Pulsed Light Application for Campylobacter Control on Poultry Meat and Its Effect on Colour and Volatile Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Preparation of Bacterial Suspensions for Inoculation
2.2. Poultry Meat Sample Preparation
2.3. Pulsed-Light Treatments
2.4. Microbial Analysis
2.5. Physicochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
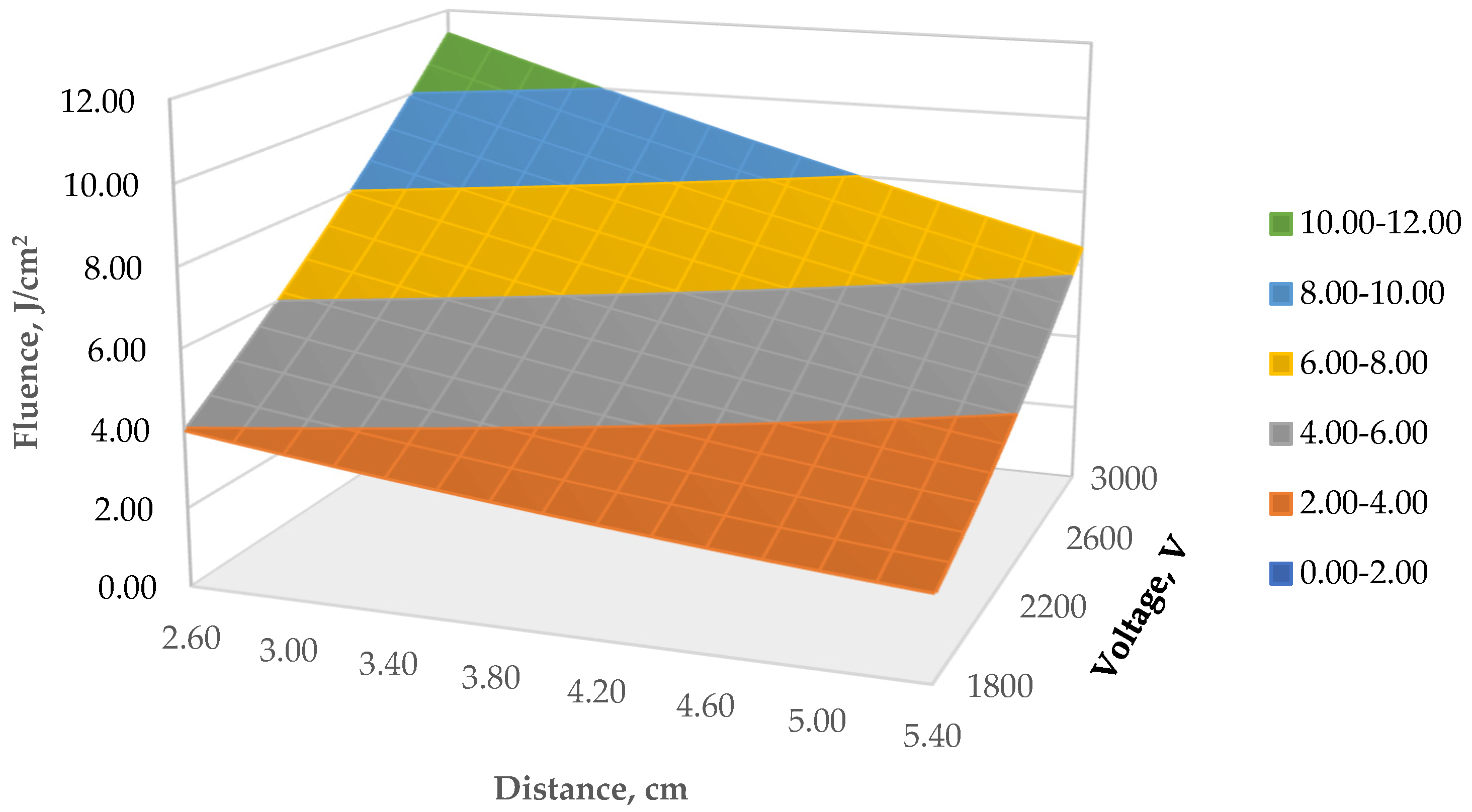
3.1. Pulsed-Light Treatment: Energy Irradiation Received on Samples
3.2. Effect of Pulsed-Light Treatment on Spoilage Microorganisms and Campylobacter
3.2.1. Spoilage Microorganisms
3.2.2. Campylobacter
3.3. Effect of Pulsed Light Treatment on Poultry Meat Colour and Volatile Aldehydes Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Treatment | Voltage (V) | Distance (cm) | Fluence (J/cm2) | Campylobacter Log cfu/g | Enterobacteriaceae Log cfu/g | Psycrotrophic Log cfu/g | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 4.9 ± 0.1 ab | 2.5 ± 0.3 ab | 3.1 ± 0.4 | 48.84 ± 1.08 | 3.76 ± 0.98 c | 22.40 ± 1.95 |
| 1 | 2414.25 | 2.6 | 7.26 ± 0.27 | 4.8 ± 0.1 abc | 2.9 ± 0.3 a | 3.2 ± 0.4 | 52.17 ± 1.08 | 6.76 ± 0.98 ab | 27.24 ± 1.95 |
| 2 | 2000.00 | 3.0 | 4.61 ± 0.13 | 4.6 ± 0.1 bc | 1.6 ± 0.3 bcd | 2.3 ± 0.3 | 53.39 ± 1.08 | 7.19 ± 0.98 ab | 28.28 ± 1.95 |
| 3 | 2828.50 | 3.0 | 9.68 ± 0.15 | 4.5 ± 0.1 c | 3.1 ± 0.3 a | 2.6 ± 0.3 | 50.85 ± 1.08 | 7.33 ± 0.98 ab | 25.40 ± 1.95 |
| 4 | 1828.41 | 4.0 | 3.15 ± 0.04 | 4.8 ± 0.1 abc | 1.7 ± 0.3 bcd | 2.8 ± 0.3 | 50.73 ± 1.08 | 7.22 ± 0.98 ab | 26.61 ± 1.95 |
