Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oleogel Preparation
2.3. Oleofoam Preparation and Characterization
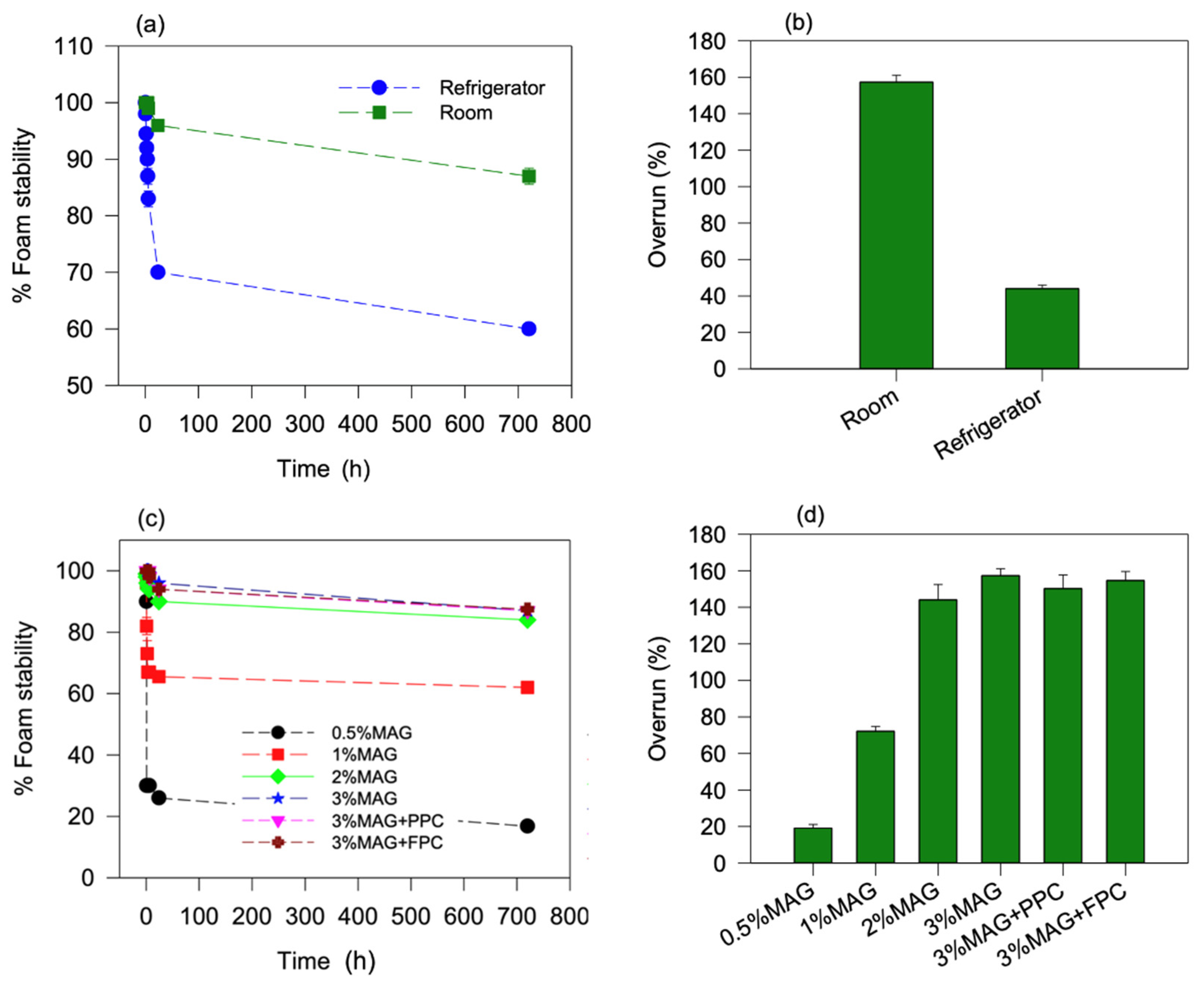
2.3.1. Overrun and Oleofoam Stability
2.3.2. Microstructure of Oleofoams
2.3.3. Viscoelasticity of Oleofoams
2.4. Cake Baking with Oleogels and Oleofoams
2.5. Characterization of Cake Batters and Cakes
2.5.1. Specific Gravity of Cake Batter
2.5.2. Microstructure of Cake Batter
2.5.3. Rheology of Cake Batter
2.5.4. X-ray Microtomography of Cake
2.5.5. Specific Volume of Cake
2.5.6. Cake Texture Analysis
2.6. Statistical Analysis
3. Results
3.1. Formation of Oleofoams from Oleogels
3.1.1. Oleofoam Stability and Overrun
3.1.2. Viscoelasticity of Oleofoams
3.1.3. Visual Observation and Microstructure of Oleofoams
3.2. Cake Baking with Oleogels and Oleofoams
3.2.1. Optimization of Cake Batter Preparation Using Oleofoams
3.2.2. Microstructure of Cake Batters
3.2.3. Rheology of Cake Batters
3.3. Properties of Cakes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirkesen, I.; Mert, B. Recent developments of oleogel utilizations in bakery products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2460–2479. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Hwang, H.-S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- De Vries, A.; Hendriks, J.; Van Der Linden, E.; Scholten, E. Protein Oleogels from Protein Hydrogels via a Stepwise Solvent Exchange Route. Langmuir 2015, 31, 13850–13859. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Mohanan, A.; Tang, Y.R.; Nickerson, M.T.; Ghosh, S. Oleogelation using pulse protein-stabilized foam, and their potential as a baking ingredient. RSC Adv. 2020, 10, 14892. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Auzanneau, F.-I.; Rogers, M. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef]
- Patel, A.R.; Dewettinck, K. Edible oil structuring: An overview and recent updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. Ethylcellulose Oleogels: Structure, Functionality, and Food Applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–56. [Google Scholar]
- de Vries, A.; Wesseling, A.; van der Linden, E.; Scholten, E. Protein oleogels from heat-set whey protein aggregates. J. Colloid Interface Sci. 2017, 486, 75–83. [Google Scholar] [CrossRef]
- Patel, A.R.; Schatteman, D.; Lesaffer, A.; Dewettinck, K. A foam-templated approach for fabricating organogels using a water-soluble polymer. RSC Adv. 2013, 3, 22900–22903. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lewille, B.; Lesaffer, A.; Dewettinck, K. Polysaccharide-Based Oleogels Prepared with an Emulsion-Templated Approach. ChemPhysChem 2014, 15, 3435–3439. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Abdollahi, M.; Goli, S.A.H.; Soltanizadeh, N. Physicochemical Properties of Foam-Templated Oleogel Based on Gelatin and Xanthan Gum. Eur. J. Lipid Sci. Technol. 2019, 122, 1900196. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. The Effect of Addition of High-Melting Monoacylglycerol and Candelilla Wax on Pea and Faba Bean Protein Foam-Templated Oleogelation. J. Am. Oil Chem. Soc. 2020, 97, 1319–1333. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.-S.; Lee, S. Utilization of Oleogels as a Replacement for Solid Fat in Aerated Baked Goods: Physicochemical, Rheological, and Tomographic Characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef]
