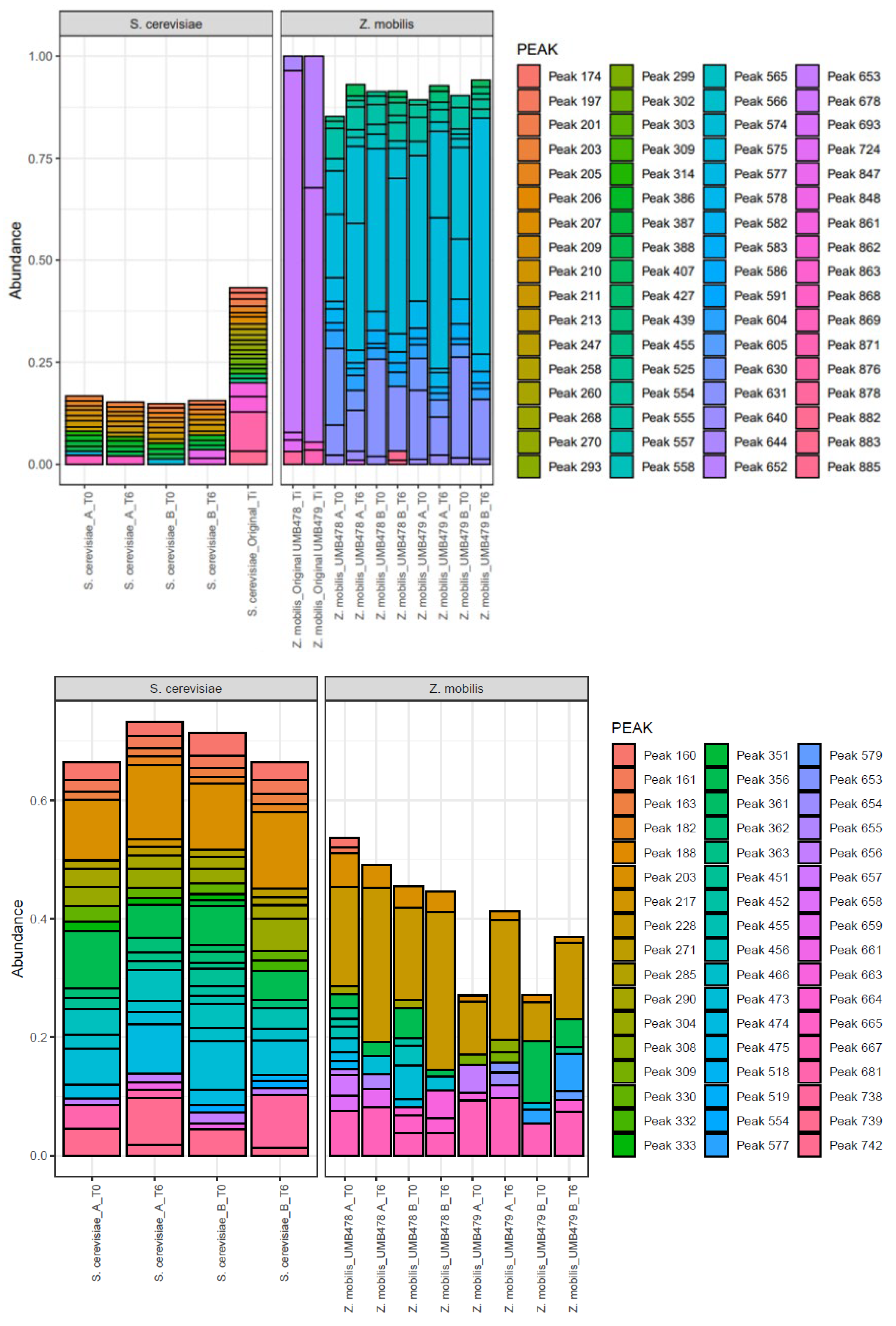
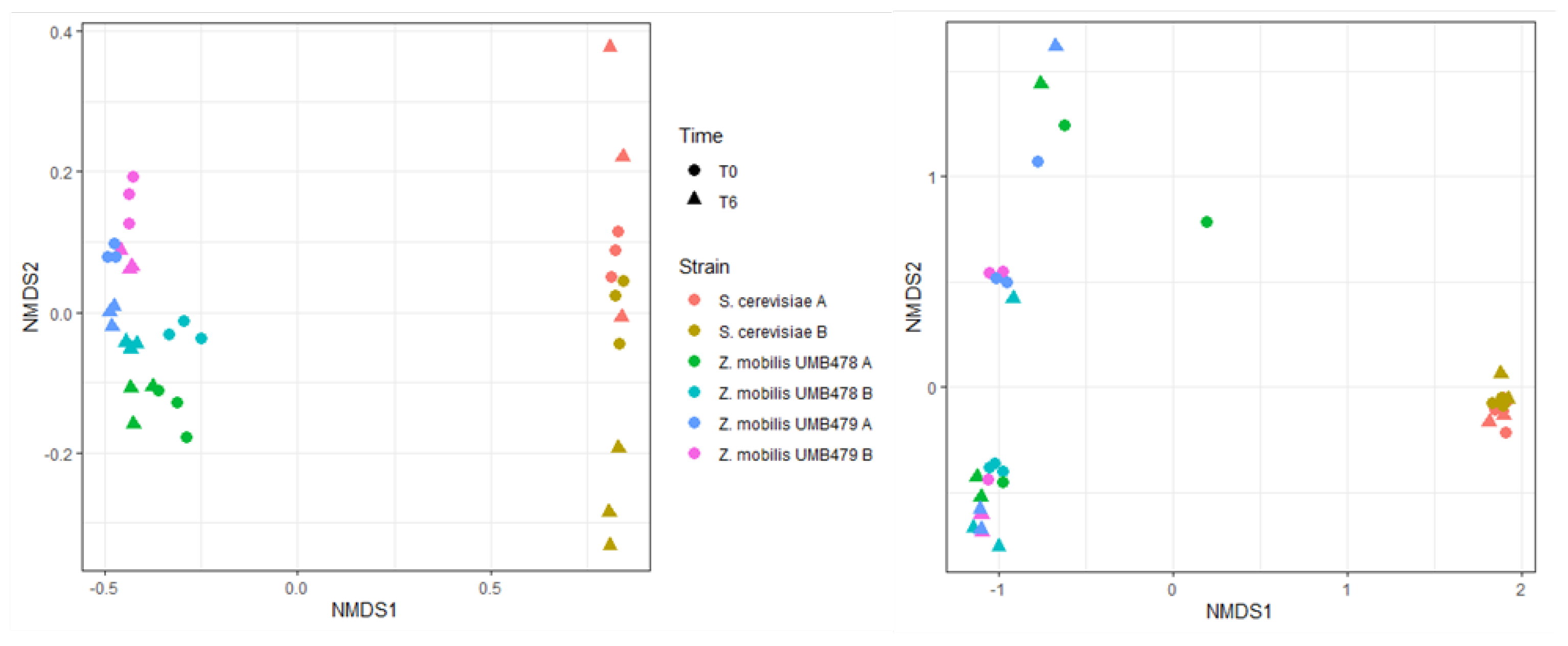
3.4. Population Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Target | Primers (Sequence 5′→3′) | PCR Condition | References |
|---|---|---|---|
| 16S rRNA (Bacteria) | F: ITSF GTCGTAACAAGGTAGCCGTA R: ITSReub-6FAM GCCAAGGCATCCACC | 94 °C 3 min; 35 cycles: 94 °C 45 s, 55 °C 1 min, 72 °C 2 min; 72 °C 10 min. 30 µL TOT: 3 µL DNA, 15 µL DreamTaq (2×) (Thermo Fisher Scientific, USA), 0.6 µL of each primer 10 µM, 10.8 µL H2O. | [33] |
| Internal Transcribed Spacer region (ITS) (Fungi) | F: ITS1F-6FAM CTTGGTCATTTAGAGGAAGTAA R: ITS4 TCCTCCGCTTATTGATATGC | 95 °C 2 min; 35 cycles: 95 °C 1 min, 53 °C 30 s, 72 °C 1 min; 72 °C 10 min. 25 µL TOT: 3 µL DNA, 15 µL DreamTaq (2×) (Thermo Fisher Scientific, USA), 0.75 µL of each primer at 10 µM, 5.5 µL H2O. | [34,35,36] |
References
- Sicard, D.; Legras, J.-L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. Comptes Rendus-Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Perricone, R.; Blank, M.; Perricone, C.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae autoantibodies in autoimmune diseases: From bread baking to autoimmunity. Clin. Rev. Allergy Immunol. 