| 5 | 2414.25 | 4.0 | 5.51 ± 0.06 | 4.6 ± 0.1 bc | 1.6 ± 0.3 abcd | 2.1 ± 0.3 | 52.69 ± 1.08 | 7.21 ± 0.98 ab | 28.27 ± 1.95 |
| 6 | 2414.25 | 4.0 | 5.92 ± 0.03 | 4.9 ± 0.1 ab | 1.9 ± 0.3 bc | 2.8 ± 0.3 | 50.65 ± 1.08 | 6.92 ± 0.98 ab | 26.60 ± 1.95 |
| 7 | 2414.25 | 4.0 | 5.37 ± 0.24 | 4.8 ± 0.1 abc | 1.4 ± 0.3 cd | 2.7 ± 0.4 | 50.39 ± 1.08 | 5.81 ± 0.98 bc | 24.81 ± 1.95 |
| 8 | 3000.09 | 4.0 | 8.53 ± 0.19 | 5.0 ± 0.1 a | 1.6 ± 0.3 cd | 3.1 ± 0.4 | 51.65 ± 1.08 | 7.32 ± 0.98 ab | 27.18 ± 1.95 |
| 9 | 2000.00 | 5.0 | 2.82 ± 0.06 | 5.0 ± 0.1 a | 1.2 ± 0.3 d | 2.7 ± 0.4 | 49.66 ± 1.08 | 7.94 ± 0.98 ab | 27.22 ± 1.95 |
| 10 | 2828.50 | 5.0 | 6.38 ± 0.06 | 5.0 ± 0.1 a | 1.5 ± 0.3 cd | 2.6 ± 0.3 | 50.34 ± 1.08 | 9.12 ± 0.98 a | 28.86 ± 1.95 |
| 11 | 2414.25 | 5.4 | 4.06 ± 0.05 | 5.0 ± 0.1 a | 1.4 ± 0.3 cd | 2.7 ± 0.5 | 52.22 ± 1.08 | 5.44 ± 0.98 bc | 24.58 ± 1.95 |
| Sig. | - | - | - | * | ** | NS | NS | * | NS |
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Walter, E.J.S.; Crim, S.M.; Bruce, B.B.; Griffin, P.M. Incidence of Campylobacter-Associated Guillain-Barré Syndrome Estimated from Health Insurance Data. Foodborne Pathog. Dis. 2020, 17, 23–28. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Regulation (E.C.) No. 2017/1495; Commission Regulation (E.U.) 2017/1495 of 23rd August 2017 Amending Regulation (E.C.) No 2073/2005 as Regards Campylobacter in Broiler Carcases. Official Journal of the European Union: Brussels, Belgium, 2017. Available online: http://data.europa.eu/eli/reg/2017/1495/oj. (accessed on 5 March 2022).
