Health Benefits and Side Effects of Short-Chain Fatty Acids
Abstract
:1. Introduction
2. The Bioactivities of SCFAs
2.1. Anti-Inflammatory Activity
2.2. Immunoregulatory Activity
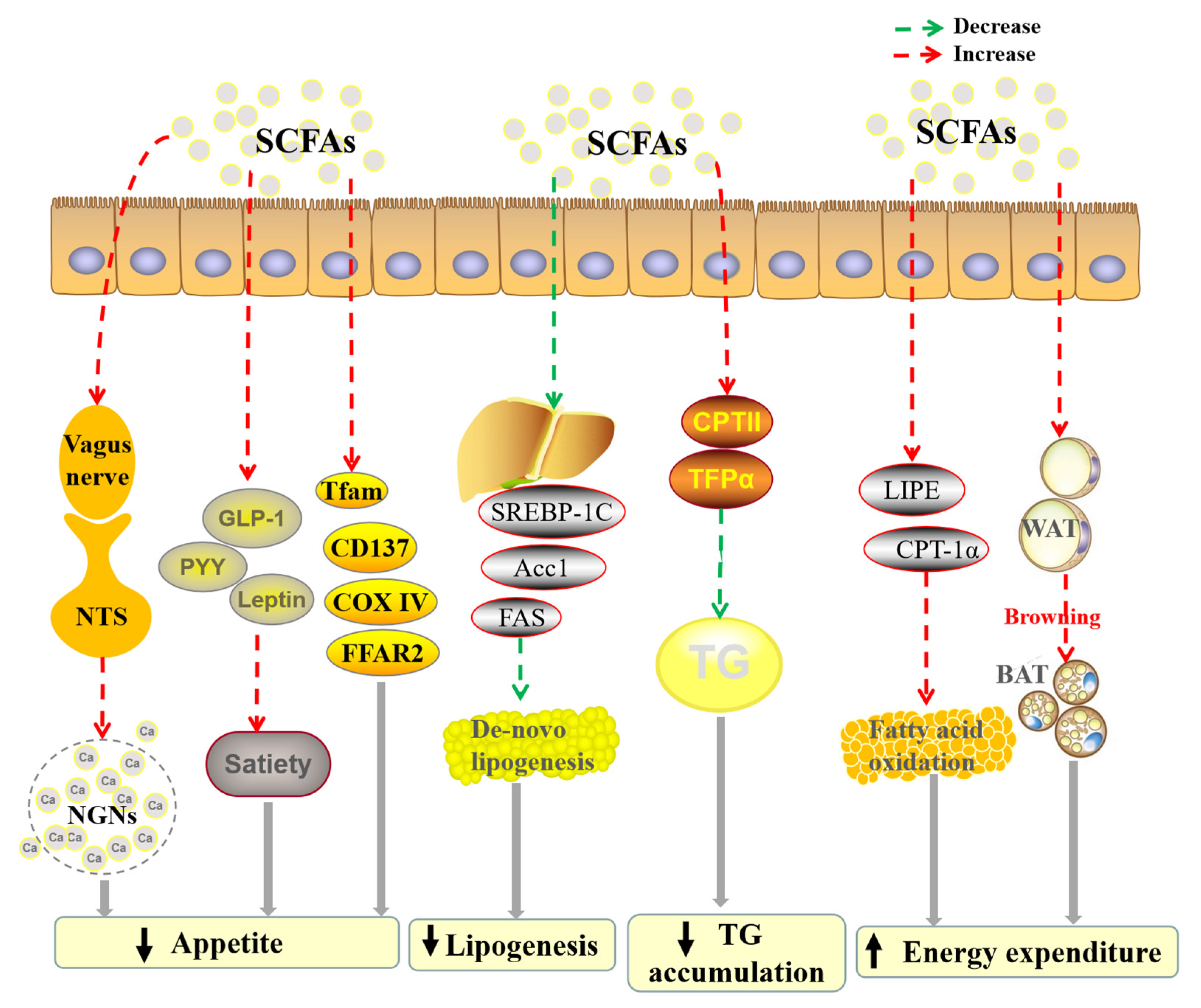
2.3. Anti-Obesity Activity
2.4. Cardio-Protective Activity
2.5. Hepatoprotective Activity
2.6. Anti-Diabetic Activity
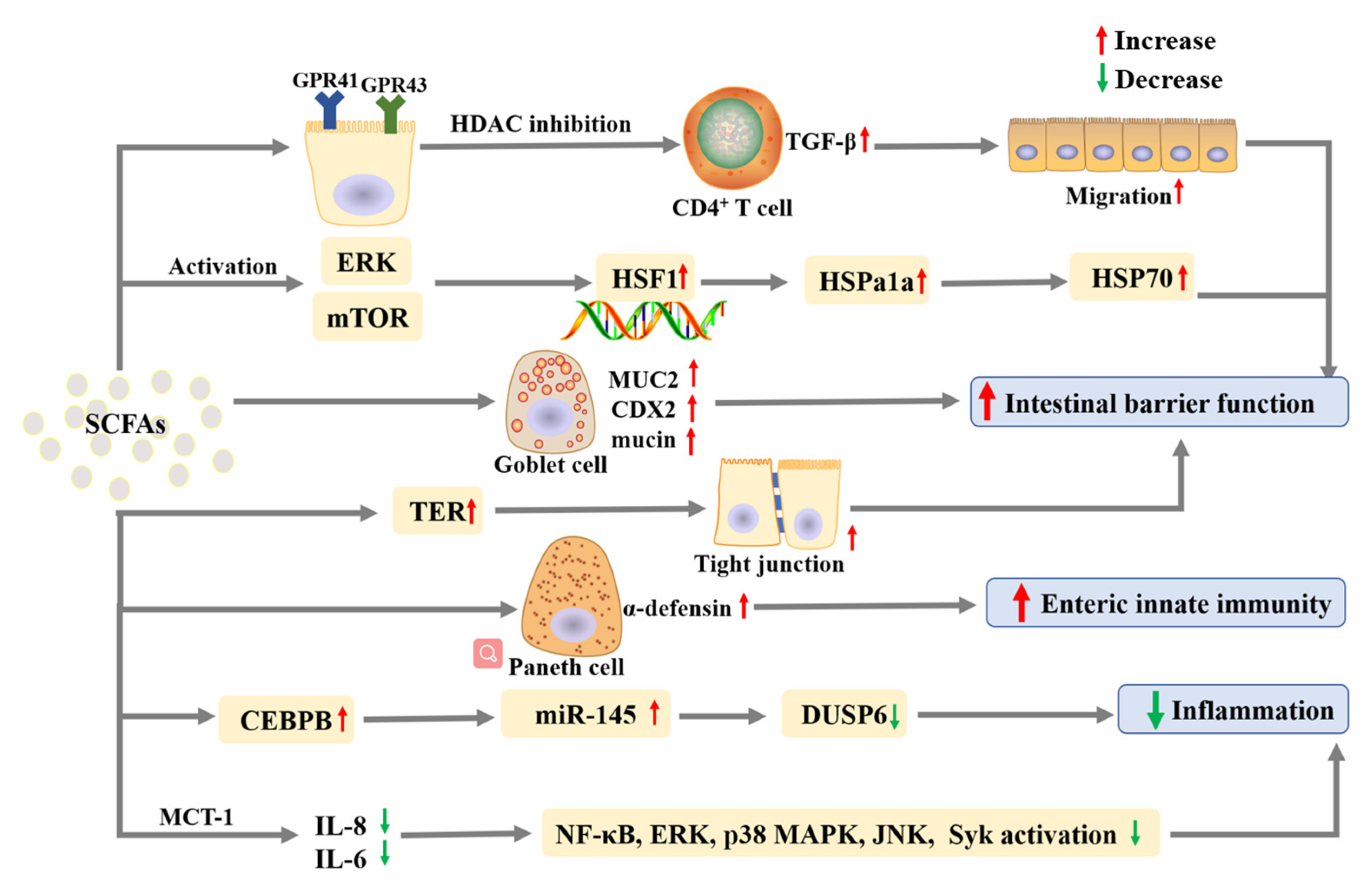
2.7. Effects on Inflammatory Bowel Diseases
2.8. Effects on Constipation
2.9. Neuroprotective Activity
2.10. Anticancer Activity
2.11. Anti-Bacterial Activity
2.12. Effects of SCFAs on Other Diseases
| Study Type | Individuals | Outcomes | Ref. |
|---|---|---|---|
| Anti-obesity | |||
| Prospective study | 1585 singleton late preterm or full-term born | Human milk SCFAs could prevent excess weight gain in infants | [31] |
| Hepatoprotective activity | |||
| Prospective study | 49 patients with cirrhosis | SCFAs were negatively correlated with cirrhosis disease severity | [54] |
| Effects on constipation | |||
| Cohort study | 30 patients with ascending colon cancer and 90 patients with mixed refractory constipation | SCFAs were negatively correlated with the severity of constipation | [84] |
| Neuroprotective activity | |||
| Cross-sectional study | 116 women | Depressive women had lower levels of acetate and propionate | [92] |
| SCFAs Species | Study Type | Subjects | Doses | Effects and Mechanisms | Ref. |
|---|---|---|---|---|---|
| Anti-inflammation | |||||
| Acetate | In vivo | C57BL/6 mice and C57BL6 GFP Het | 150 mM in drinking water | Induce caspase-dependent apoptosis of neutrophils; Decrease the activity of NF-κB; Enhance production of IL-10, TGF-β, and annexin A1. | [22] |
| Propionate | In vitro | HPMECs | 0.6 mM | Accelerate Nrf2 nuclear translocation; Protect cells and promote angiogenesis; Reduce inflammatory response via NF-κB pathway. | [23] |
| Propionate | In vivo | C57BL/6J and Nrf2−/− mice | 1.2 mg/g i.p. | Reduce pulmonary inflammation and oxidative stress. | [23] |
| Propionate | In vivo | BALB/c and C57BL/6 mice | 150 mM in drinking water | Interfere with the production and migration of inflammatory mediators. | [24] |
| Butyrate | In vivo | ICR mice | 200 mg/kg i.p. | Up-regulate the IL-10 in septic shock. | [21] |
| Butyrate | In vitro | RAW 264.7 cells | 100 μM | Down-regulate the IL-6 and IL-1β; Increase the IL-10. | [21] |
| Propionate; Butyrate | In vitro | THP-1 cells | 10 µM | Inhibit the expressions of IL-4, IL-6, and ROS; Enhance the expressions of IL-10 and IFN-γ. | [20] |
| Immunoregulation | |||||
| Butyrate | In vivo | C57BL/6J mice | 200 mM in drinking water | Promote IL-22 production by CD4+ T cells and ILCs. | [28] |
| Acetate; Propionate; Butyrate | In vitro | CD4+ T cells | 10 mM acetate; 0.5 mM propionate; 0.5 mM butyrate | Promote CD4+ T cell and ILC production of IL-22 through GPR41 and HDAC inhibition. | [28] |
| Acetate; Propionate; Butyrate | In vitro | Synovial fibroblasts | 250 µM propionate or the mixture (300 µM acetate, 100 µM propionate, 100 µM butyrate) | Interfere with arthritogenic properties of synovial fibroblasts; Induce cellular senescence. | [24] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J and C.129-IL4tm1Lky/J (4get) mice | 40 mM butyrate, 67.5 mM acetate and 25.9 mM propionate in drinking water | Regulate T cells and DC activities; Reduce the production of IL4-producing CD4+ T cells; Decrease circulating IgE level. | [27] |
| Acetate; Propionate; Butyrate; Pentanoate | In vitro | Splenic B cells isolated from C57BL/6J mice | 0.5 mM of NaAc, NaPr, NaBu or NaPe | Promote B10 cell generation; Enhance B10 cell function. | [29] |
| Acetate; Propionate; Butyrate; Pentanoate | In vivo | C57BL/6J mice | 150 mM acetate, propionate, butyrate or pentanoate in drinking water | Promote B10 cell generation via activation of GPCR. | [29] |
| Anti-obesity | |||||
| Propionate | In vitro | YAMC cells | 5 mM | Repress the TG accumulation via modulating the expression levels of PPARα-responsive genes. | [36] |