2013, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Salamati, S.; Martins, C.; Kulseng, B. Baker’s yeast (Saccharomyces cerevisiae) antigen in obese and normal weight subjects. Clin. Obes. 2015, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.A.; Tadros, S.; Bansal, A.S. Beer, Cider, and Wine Allergy. Case Reports Immunol. 2017, 2017, 7958924. [Google Scholar] [CrossRef]
- Colboc, H.; Fite, C.; Cannistra, C.; Chaby, G.; Maillard, H.; Bouscarat, F.; Crickx, B.; Gabison, G.; Ezzedine, K.; Descamps, V.; et al. Interest of Brewer’s Yeast-Exclusion Diet in the Management of Hidradenitis Suppurativa. J. Clin. Exp. Dermatol. Res. 2016, 7, 1000371. [Google Scholar]
- Panesar, P.S.; Marwaha, S.S.; Kennedy, J.F. Zymomonas mobilis: An alternative ethanol producer. J. Chem. Technol. Biotechnol. 2006, 81, 623–635. [Google Scholar] [CrossRef]
- Vriesekoop, F.; Rasmusson, M.; Pamment, N.B. Respective effects of sodium and chloride ions on filament formation and growth and ethanol production in Zymomonas mobilis fermentations. Lett. Appl. Microbiol. 2002, 35, 27–31. [Google Scholar] [CrossRef]
- Musatti, A.; Rollini, M.; Sambusiti, C.; Manzoni, M. Zymomonas mobilis: Biomass production and use as a dough leavening agent. Ann. Microbiol. 2015, 65, 1583–1589. [Google Scholar] [CrossRef]
- Musatti, A.; Cappa, C.; Mapelli, C.; Alamprese, C.; Rollini, M. Zymomonas mobilis in Bread Dough: Characterization of Dough Leavening Performance in Presence of Sucrose. Foods 2020, 9, 89. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Rollini, M.; Foschino, R.; Picozzi, C. Can Zymomonas mobilis Substitute Saccharomyces cerevisiae in Cereal Dough Leavening? Foods 2018, 7, 61. [Google Scholar] [CrossRef]
- Nissen, L.; Rollini, M.; Picozzi, C.; Musatti, A.; Foschino, R.; Gianotti, A. Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach. Microorganisms 2020, 8, 792. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Foschino, R.; Picozzi, C.; Rollini, M. Unconventional bacterial association for dough leavening. Int. J. Food Microbiol. 2016, 237, 28–34. [Google Scholar] [CrossRef]
- Pancher, M.; Ceol, M.; Corneo, P.E.; Longa, C.M.O.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal endophytic communities in grapevines (Vitis vinifera L.) Respond to crop management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef]
- Rees, G.N.; Baldwin, D.S.; Watson, G.O.; Perryman, S.; Nielsen, D.L. Ordination and significance testing of microbial community composition derived from terminal restriction fragment length polymorphisms: Application of multivariate statistics. Int. J. Gen. Mol. Microbiol. 2005, 86, 339–347. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 September 2021).
- Martinez Arbizu, P. PairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.4. 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 1 September 2021).