- Lindqvist, R.; Lindblad, M. Quantitative risk assessment of thermophilic Campylobacter spp. and cross-contamination during handling of raw broiler chickens evaluating strategies at the producer level to reduce human Campylobacteriosis in Sweden. Int. J. Food Microbiol. 2008, 121, 41–52. [Google Scholar] [CrossRef]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Fanning, S.; Whyte, P. Effect of crust freezing applied alone and in combination with ultraviolet light on the survival of Campylobacter on raw chicken. Food Micro. 2012, 32, 147–151. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Health and Food Safety. Mitigation Measures in Place for Campylobacter spp. in Poultry: Overview Report; Publications Office: Brussels, Belgium, 2018; p. 44. Available online: https://data.europa.eu/doi/10.2875/894648 (accessed on 13 January 2022).
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar] [CrossRef]
- Roobab, U.; Afzal, R.; Ranjha, M.M.A.N.; Zeng, X.-A.; Ahmed, Z.; Aadil, R.M. High pressure-based hurdle interventions for raw and processed meat: A clean-label prospective. Int. J. Food Sci. Technol. 2022, 57, 816–826. [Google Scholar] [CrossRef]
- Rahman, U.; Sahar, A.; Ishaq, A.; Aadil, R.M.; Zahoor, T.; Ahmad, M.H. Advanced meat preservation methods: A mini review. J. Food Saf. 2018, 38, e12467. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J.; Pulsed, U.V. As a potential surface sanitizer in food production processes to ensure consumer safety. Curr. Opin. Food Sci. 2019, 26, 65–70. [Google Scholar] [CrossRef]
- Cheigh, C.-I.; Hwang, H.-J.; Chung, M.-S. Intense pulsed light (IPL) and UV-C treatments for inactivating Listeria monocytogenes on solid medium and seafoods. Food Res. Int. 2013, 54, 745–752. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Seo, J.-H.; Jeong, C.; Cheigh, C.-I.; Chung, M.-S. Analysis of bacterial inactivation by intense pulsed light using a double-Weibull survival model. Innov. Food Sci. Emerg. Technol. 2019, 56, 102185. [Google Scholar] [CrossRef]
- Kramer, B.; Wunderlich, J.; Muranyi, P. Recent findings in pulsed light disinfection. J. Appl. Microbiol. 2017, 122, 830–856. [Google Scholar] [CrossRef]
- McLeod, A.; Hovde Liland, K.; Haugen, J.-E.; Sørheim, O.; Myhrer, K.S.; Holck, A.L. Chicken fillets subjected to UV-C and pulsed U.V. light: Reduction of pathogenic and spoilage bacteria, and changes in sensory quality. J. Food Saf. 2018, 38, e12421. [Google Scholar] [CrossRef]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J.; Fanning, S.; Whyte, P. Efficacy of U.V. Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J. Food Prot. 2011, 74, 565–572. [Google Scholar] [CrossRef]
- Cassar, J.R.; Mills, E.W.; Campbell, J.A.; Demirci, A. Decontamination of Chicken Thigh Meat by Pulsed Ultraviolet Light. Meat Muscle Biol. 2019, 3, 479–487. [Google Scholar] [CrossRef]
- Araújo, P.M.; Batista, E.; Fernandes, M.H.; Fernandes, M.J.; Gama, L.T.; Fraqueza, M.J. Assessment of biofilm formation by Campylobacter spp. isolates mimicking poultry slaughterhouse conditions. Poult. Sci. 2022, 101, 101586. [Google Scholar] [CrossRef]
- Santos, D.I.; Fraqueza, M.J.; Pissarra, H.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut. Foods 2020, 9, 1752. [Google Scholar] [CrossRef]
- ISO 6887-1; Microbiology of the Food Chain—Preparation of Test Samples. Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 2: Specific Rules for the Preparation of Meat and Meat Products. International Organization for Standardization ISO Standards: Geneva, Switzerland, 2019.
- ISO 21528-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization ISO Standards: Geneva, Switzerland, 2017.
- ISO 10272-2; Microbiology of the Food Chain–Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 2: Colony-Count Technique. International Organization for Standardization ISO Standards: Geneva, Switzerland, 2017.