| Acetate; Propionate; Butyrate | In vitro | 3T3-L1 cells | 6.4 mM acetic acid; 3.2 mM propionic acid or 0.4 mM butyric acid | Accelerate the 3T3-L1 adipocyte differentiation; Promote lipid accumulation via modulation of the expression of LPL, adipocyte FABP4, FATP4, and FAS. | [115] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J mice | 5% acetate, propionate, or butyrate in the diet | Protect against high-fat diet-induced obesity; Suppress hepatic weight and lipid synthesis. | [37] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6 mice | 6 mmol/kg acetate; 6 mmol/kg propionate; 1–6 mmol/kg butyrate, 10 mL/kg i.p. | Activate vagal afferent neurons. | [32] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J mice | 5% sodium acetate; 5% sodium propionate or 5% sodium butyrate in a high-fat diet | Reduce appetite and fat accumulation via modulating relevant genes and hormones; Regulate the expressions of several mRNA. | [34] |
| Acetate; Propionate; Butyrate | In vivo | Barrows (Duroc × Landrace × Yorkshire) | 0.1% acetate; 0.1% propionate; 0.1% butyrate; 0.1% mixed SCFAs (3:1:1) in diet supplement | Increase the concentrations of the serum GLP-1, PYY and leptin to regulate the appetite; Down-regulate of the mRNA expressions of FAS, ACC, and SREBP-1C to participate the de novo synthesis of fatty acids; Enhance the mRNA expressions of LIPE and CPT-1α to participate in fatty acids oxidation. | [33] |
| Acetate; Propionate; Butyrate | In vivo | Long–Evans rats | MNaAc:MNaPr:MNaBu = 60:20:20, dissolve in 0.1 M PBS, 500 mg/kg i.p. | Suppress the de novo lipogenesis by reducing mRNA expression of hepatic Acc1. | [35] |
| Cardiovascular protection | |||||
| Acetate | In vivo | SD rats | 200 mmol/L magnesium acetate in drinking water | Up-regulate SCFAs receptors Olfr78, GPR41, and GPR43 to keep the balance of vasoconstriction and vasodilation shifts. | [47] |
| Propionate | In vivo | NMRI and ApoE−/− mice | 200 mmol/L in drinking water | Reduce blood pressure; Attenuate cardiac hypertrophy, fibrosis, and vascular dysfunction. | [48] |
| Propionate | In vivo | ApoE−/− mice | 200 mg/kg i.g. | Reduce intestinal cholesterol absorption and aortic atherosclerotic lesion area; Increase levels of regulatory T-cell numbers and IL-10; Reduce the levels of NPC1l1. | [44] |
| Butyrate | In vitro | Caco-2 cells | 0, 0.1, 1 and 10 mmol/L | Inhibit cholesterol absorption; Reduce the levels of NPC1l1; Increase the levels of ABCG5 and ABCG8. | [43] |
| Butyrate | In vivo | SD rats | 200 mmol/L in drinking water | Improves myocardial I/R injury via gut-brain neural circuit. | [49] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J mice | 100 mmol/L in drinking water | Reduce blood pressure. | [46] |
| Acetate; Propionate; Butyrate; valerate | In vivo | Golden Syrian hamsters | 0.5 mol/kg sodium acetate, sodium propionate, sodium butyrate, and valeric acid in high-cholesterol diet | Enhance fecal excretion of bile acids; Up-regulate the gene expressions of SREBP2, LDLR, and CYP7A1 in the liver. | [45] |
| Hepatoprotection | |||||
| Acetate | In vivo | C57BL/6 mice | 200µL LITA-Rhd i.p. | Decrease lipid accumulation; Improve hepatic function; Increase mitochondrial efficiency. | [39] |
| Propionate | In vitro | HepG2 cells | 0.2, 0.4, 0.8 mM | Enhance hepatic function; Alleviate ethanol-induced hepatic steatosis. | [56] |
| Propionate | In vivo | C57BL/6J mice | 100 or 200 mM in the diet | Prevent ethanol-induced loss of hepatic function; Alleviate ethanol-induced hepatic steatosis. | [56] |
| Acetate; Propionate; Butyrate | In vitro | BRL 3A cell | 10 mM NaAc, 5 mM NaPr, 2.5 Mm NaBu or 10 mM mixture (MNaAc:MNaPr:MNaBu = 3:1:1) | Reduce the production of ROS and MDA; Activate AMPK and PPAR signaling pathways; Down-regulate the expression of genes related to lipid synthesis. | [59] |
| Acetate; Propionate; Butyrate | In vitro | human-iPSC-derived liver organoids | 1 μM acetate; 1 μM propionate; 1 μM butyrate | Increase the expression of CYP3A4 and ALB. | [63] |
| Anti-diabetes | |||||
| Propionate | In vitro | HepG2 cells | 0, 0.25, 0.5 mM | Suppress gluconeogenesis by down-regulation of gluconeogenic enzymes; Suppress hepatic gluconeogenesis by activating AMPK; Activate AMPK via Ca2+/CaMKKβ pathway. | [70] |