- Sprenger, G.A. Carbohydrate metabolism in Zymomonas mobilis: A catabolic highway with some scenic routes. FEMS Microbiol. Lett. 1996, 145, 301–307. [Google Scholar] [CrossRef]
- Fuchino, K.; Bruheim, P. Increased salt tolerance in Zymomonas mobilis strain generated by adaptative evolution. Microb. Cell Fact. 2020, 19, 147. (accessed on 1 September 2021). [Google Scholar] [CrossRef]
- Oda, Y.; Tonomura, K. Dough-leavening by Zymomonas mobilis and Its Application to Breadmaking. J. Food Sci. 1994, 59, 171–174. [Google Scholar] [CrossRef]
- Wang, J.L.; Wu, B.; Qin, H.; You, Y.; Liu, S.; Shui, Z.X.; Tan, F.R.; Wang, Y.W.; Zhu, Q.L.; Li, Y.; et al. Engineered Zymomonas mobilis for salt tolerance using EZ-Tn5-based transposon insertion mutagenesis system. Microb. Cell Fact. 2016, 15, 101. [Google Scholar] [CrossRef]
- van Dorst, J.; Bissett, A.; Palmer, A.S.; Brown, M.; Snape, I.; Stark, J.S.; Raymond, B.; McKinlay, J.; Ji, M.; Winsley, T.; et al. Community fingerprinting in a sequencing world. FEMS Microbiol. Ecol. 2014, 89, 316–330. [Google Scholar] [CrossRef]
- Pioli, S.; Antonucci, S.; Giovannelli, A.; Traversi, M.L.; Borruso, L.; Bani, A.; Brusetti, L.; Tognetti, R. Community fingerprinting reveals increasing wood-inhabiting fungal diversity in unmanaged Mediterranean forests. For. Ecol. Manag. 2018, 408, 202–210. [Google Scholar] [CrossRef]
- Arteau, M.; Labrie, S.; Roy, D. Terminal-restriction fragment length polymorphism and automated ribosomal intergenic spacer analysis profiling of fungal communities in Camembert cheese. Int. Dairy J. 2010, 20, 545–554. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.F.; Setati, M.E. The impact of Saccharomyces cerevisiae on a wine yeast consortium in natural and inoculated fermentations. Front. Microbiol. 2017, 8, 1988. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Di Cagno, R.; Gallo, G.; Curci, M.; Siragusa, S.; Crecchio, C.; Parente, E.; Gobbetti, M. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 2007, 114, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P.; Brandt, M.J.; Francis, K.L.; Rosenheim, J.; Seitter, M.F.H.; Vogelmann, S.A. Microbial ecology of cereal fermentations. Trends Food Sci. Technol. 2005, 16, 4–11. [Google Scholar] [CrossRef]
- Chiva, R.; Celador-Lera, L.; Uña, J.A.; Jiménez-López, A.; Espinosa-Alcantud, M.; Mateos-Horganero, E.; Vega, S.; Santos, M.Á.; Velázquez, E.; Tamame, M. Yeast biodiversity in fermented doughs and raw cereal matrices and the study of technological traits of selected strains isolated in Spain. Microorganisms 2021, 9, 47. [Google Scholar] [CrossRef]
- Alcántara-Hernández, R.J.; Rodríguez-Álvarez, J.A.; Valenzuela-Encinas, C.; Gutiérrez-Miceli, F.A.; Castañón-González, H.; Marsch, R.; Ayora-Talavera, T.; Dendooven, L. The bacterial community in “taberna” a traditional beverage of Southern Mexico. Lett. Appl. Microbiol. 2010, 51, 558–563. [Google Scholar] [CrossRef]
- Misery, B.; Legendre, P.; Rue, O.; Bouchart, V.; Guichard, H.; Laplace, J.M.; Cretenet, M. Diversity and dynamics of bacterial and fungal communities in cider for distillation. Int. J. Food Microbiol. 2021, 339, 108987. [Google Scholar] [CrossRef]