- Serra, A.; Buccioni, A.; Rodriguez-Estrada, M.T.; Conte, G.; Cappucci, A.; Mele, M. Fatty acid composition, oxidation status and volatile organic compounds in “Colonnata” lard from Large White or Cinta Senese pigs as affected by curing time. Meat Sci. 2014, 97, 504–512. [Google Scholar] [CrossRef]
- Hsu, L.; Moraru, C.I. Quantifying and mapping the spatial distribution of fluence inside a pulsed light treatment chamber and various liquid substrates. J. Food Eng. 2011, 103, 84–91. [Google Scholar] [CrossRef]
- Wambura, P.; Verghese, M. Effect of pulsed ultraviolet light on quality of sliced ham. LWT-Food Sci. Technol. 2011, 44, 2173–2179. [Google Scholar] [CrossRef]
- Lauritsen, C.V.; Kjeldgaard, J.; Ingmer, H.; Bisgaard, M.; Christensen, H. Microbiota encompassing putative spoilage bacteria in retail packaged broiler meat and commercial broiler abattoir. Int. J. Food Microbiol. 2019, 300, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.T.; Välitalo, H.; Säde, E.; Paloranta, A.; Koskinen, K.; Björkroth, J. The effect of marination on lactic acid bacteria communities in raw broiler fillet strips. Front. Microbiol. 2012, 3, 376. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 18, 464–473. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Tewari, J.C.; Irudayaraj, J.; Demirci, A. Microscopic and Spectroscopic Evaluation of Inactivation of Staphylococcus aureus by Pulsed, U.V. Light and Infrared Heating. Food Bioprocess Technol. 2010, 3, 93–104. [Google Scholar] [CrossRef]
- Chintagari, S.; Jadeja, R.; Hung, Y.-C. Resistance of various shiga-toxin producing Escherichia coli (STEC) strains and serogroups to infra-red and pulsed U.V. radiation and effect of nalidixic acid adaptation. LWT-Food Sci. Tech. 2019, 102, 356–363. [Google Scholar] [CrossRef]
- Keklik, N.M.; Demirci, A.; Puri, V.M. Decontamination of unpackaged and vacuum-packaged boneless chicken breast with pulsed ultraviolet light. Poult. Sci. 2010, 89, 570–581. [Google Scholar] [CrossRef]
- Haughton, P.N.; Grau, E.G.; Lyng, J.G.; Cronin, D.A.; Fanning, S.; Whyte, P. Susceptibility of Campylobacter to high intensity near ultraviolet/visible 395 ± 5 nm light and its effectiveness for the decontamination of raw chicken and contact surfaces. Int. J. Food Microbiol. 2012, 159, 267–273. [Google Scholar] [CrossRef]
- Sauer, A.; Moraru, C.I. Inactivation of Escherichia coli ATCC 25922 and Escherichia coli O157: H7 in Apple Juice and Apple Cider, Using Pulsed Light Treatment. J. Food Prot. 2009, 72, 937–944. [Google Scholar] [CrossRef]
- Araújo, P.D.; Araújo, W.M.C.; Patarata, L.; Fraqueza, M.J. Understanding the main factors that influence consumer quality perception and attitude towards meat and processed meat products. Meat Sci. 2022, 193, 108952. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.M.; Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Salim, A.P.A.A.; Conte, C.A. Color stability and lipid oxidation of broiler breast meat from animals raised on organic versus non-organic production systems. Poult. Sci. 2017, 96, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Ferreira, M.C.; Barreto, A.S. Spoilage of light (PSE-like) and dark turkey meat under aerobic or modified atmosphere package: Microbial indicators and their relationship with total volatile basic nitrogen. Br. Poult. Sci. 2008, 49, 12–20. [Google Scholar] [CrossRef]
- Bianchi, M.; Petracci, M.; Cavani, C. The Influence of Genotype, Market Live Weight, Transportation, and Holding Conditions Prior to Slaughter on Broiler Breast Meat Color. Poult. Sci. 2006, 85, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Betti, M.; Bianchi, M.; Cavani, C. Color variation and characterization of broiler breast meat during processing in Italy. Poult. Sci. 2004, 83, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ha, S.-D. Ultraviolet-C Radiation on the Fresh Chicken Breast: Inactivation of Major Foodborne Viruses and Changes in Physicochemical and Sensory Qualities of Product. Food Bioproc. Tech. 2015, 8, 895–906. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef]