| Acetate; Propionate | In vivo | C3H/HeOuJ mice | 5% SCFA (Ac:Pr, 2.5:1 or Ac:Pr, 1:2.5) in the diet | Attenuate high-fat diet-induced insulin resistance. | [38] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6 WT and IL22 KO mice | 67.5 mM acetate, 40 mM butyrate and 25.9 mM propionate in drinking water | Prevent type 1 diabetes; Promote development of regulatory T cells. | [116] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6 mice | Acetate (5% w/w of diet), propionate (10% w/w of diet), butyrate (10% w/w of diet), acetate + propionate (5% + 10% w/w of diet) | Improve insulin sensitivity. | [67] |
| Prevention and management of inflammatory bowel disease | |||||
| Propionate | In vivo | WT C57BL / 6 mice | 200 mM in drinking water | Promote intestinal epithelial cell migration; Increase cell speed and persistence. | [74] |
| Butyrate | In vivo | WT C57BL/6J mice | 200 mM in drinking water | promote IL-22; Protect the intestines from Citrobacter rodentium infection. | [28] |
| Butyrate | In vitro | ICR mice crypt | 100μM | Elicit α-defensin secretion by Paneth cells; Improve enteric innate immunity through potent microbicidal activities. | [79] |
| Acetate; Propionate; Butyrate | In vitro | Sheep ruminal tissue | 60 mM NaAc, 30 mM NaPr and 10 mM NaBu | Release protons; Induce subacute ruminal acidosis. | [117] |
| Acetate; Propionate; Butyrate | In vitro | Caco-2 cells | acetate (12.5, 25, and 50 mM), butyrate (5, 10, and 20 mM) and propionate (5, 10, and 20 mM) | Increase the level of Hspa1a expression. Up-regulate HSP70; phosphorylate HSP1. | [76] |
| Acetate; Propionate; Butyrate | In vitro | Caco-2 and T84 cells | Acetate: 0–20 mM, propionate: 0–10 mM, butyrate: 0–2.5 mM | Reduce IL-8 and IL-6 expression levels; Reduce the activation of NF-κB, ERK, p38 MAPK, JNK, and Syk. | [81] |
| Acetate; Propionate; Butyrate | In vitro | MODE-K and MC38 cell lines | A mixture of 0.5 mM acetate, 0.01 mM propionate, and 0.01 mM butyrate | Inhibit DUSP6 by up-regulating miR-145 through decreasing the CEBPB expression. | [82] |
| Acetate; Propionate; Butyrate | In vitro | Caco-2 cells | 0.5 mM acetate, 0.01 mM butyrate, 0.01 mM propionate | Increase TER; Improve the formation of tight junction; Inhibit the activation of NLRP3 inflammasome and autophagy induced by LPS. | [78] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J mice | 200 mM propionate, 200 mM acetate or 100 mM butyrate in the drinking water | Repress IL-17- and IL-22-producing γδ T cells; Reduce IL-17 production by γδ T cells by inhibiting HDAC. | [80] |
| Acetate; Propionate; Butyrate | In vivo | C57BL/6J mice | 25 mM propionate, 40 mM butyrate and 67.5 mM acetate in drinking water | Inhibit DUSP6 via up-regulating miR-145 by suppressing CEBPB; Improve DAI. | [82] |
| Prevention and management of constipation | |||||
| Butyrate | In vitro | ICCs | 0, 0.00005, 0.0005, 0.005, 0.05 and 0.5 mmol/L | Promote mouse ICC proliferation by activating AKT/NF-κB signaling. | [88] |
| Butyrate | In vivo | Kunming mice | 1.1% in the drinking water | Promote defecation; Improve intestinal mobility; Activate the AKT-NF-κB signaling pathway. | [88] |
| Acetate; Propionate; Butyrate | In vivo | BALB/c mice | Diets supplemented with 150 g/kg of either acetylated starch, propylated starch, butylated starch | Acetylated starch and butylated starch relieve constipation; Acetic acid increases WCF and SITR; Butyric acid decreases the transit time through the gut. | [86] |
| Neuroprotection | |||||
| Acetate | In vitro | BV2 cells | 1200 μM | Improve cognitive impairment; Decrease the CD11b level; Suppress neuroinflammation. | [93] |
| Acetate | In vivo | APP/PS1 transgenic and matched WT mice | 1.5 g/kg i.g. | Inhibit the phosphorylation of NF-κB p65, ERK, and JNK; Decrease COX-2 and IL-1β levels; Increase GPR41 level. | [93] |
| Propionate | In vivo | Western albino rats | 75 mg/kg or 250 mg/kg i.g. | Increase the levels of IFN-γ and caspase-3; Decrease levels of nor-adrenaline, dopamine, and 5-HT. | [118] |
| Propionate | In vivo | Western albino rats | 75 mg/kg or 250 mg/kg i.g. | Increase the levels of glutamate and the glutamate/glutamine ratio; Decrease GABA, glutamine, and the GABA/glutamate ratio. | [119] |
| Anticancer | |||||