- Weir, P.M. The ecology of Zymomonas: A review. Folia Microbiol. 2016, 61, 385–392. [Google Scholar] [CrossRef]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Zanardini, E.; Sorlini, C.; Corselli, C.; Daffonchio, D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef] [Green Version]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Gleeson, D.B.; Clipson, N.; Melville, K.; Gadd, G.M.; McDermott, F.P. Characterization of fungal community structure on a weathered pegmatitic granite. Microb. Ecol. 2005, 50, 360–368. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar]

| Carbon Source | Strain | Inoculum (% v/v) | Lag Phase (h) | Growth Rate (OD/h) | Yend (OD) |
|---|---|---|---|---|---|
| Glucose | UMB478 | 5 | 4.32 ± 0.05 | 0.12 ± 0.00 | 0.887 ± 0.008 |
| 10 | 2.76 ± 0.18 | 0.12 ± 0.01 | 0.949 ± 0.035 | ||
| UMB479 | 5 | 4.47 ± 0.27 | 0.10 ± 0.01 | 0.810 ± 0.046 | |
| 10 | 2.60 ± 0.42 | 0.10 ± 0.02 | 0.832 ± 0.074 | ||
| Fructose | UMB478 | 5 | 6.06 ± 0.20 | 0.09 ± 0.01 | 0.850 ± 0.057 |
| 10 | 3.49 ± 0.18 | 0.09 ± 0.00 | 0.909 ± 0.018 | ||
| UMB479 | 5 | 5.19 ± 0.55 | 0.08 ± 0.01 | 0.815 ± 0.077 | |
| 10 | 4.10 ± 0.44 | 0.08 ± 0.00 | 0.736 ± 0.020 | ||
| Sucrose | UMB478 | 5 | 5.14 ± 0.09 | 0.11 ± 0.01 | 0.923 ± 0.049 |
| 10 | 3.51 ± 0.26 | 0.14 ± 0.02 | 1.023 ± 0.041 | ||
| UMB479 | 5 | 5.20 ± 0.17 | 0.11 ± 0.01 | 0.985 ± 0.018 | |
| 10 | 3.20 ± 0.34 | 0.12 ± 0.00 | 1.078 ± 0.012 |
| NaCl (g/L) | Lag Phase (%) | Growth Rate (%) | Yend (OD) (%) | R2 |
|---|---|---|---|---|
| 1.25 | −1.1 ± 8.1 a | −14.5 ± 4.5 c | −17.5 ± 0.3 d | 0.995 |
| 2.5 | −7.4 ± 7.9 a | −25.3 ± 3.0 b | −23.0 ± 0.3 b | 0.996 |
| 3.75 | −6.4 ± 11.0 a | −33.7 ± 4.0 b | −18.0 ± 0.5 d | 0.989 |
| 5 | +8.2 ± 5.9 a | −21.7 ± 3.1 b | −20.7 ± 0.3 c | 0.999 |
| 7.5 | +13.7 ± 9.8 a | −36.1 ± 2.7 b | −23.2 ± 0.3 b | 0.994 |
| 10 | +55.6 ± 11.3 b | −37.4 ± 3.9 b | −14.9 ± 0.3 e | 0.994 |
| 15 | +75.2 ± 13.9 b | −79.5 ± 1.7 a | −61.0 ± 0.3 a | 0.994 |
| NaCl (g/L) | Lag Phase (%) | Growth Rate (%) | Yend (OD) (%) | R2 |
|---|---|---|---|---|
| 1.25 | −8.1 ± 3.8 a | −6.4 ± 3.1 c | −3.9 ± 0.5 c | 0.998 |
| 2.5 | −13.5 ± 3.6 a | −12.8 ± 2.9 b | −9.7 ± 0.5 a | 0.998 |
| 3.75 | −14.4 ± 3.6 a | −13.8 ± 2.9 b | −10.6 ± 0.5 a | 0.998 |
| 5 | −12.3 ± 3.7 a | −7.5 ± 4.1 bc | −3.6 ± 0.5 c | 0.998 |
| 7.5 | +3.5 ± 4.3 b | −6.4 ± 4.1 bc | −7.2 ± 0.5 b | 0.997 |
| 10 | +12.1 ± 4.9 c | −3.2 ± 4.2 c | +8.8 ± 0.7 d | 0.997 |
| 15 | +47.0 ± 5.3 d | −46.8 ± 2.2 a | −5.2 ± 0.5 c | 0.999 |
| Microrganism | LLT (h) | LR (mL/h) | DV Max (mL) | R2 |
|---|---|---|---|---|
| Z. mobilis UMB478 | 1.2 ± 0.1 | 92.1 ± 12.8 | 586.5 ± 13.8 | 0.999 |
| Z. mobilis UMB479 | 0.6 ± 0.0 | 139.1 ± 2.3 | 642.7 ± 8.2 | 1.000 |
| S. cerevisiae | 0.6 ± 0.1 | 153.6 ± 10.6 | 551.1 ± 5.2 | 0.999 |
| Strain | Time (h) | TBC (Log CFU/g) | Y&M (Log CFU/g) | Z. mobilis (Log CFU/g) | pH |
|---|---|---|---|---|---|
| Z. mobilis UMB478 | 0 | 5.21 ± 0.07 | 2.72 ± 0.09 | 5.66 ± 0.19 | 5.89± 0.01 c |
| 6 | 4.71 ± 0.08 ab | 2.32 ± 0.19 | 5.65 ± 0.02 | 5.27 ± 0.04 | |
| Z. mobilis UMB479 | 0 | 5.15 ± 0.11 | 2.23 ± 0.14 | 8.41 ± 0.17 | 5.73 ± 0.01 b |
| 6 | 4.63 ± 0.16 b | 1.57 ± 0.24 | 8.39 ± 0.19 | 5.14 ± 0.01 | |
| S. cerevisiae | 0 | 5.28 ± 0.09 | 7.28 ± 0.29 | - | 5.31 ± 0.02 a |