- Fricano, A.; Librizzi, F.; Rao, E.; Alfano, C.; Vetri, V. Blue autofluorescence in protein aggregates “lighted on” by U.V. induced oxidation. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 140258. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry—A critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Dawson, P.L.; Spinelli, N. Poultry Meat Flavor. In Handbook of Meat, Poultry and Seafood Quality; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 341–359. [Google Scholar] [CrossRef]
- Jayasena, D.; Ahn, D.; Nam, K.; Jo, C. Flavour Chemistry of Chicken Meat: A Review. Asian-Australas J. Anim. Sci. (AJAS) 2013, 26, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.C.; Silletti, S.; Mattioli, E.; Dal Bosco, A.; Sebastiani, B.; Menchetti, L.; Koot, A.; van Ruth, S.; Castellini, C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2021, 100, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Fogerty, A.C.; Whitfield, F.B.; Svoronos, D.; Ford, G.L. Changes in the composition of the fatty acids and aldehydes of meat lipids after heating. Int. J. Food Sci. Tech. 1990, 25, 304–312. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Wei, X.; Wang, C.; Zhang, C.; Li, X.; Wang, J.; Li, H.; Tang, C. A combination of quantitative marinating and Maillard reaction to enhance volatile flavor in Chinese marinated chicken. J. Sci. Food Agric. 2017, 97, 823–831. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.P.; Deng, S.; Li, C.; Xu, X.; Zhou, G.; Liu, Y. Application of sensory evaluation, GC-ToF-MS, and E-nose to discriminate the flavor differences among five distinct parts of the Chinese blanched chicken. Int. Food Res. J. 2020, 137, 109669. [Google Scholar] [CrossRef]
- Gardner, H.K.; Bourland, C.T.; Smith, M.C.; Huber, C.S. Identification and quantitation of hexadecanal and octadecanal in broiler muscle phospholipids. Poult. Sci. 1972, 51, 1056–1058. [Google Scholar] [CrossRef]
- Salih, A.M.; Price, J.F.; Smith, D.M.; Dawson, L.E. Identification and quantitation of dimethyl acetals of hexadecanal and octadecanal in turkey breast muscle phospholipids. J. Food Sci. 1988, 53, 654–655. [Google Scholar] [CrossRef]
- Rymer, C.; Gibbs, R.A.; Givens, D.I. Comparison of algal and fish sources on the oxidative stability of poultry meat and its enrichment with omega-3 polyunsaturated fatty acids. Poult. Sci. 2010, 89, 150–159. [Google Scholar] [CrossRef]
- Chen, C.-W.; Ho, C.-T. Photochemical Reactions of Flavor Compounds. In Process-Induced Chemical Changes in Food; Shahidi, F., Ho, C.-T., van Chuyen, N., Eds.; Springer: Boston, MA, USA, 1998; pp. 341–355. [Google Scholar]
- Theodoropoulou, M.A.; Nikitas, N.F.; Kokotos, C.G. Aldehydes as powerful initiators for photochemical transformations. Beilstein J. Org. Chem. 2020, 16, 833–857. [Google Scholar] [CrossRef]
- Min, B.; Cordray, J.C.; Ahn, D.U. Effect of NaCl, myoglobin, Fe(II), and Fe(III) on lipid oxidation of raw and cooked chicken breast and beef loin. J. Agric. Food Chem. 2010, 58, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| No | Voltage/V | Distance/cm |
|---|---|---|
| Control | 0 | 0 |
| 1 | 2414 | 2.6 |
| 2 | 2000 | 3 |
| 3 | 2828 | 3 |
| 4 | 1828 | 4 |
| 5 | 2414 | 4 |
| 6 | 2414 | 4 |
| 7 | 2414 | 4 |
| 8 | 3000 | 4 |
| 9 | 2000 | 5 |
| 10 | 2828 | 5 |
| 11 | 2414 | 5.4 |