| Valerate | In vitro | Hep3B, SNU-449, HepG2, THLE-3, MCF-7, MDA-MB-231, MCF-10A, A549, U-87 and A172, HeLa, DU145, and HL-60 cells | 0.5, 1, 2, 4, 8 mM | Suppress colony formation, migration, and invasion of liver cancer cells; Suppresses 3D spheroid formation of liver cancer cells. | [100] |
| Valerate | In vivo | Athymic nude mice | 100 mg/kg tail injection | Suppress HCC development; Improve the survival rate. | [100] |
| Butyrate; Propionate | In vivo | C57BL/6 mice | 300 mg/kg acetate, 150 mg/kg propionate or 88 mg/kg butyrate i.p. | Increase the expression of CCL20; Reduce the recruitment of Th17 cells; Inhibit the lung metastasis of melanoma cells. | [99] |
| Acetate; Butyrate; Propionate | In vivo | BALB/c mice | 67.5 mM acetate, 40 mM butyrate and 25.9 mM propionate in drinking water | Decrease cell proliferation. | [98] |
| Acetate; Propionate; Butyrate Pentanoate | In vivo | CD45.1 WT; CD45.2 WT; CD45.1 OT-I; CD45.2 Ffar2−/− Ffar3−/− mice; CD45.2 FIR × tiger; Rag1−/− mice | 0.5, 1.0, 2.5 mM | Increase the anticancer activity of cytotoxic T lymphocytes and chimeric antigen receptor T cells via metabolic and epigenetic reprogramming. | [101] |
| Prevention and management of arthritis | |||||
| Acetate; Propionate; Butyrate | In vivo | WT C57BL/6J mice and DBA/1J mice | 150 mM acetate, propionate or butyrate in drinking water | Increase systemic bone mass via inducing the reprogramming of osteoclasts metabolism, enhancing glycolysis, and down-regulating TRAF6 and NFATc1; Prevent bone loss after menopause; Alleviate arthritis. | [112] |
| Acetate; Propionate; Butyrate | In vivo | DBA/1JGpt, Ffar2fl/fl and Ffar2fl/fl/CD19-Cre mice | 150 mM acetate, propionate or butyrate in drinking water | Synergistic treatment of CIA; Regulate B cell differentiation via FFA2 receptors; Suppress the inflammatory response. | [113] |
| SCFAs Species | STUDY TYPE | Individuals | Administration Methods | Outcomes | Ref. |
|---|---|---|---|---|---|
| Anti-obesity | |||||
| Propionate | Single-blind crossover RCT | 20 healthy men | Inulin-propionate ester (10 g/day) for 24 weeks | Reduce anticipatory reward responses in the human striatum to high-energy foods | [40] |
| Cardio-protective activity | |||||
| Propionate | Double-blind RCT | 62 participants | Calcium-Propionate (500 mg, twice daily) for 8 weeks | Reduce levels of LDL and non-high-density lipoprotein cholesterol | [44] |
| Anti-diabetic activity | |||||
| SCFAs | RCT | 29 overweight/obese individuals | Med-D intervention for 8 weeks | Increase plasma butyric acid Improve postprandial glucose metabolism and insulin sensitivity | [72] |
3. The Side Effects of SCFAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Nakamura, K.; Kitada, N.; Aizawa, T.; Shimizu, Y.; Nakamura, K.; Ayabe, T.; Kimura, T.; Tamakoshi, A. Associations of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci Microbiota Food Health 2020, 39, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.M. Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int. J. Mol. Sci. 2019, 20, 4477. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Chen, X.F.; Chen, X.; Tang, X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. 2020, 134, 657–676. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 produced more butyric acid through regulating the microbial community of human gut microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Zhu, Y.; Li, X.T.; Sun, B.G. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Somogyi, A.; Bailey, M.T. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 533–540. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S. Gut-derived short-chain fatty acids: A friend or foe for hepatic lipid metabolism? Nutr. Bull. 2019, 44, 154–159. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Fernandez, J.; Redondo-Blanco, S.; Gutierrez-del-Rio, I.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, Y.; Kimura, I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci. Microbiota Food Health 2017, 36, 135–140. [Google Scholar] [CrossRef]