| 6 | 5.07 ± 0.02 a | 7.37 ± 0.17 | - | 5.13 ± 0.10 |
| Dough Sample | Fungal | Bacterial | ||||||
|---|---|---|---|---|---|---|---|---|
| Species Richness | Shannon | Simpson | Pielou’s Evenness | Species Richness | Shannon | Simpson | Pielou’s Evenness | |
| Z. mobilis UMB478 A t0 | 106 ± 49 | 2.7 ± 0.5 | 0.8 ± 0.1 | 0.6 ± 0.1 | 274 ± 22 | 4.4 ± 0.1 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| Z. mobilis UMB478 A t6 | 40 ± 2 | 2.2 ± 0.1 | 0.7 ± 0.0 | 0.6 ± 0.0 | 291 ± 13 | 4.2 ± 0.0 | 0.9 ± 0.0 | 0.7 ± 0.0 |
| Z. mobilis UMB478 B t0 | 63 ± 3 | 2.3 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 278 ± 5 | 4.6 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| Z. mobilis UMB478 B t6 | 55 ± 29 | 2.3 ± 0.5 | 0.7 ± 0.1 | 0.6 ± 0.0 | 269 ± 11 | 4.3 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 |
| Z. mobilis UMB479 A t0 | 70 ± 4 | 2.4 ± 0.1 | 0.8 ± 0.0 | 0.6 ± 0.0 | 344 ± 11 | 5.1 ± 0.2 | 1.0 ± 0.0 | 0.9 ± 0.0 |
| Z. mobilis UMB479 A t6 | 34 ± 3 | 1.9 ± 0.1 | 0.7 ± 0.0 | 0.5 ± 0.0 | 310 ± 13 | 4.6 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| Z. mobilis UMB479 B t0 | 68 ± 15 | 2.3 ± 0.2 | 0.8 ± 0.0 | 0.6 ± 0.0 | 313 ± 12 | 5.1 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.0 |
| Z. mobilis UMB479 B t6 | 35 ± 13 | 1.9 ± 0.1 | 0.7 ± 0.0 | 0.5 ± 0.0 | 326 ± 11 | 4.9 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.0 |
| S. cerevisiae | 162 ± 0 | 4.7 ± 0 | 1.0 ± 0 | 0.9 ± 0 | - | - | - | - |
| S. cerevisiae A t0 | 214 ± 29 | 5.2 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.0 | 236 ± 23 | 4.2 ± 0.1 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| S. cerevisiae A t6 | 219 ± 19 | 5.2 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 | 179 ± 17 | 4.1 ± 0.1 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| S. cerevisiae B t0 | 243 ± 13 | 5.3 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 245 ± 2 | 4.3 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 |
| S. cerevisiae B t6 | 274 ± 29 | 5.4 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 207 ± 15 | 4.2 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picozzi, C.; Clagnan, E.; Musatti, A.; Rollini, M.; Brusetti, L. Characterization of Two Zymomonas mobilis Wild Strains and Analysis of Populations Dynamics during Their Leavening of Bread-like Doughs. Foods 2022, 11, 2768. https://doi.org/10.3390/foods11182768
Picozzi C, Clagnan E, Musatti A, Rollini M, Brusetti L. Characterization of Two Zymomonas mobilis Wild Strains and Analysis of Populations Dynamics during Their Leavening of Bread-like Doughs. Foods. 2022; 11(18):2768. https://doi.org/10.3390/foods11182768
Chicago/Turabian StylePicozzi, Claudia, Elisa Clagnan, Alida Musatti, Manuela Rollini, and Lorenzo Brusetti. 2022. "Characterization of Two Zymomonas mobilis Wild Strains and Analysis of Populations Dynamics during Their Leavening of Bread-like Doughs" Foods 11, no. 18: 2768. https://doi.org/10.3390/foods11182768
APA StylePicozzi, C., Clagnan, E., Musatti, A., Rollini, M., & Brusetti, L. (2022). Characterization of Two Zymomonas mobilis Wild Strains and Analysis of Populations Dynamics during Their Leavening of Bread-like Doughs. Foods, 11(18), 2768. https://doi.org/10.3390/foods11182768