| Treatment | Voltage (V) | Distance (cm) | Fluence (mJ/cm2) | Hexanal % | Heptanal % | Benzaldehyde % | Octanal % | Nonanal % | (E,E)-2,4-Decadienal % | Hexadecanal % | Octadecanal % | Other Aldehydes % | Total Aldehydes % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 1.06 ± 1.74 ab | 0.71 ± 0.23 a | 2.54 ± 0.30 a | 0.65 ± 0.18 a | 6.47 ± 1.69 a | 1.45 ± 0.19 a | 6.55 ± 0.78 a | 0.69 ± 0.19 a | 3.52 ± 0.82 a | 23.64 ± 4.26 a |
| 1 | 2414.25 | 2.6 | 7.26 ± 0.27 | 0.85 ± 1.74 ab | 0.00 ± 0.23 b | 0.26 ± 0.30 b | 0.25 ± 0.18 ab | 3.08 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 4.44 ± 4.26 b |
| 2 | 2000.00 | 3.0 | 4.61 ± 0.13 | 5.76 ± 1.74 a | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.34 ± 0.18 ab | 1.73 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 7.82 ± 4.26 b |
| 3 | 2828.50 | 3.0 | 9.68 ± 0.15 | 0.00 ± 1.74 b | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.08 ± 0.18 b | 1.05 ± 1.69 b | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 1.13 ± 4.26 b |
| 4 | 1828.41 | 4.0 | 3.15 ± 0.04 | 0.00 ± 1.74 b | 0.00 ± 0.23 b | 0.22 ± 0.30 b | 0.20 ± 0.18 ab | 1.50 ± 1.69 b | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 1.91 ± 4.26 b |
| 5 | 2414.25 | 4.0 | 5.51 ± 0.06 | 0.38 ± 1.74 b | 0.26 ± 0.23 ab | 0.59 ± 0.30 b | 0.30 ± 0.18 ab | 4.50 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 6.02 ± 4.26 b |
| 6 | 2414.25 | 4.0 | 5.92 ± 0.03 | 0.00 ± 1.74 b | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.04 ± 0.18 b | 2.80 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 2.83 ± 4.26 b |
| 7 | 2414.25 | 4.0 | 5.37 ± 0.24 | 0.00 ± 1.74 b | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.39 ± 0.18 ab | 3.92 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 4.31 ± 4.26 b |
| 8 | 3000.09 | 4.0 | 8.53 ± 0.19 | 0.81 ± 1.74 ab | 0.21 ± 0.23 ab | 0.38 ± 0.30 b | 0.17 ± 0.18 ab | 5.21 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 7.23 ± 4.26 b |
| 9 | 2000.00 | 5.0 | 2.82 ± 0.06 | 0.00 ± 1.74 b | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.43 ± 0.18 ab | 4.31 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 4.74 ± 4.26 b |
| 10 | 2828.50 | 5.0 | 6.38 ± 0.06 | 0.99 ± 1.74 ab | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.00 ± 0.18 b | 2.43 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 3.42 ± 4.26 b |
| 11 | 2414.25 | 5.4 | 4.06 ± 0.05 | 0.65 ± 1.74 b | 0.00 ± 0.23 b | 0.00 ± 0.30 b | 0.00 ± 0.18 b | 4.55 ± 1.69 ab | 0.00 ± 0.19 b | 0.00 ± 0.78 b | 0.00 ± 0.19 b | 0.00 ± 0.82 b | 5.20 ± 4.26 b |
| Sig. | - | - | - | * | ** | *** | * | *** | *** | *** | ** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, E.; Borges, A.; Aymerich, T.; Alves, S.P.; Gama, L.T.d.; Fernandes, H.; Fernandes, M.J.; Fraqueza, M.J. Pulsed Light Application for Campylobacter Control on Poultry Meat and Its Effect on Colour and Volatile Profile. Foods 2022, 11, 2848. https://doi.org/10.3390/foods11182848
Baptista E, Borges A, Aymerich T, Alves SP, Gama LTd, Fernandes H, Fernandes MJ, Fraqueza MJ. Pulsed Light Application for Campylobacter Control on Poultry Meat and Its Effect on Colour and Volatile Profile. Foods. 2022; 11(18):2848. https://doi.org/10.3390/foods11182848
Chicago/Turabian StyleBaptista, Esther, Ana Borges, Teresa Aymerich, Susana P. Alves, Luís Telo da Gama, Helena Fernandes, Maria José Fernandes, and Maria João Fraqueza. 2022. "Pulsed Light Application for Campylobacter Control on Poultry Meat and Its Effect on Colour and Volatile Profile" Foods 11, no. 18: 2848. https://doi.org/10.3390/foods11182848
APA StyleBaptista, E., Borges, A., Aymerich, T., Alves, S. P., Gama, L. T. d., Fernandes, H., Fernandes, M. J., & Fraqueza, M. J. (2022). Pulsed Light Application for Campylobacter Control on Poultry Meat and Its Effect on Colour and Volatile Profile. Foods, 11(18), 2848. https://doi.org/10.3390/foods11182848