- Xia, W.; Dai, X.Y.; Ding, L.K.; Xi, Y.; Yan, M.; Zhang, M.; Wu, L.; Yi, C.X.; Xu, H.X. Three main short-chain fatty acids inhibit the activation of THP-1 cells by Mycoplasma pneumoniae. Biosci. Biotechnol. Biochem. 2021, 85, 923–930. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S. The inflammation induced by lipopolysaccharide can be mitigated by short-chain fatty acid, butyrate, through upregulation of il-10 in septic shock. Scand. J. Immunol. 2017, 85, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Galvao, I.; Macia, L.M.; Sernaglia, E.M.; Vinolo, M.A.R.; Garcia, C.C.; Tavares, L.P.; Amaral, F.A.; Sousa, L.P.; Martins, F.S.; et al. Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J. Leukoc. Biol. 2017, 101, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gao, Z.Q.; Wang, Y.Y.; Wan, B.B.; Liu, G.; Chen, J.L.; Wu, Y.X.; Zhou, Q.; Jiang, S.Y.; Yu, R.Q.; et al. Sodium propionate enhances Nrf2-mediated protective defense against oxidative stress and inflammation in lipopolysaccharide-induced neonatal mice. J. Inflamm. Res. 2021, 14, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Friscic, J.; Durholz, K.; Chen, X.; Engdahl, C.; Moller, L.; Schett, G.; Zaiss, M.M.; Hoffmann, M.H. Dietary derived propionate regulates pathogenic fibroblast function and ameliorates experimental arthritis and inflammatory tissue priming. Nutrients 2021, 13, 1643. [Google Scholar] [CrossRef]
- Olsson, A.; Gustavsen, S.; Nguyen, T.D.; Nyman, M.; Langkilde, A.R.; Hansen, T.H.; Sellebjerg, F.; Oturai, A.B.; Sondergaard, H.B. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front. Immunol. 2021, 12, 661493. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.H. Regulation of humoral immunity by gut microbial products. Gut Microbes 2017, 8, 392–399. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Yang, W.J.; Yu, T.M.; Huang, X.S.; Bilotta, A.J.; Xu, L.Q.; Lu, Y.; Sun, J.R.; Pan, F.; Zhou, J.; Zhang, W.B.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Zou, F.; Qiu, Y.; Huang, Y.; Zou, H.; Cheng, X.; Niu, Q.; Luo, A.; Sun, J. Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function. Cell Death Dis. 2021, 12, 582. [Google Scholar] [CrossRef]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y.; Tang, G.Y.; Corke, H.; Gan, R.Y.; Li, H.B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human milk short-chain fatty acid composition is associated with adiposity outcomes in infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Iwasaki, Y.; Yada, T. Short-chain fatty acids suppress food intake by activating vagal afferent. J. Nutr. Biochem. 2018, 57, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.R.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.H.; Luo, J.Q.; Mao, X.B.; Chen, D.W. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.R.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.H.; Luo, J.Q.; Yan, H.; Wang, Q.Y.; Wang, H.F.; et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87, 111198. [Google Scholar] [CrossRef]
- Shah, S.; Fillier, T.; Pham, T.H.; Thomas, R.; Cheema, S.K. Intraperitoneal administration of short-chain fatty acids improves lipid metabolism of long-evans rats in a sex-specific manner. Nutrients 2021, 13, 892. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Yoshikawa, T. Propionate promotes fatty acid oxidation through the up-regulation of peroxisome proliferator-activated receptor α in intestinal epithelial cells. J. Nutr. Sci. Vitaminol. 2015, 61, 511–515. [Google Scholar] [CrossRef]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Kappo, K.A.; Petzke, K.J.; Kipp, A.P.; Blaut, M.; Klaus, S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016, 60, 2611–2621. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ’browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.Y.; Zhao, C.N.; Gan, R.Y.; Xu, X.Y.; Wei, X.L.; Corke, H.; Atanasov, A.G.; Li, H.B. Effects and Mechanisms of Tea and Its Bioactive Compounds for the Prevention and Treatment of Cardiovascular Diseases: An Updated Review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Petersen, K.S.; Kris-Etherton, P.M. Dietary patterns affect the gut microbiome-the link to risk of cardiometabolic diseases. J. Nutr. 2018, 148, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Liu, J.H.; Hao, W.J.; Zhu, H.Y.; Liang, N.; He, Z.Y.; Ma, K.Y.; Chen, Z.Y. Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. J. Agric. Food Chem. 2017, 65, 10984–10992. [Google Scholar] [CrossRef]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on gut microbial metabolite trimethylamine-N-oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Yu, Z.; Han, J.; Chen, H.; Wang, Y.; Zhou, L.; Wang, M.; Zhang, R.; Jin, X.; Zhang, G.; Wang, C.; et al. Oral supplementation with butyrate improves myocardial ischemia/reperfusion injury via a gut-brain neural circuit. Front. Cardiovasc. Med. 2021, 8, 718674. [Google Scholar] [CrossRef]
- Tang, T.W.H.; Chen, H.C.; Chen, C.Y.; Yen, C.Y.T.; Lin, C.J.; Prajnamitra, R.P.; Chen, L.L.; Ruan, S.C.; Lin, J.H.; Lin, P.J.; et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef]
- Huang, W.F.; Kong, D.S. The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed. Pharmacother. 2021, 135, 111235. [Google Scholar] [CrossRef]
- Li, B.Y.; Mao, Q.Q.; Zhou, D.D.; Luo, M.; Gan, R.Y.; Li, H.Y.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Effects of tea against alcoholic fatty liver disease by modulating gut microbiota in chronic alcohol-exposed mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef]
- Meng, X.; Li, S.; Li, Y.; Gan, R.Y.; Li, H.B. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients 2018, 10, 1457. [Google Scholar] [CrossRef]
- Bloom, P.P.; Luévano, J.M., Jr.; Miller, K.J.; Chung, R.T. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann. Hepatol. 2021, 25, 100333. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Mu, Y.; Song, Y.; Hao, N.; Wei, Y.; Wang, Q.; Mackay, C.R. Propionate ameliorates alcohol-induced liver injury in mice via the gut-liver axis: Focus on the improvement of intestinal permeability. J. Agric. Food Chem. 2022, 70, 6084–6096. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef]
- Liu, W.X.; Luo, X.L.; Tang, J.; Mo, Q.F.; Zhong, H.; Zhang, H.; Feng, F.Q. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: By changing gut barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, F.F.; Strappe, P.; Liu, W.T.; Zheng, J.X.; Zhou, Z.K.; Zhang, Y. Microbiota fermentation characteristics of acylated starches and the regulation mechanism of short-chain fatty acids on hepatic steatosis. Food Funct. 2021, 12, 8659–8668. [Google Scholar] [CrossRef]
- Batista, A.G.; da Silva-Maia, J.K.; Mendonca, M.C.P.; Soares, E.S.; Lima, G.C.; Bogusz, S.; da Cruz-Hofling, M.A.; Marostica, M.R. Jaboticaba berry peel intake increases short chain fatty acids production and prevent hepatic steatosis in mice fed high-fat diet. J. Funct. Foods 2018, 48, 266–274. [Google Scholar] [CrossRef]
- Li, W.F.; Zhang, K.; Yang, H.Y. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: Possible role of short-chain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Yang, F.; Feng, B.; Niu, Y.J.; Hu, C.Y.; Meng, Y.H. Fu instant tea ameliorates fatty liver by improving microbiota dysbiosis and elevating short-chain fatty acids in the intestine of mice fed a high-fat diet. Food Biosci. 2021, 42, 101207. [Google Scholar] [CrossRef]
- Mun, S.J.; Lee, J.; Chung, K.S.; Son, M.Y.; Son, M.J. Effect of microbial short-chain fatty acids on CYP3A4-mediated metabolic activation of human pluripotent stem cell-derived liver organoids. Cells 2021, 10, 126. [Google Scholar] [CrossRef]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M.; Shang, A.; Mao, Q.Q.; Li, H.B. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 2019, 8, 440. [Google Scholar] [CrossRef]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: An updated review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. The combinatorial effect of acetate and propionate on high-fat diet induced diabetic inflammation or metaflammation and T cell polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.F.; Wang, J.; Wang, A.Q.; Yao, X.; Strappe, P.; Zhou, Z.K.; Wu, Q.H.; Guo, T.L. Starch acylation of different short-chain fatty acids and its corresponding influence on gut microbiome and diabetic indexes. Food Chem. 2022, 389, 133089. [Google Scholar] [CrossRef]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2021, 40, 428–437. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Bilotta, A.J.; Ma, C.; Yang, W.; Yu, Y.; Yu, Y.; Zhao, X.; Zhou, Z.; Yao, S.; Dann, S.M.; Cong, Y. Propionate enhances cell speed and persistence to promote intestinal epithelial turnover and repair. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1023–1044. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef]
- Adesina, P.A.; Isayama, K.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Propionate and dietary fermentable fibers upregulate intestinal heat shock protein70 in intestinal Caco-2 cells and mouse colon. J. Agric. Food Chem. 2021, 69, 8460–8470. [Google Scholar] [CrossRef]
- Chang, Y.H.; Jeong, C.H.; Cheng, W.N.; Choi, Y.; Shin, D.M.; Lee, S.; Han, S.G. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 2021, 104, 7415–7425. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Takakuwa, A.; Nakamura, K.; Kikuchi, M.; Sugimoto, R.; Ohira, S.; Yokoi, Y.; Ayabe, T. Butyric acid and leucine induce α-defensin secretion from small intestinal paneth cells. Nutrients 2019, 11, 2817. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Short-chain fatty acids suppress inflammatory reactions in Caco-2 cells and mouse colons. J. Agric. Food Chem. 2018, 66, 108–117. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Z.; Zhou, L.; Peng, R.; Li, X.; Zuo, W.; Gou, J.; Zhou, F.; Yu, S.; Huang, M.; et al. Short-chain fatty acid decreases the expression of CEBPB to inhibit mir-145-mediated DUSP6 and thus further suppresses intestinal inflammation. Inflammation 2022, 45, 372–386. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut microbiota and chronic constipation: A review and update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Q.; Huang, Y.; Ni, L.; Liu, J.; Jiang, J.; Li, N. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Colorectal Dis. 2016, 18, 803–810. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.T.; Ma, Q.C.; Strappe, P.; Zhou, Z.K. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. [Google Scholar] [CrossRef]
- Wang, L.L.; Cen, S.; Wang, G.; Lee, Y.K.; Zhao, J.X.; Zhan, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, L.J.; Yan, S.; Jiang, T.; Fang, S.G.; Wang, G.; Zhao, J.X.; Zhang, H.; Chen, W. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 2017, 8, 1966–1978. [Google Scholar] [CrossRef]
- He, Q.L.; Han, C.P.; Huang, L.; Yang, H.J.; Hu, J.C.; Chen, H.X.; Dou, R.X.; Ren, D.L.; Lin, H.C. Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J. Cell. Mol. Med. 2020, 24, 9349–9361. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Ling, Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.Y.; Zhao, C.N.; Meng, X.; Li, H.B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Matsuya-Ogawa, M.; Shibata, T.; Itoh, H.; Murakami, H.; Yaguchi, C.; Sugihara, K.; Kanayama, N. Oncoprotective effects of short-chain fatty acids on uterine cervical neoplasia. Nutr. Cancer 2019, 71, 312–319. [Google Scholar] [CrossRef]
- Casanova, M.R.; Azevedo-Silva, J.; Rodrigues, L.R.; Preto, A. Colorectal cancer cells increase the production of short chain fatty acids by propionibacterium freudenreichii impacting on cancer cells survival. Front. Nutr. 2018, 5, 44. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018, 57, 103–109. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and butyrate produced by gut microbiota after probiotic supplementation attenuate lung metastasis of melanoma cells in mice. Mol. Nutr. Food Res. 2021, 65, e2100096. [Google Scholar] [CrossRef]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol. Ther.-Oncolytics 2020, 19, 8–18. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, Y.; Heidari, H.R.; Khosroushahi, A.Y. Review of short-chain fatty acids effects on the immune system and cancer. Food Biosci. 2020, 38, 100793. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Subramanian, U.; Venkidasamy, B.; Thirupathi, P.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Chung, I.M.; Rengasamy, K.R.R. Emerging role of nutritional short-chain fatty acids (SCFAs) against cancer via modulation of hematopoiesis. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Ithakisiou, G.N.; Henricks, P.A.J.; Pieters, R.; Folkerts, G.; Braber, S. Non-digestible oligosaccharides and short chain fatty acids as therapeutic targets against enterotoxin-producing bacteria and their toxins. Toxins 2021, 13, 175. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3987–4002. [Google Scholar] [CrossRef]
- Venditti, T.; Ladu, G.; Cubaiu, L.; Myronycheva, O.; D’Hallewin, G. Repeated treatments with acetic acid vapors during storage preserve table grapes fruit quality. Postharvest. Biol. Technol. 2017, 125, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Loor, J.J.; Jiang, Q.; Zhu, Y.; Li, W.; Du, X.; Song, Y.; Gao, W.; Lei, L.; et al. Propionate alleviates fatty acid-induced mitochondrial dysfunction, oxidative stress, and apoptosis by upregulating PPARG coactivator 1 alpha in hepatocytes. J. Dairy Sci. 2022, 105, 4581–4592. [Google Scholar] [CrossRef]
- Heath, A.M.; Haszard, J.J.; Galland, B.C.; Lawley, B.; Rehrer, N.J.; Drummond, L.N.; Sims, I.M.; Taylor, R.W.; Otal, A.; Taylor, B.; et al. Association between the faecal short-chain fatty acid propionate and infant sleep. Eur. J. Clin. Nutr. 2020, 74, 1362–1365. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Kondo, T.; Chiba, T.; Tousen, Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int. J. Food Sci. Nutr. 2022; in press. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Zheng, Y.; Zhang, M.; Fei, W.; Sun, D.; Zhao, M.; Ye, Y.; Zheng, C. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br. J. Pharmacol. 2022; in press. [Google Scholar] [CrossRef]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell. Microbiol. 2021, 23, e13348. [Google Scholar] [CrossRef]
- Yu, H.N.; Li, R.; Huang, H.Y.; Yao, R.; Shen, S.R. Short-chain fatty acids enhance the lipid accumulation of 3T3-L1 cells by modulating the expression of enzymes of fatty acid metabolism. Lipids 2018, 53, 77–84. [Google Scholar] [CrossRef]
- Zou, J.; Reddivari, L.; Shi, Z.; Li, S.; Wang, Y.; Bretin, A.; Ngo, V.L.; Flythe, M.; Pellizzon, M.; Chassaing, B.; et al. Inulin fermentable fiber ameliorates type I diabetes via il22 and short-chain fatty acids in experimental models. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 983–1000. [Google Scholar] [CrossRef]
- Meissner, S.; Hagen, F.; Deiner, C.; Günzel, D.; Greco, G.; Shen, Z.; Aschenbach, J.R. Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 2017, 100, 6662–6675. [Google Scholar] [CrossRef]
- Al-Salem, H.S.; Bhat, R.S.; Al-Ayadhi, L.; El-Ansary, A. Therapeutic potency of bee pollen against biochemical autistic features induced through acute and sub-acute neurotoxicity of orally administered propionic acid. BMC Complement. Altern. Med. 2016, 16, 120. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Salem, H.S.; Asma, A.; Al-Dbass, A. Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis. 2017, 16, 96. [Google Scholar] [CrossRef]
- Cherta-Murillo, A.; Pugh, J.E.; Alaraj-Alshehhi, S.; Hajjar, D.; Chambers, E.S.; Frost, G.S. The effect of short-chain fatty acids on glycemic control in humans: A systematic review and Meta-analysis. Am. J. Clin. Nutr. 2022; in press. [Google Scholar] [CrossRef]
- Trapecar, M.; Communal, C.; Velazquez, J.; Maass, C.A.; Huang, Y.J.; Schneider, K.; Wright, C.W.; Butty, V.; Eng, G.; Yilmaz, O.; et al. Gut-liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Systems 2020, 10, 223–239.e9. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. https://doi.org/10.3390/foods11182863
Xiong R-G, Zhou D-D, Wu S-X, Huang S-Y, Saimaiti A, Yang Z-J, Shang A, Zhao C-N, Gan R-Y, Li H-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods. 2022; 11(18):2863. https://doi.org/10.3390/foods11182863
Chicago/Turabian StyleXiong, Ruo-Gu, Dan-Dan Zhou, Si-Xia Wu, Si-Yu Huang, Adila Saimaiti, Zhi-Jun Yang, Ao Shang, Cai-Ning Zhao, Ren-You Gan, and Hua-Bin Li. 2022. "Health Benefits and Side Effects of Short-Chain Fatty Acids" Foods 11, no. 18: 2863. https://doi.org/10.3390/foods11182863
APA StyleXiong, R.-G., Zhou, D.-D., Wu, S.-X., Huang, S.-Y., Saimaiti, A., Yang, Z.-J., Shang, A., Zhao, C.-N., Gan, R.-Y., & Li, H.-B. (2022). Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods, 11(18), 2863. https://doi.org/10.3390/foods11182863