- Gunes, D.Z.; Murith, M.; Godefroid, J.; Pelloux, C.; Deyber, H.; Schafer, O.; Breton, O. Oleofoams: Properties of Crystal-Coated Bubbles from Whipped Oleogels—Evidence for Pickering Stabilization. Langmuir 2017, 33, 1563–1575. [Google Scholar] [CrossRef]
- Fameau, A.-L.; Lam, S.; Arnould, A.; Gaillard, C.; Velev, O.; Saint-Jalmes, A. Smart Nonaqueous Foams from Lipid-Based Oleogel. Langmuir 2015, 31, 13501–13510. [Google Scholar] [CrossRef]
- Truong, T.; Prakash, S.; Bhandari, B. Effects of crystallisation of native phytosterols and monoacylglycerols on foaming properties of whipped oleogels. Food Chem. 2019, 285, 86–93. [Google Scholar] [CrossRef]
- Fameau, A.-L.; Saint-Jalmes, A. Recent Advances in Understanding and Use of Oleofoams. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Gehin-Delval, C.; Chisholm, H.; Chung, W.; Deyber, H.; Destribats, M.J.; Gunes, Z.D.; Pelloux, C. Method for Forming a Laminated Pastry. U.S. Patent No. US 2019/0200625 A1, 19 July 2019. [Google Scholar]
- Chisholm, H.; Gunes, Z.D.; Gehin-Delval, C.; Nouzille, C.A.; Garvey, E.; Destribats, M.J.; Chandrasekaran, S.N.; Vieira, J.B.; German, J.; Binks, B.P. Aerated Confectionery Material. U.S. Patent US20180064127A1, 8 March 2018. [Google Scholar]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Utilization of pulse protein-xanthan gum complexes for foam stabilization: The effect of protein concentrate and isolate at various pH. Food Chem. 2020, 316, 126282. [Google Scholar] [CrossRef]
- Liu, S.; Elmer, C.; Low, N.; Nickerson, M. Effect of pH on the functional behaviour of pea protein isolate-gum Arabic complexes. Food Res. Int. 2010, 43, 489–495. [Google Scholar] [CrossRef]
- AACC. AACC International. Approved Methods of Analysis, 11th ed.; Method 90-10.01. Baking Quality of Cake Flour; AACC International: St. Paul, MN, USA, 1999; Available online: https://methods.aaccnet.org/methods/10-90.pdf (accessed on 4 March 2020).
- AACC. Approved Methods of Analysis, 11th ed.; Method 10-05.01. Guidelines for Measurement of Volume by Rapeseed Displacement; AACC International: St. Paul, MN, USA, 2001; Available online: https://methods.aaccnet.org/methods/10-90.pdf (accessed on 4 March 2020).
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The Texturometer? A New Instrument for Objective Texture Measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Heymans, R.; Tavernier, I.; Danthine, S.; Rimaux, T.; Van der Meeren, P.; Dewettinck, K. Food-grade monoglyceride oil foams: The effect of tempering on foamability, foam stability and rheological properties. Food Funct. 2018, 9, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Aramaki, K.; Kato, H.; Takase, Y.; Kunieda, H. Foaming Properties of Monoglycerol Fatty Acid Esters in Nonpolar Oil Systems. Langmuir 2006, 22, 8337–8345. [Google Scholar] [CrossRef]
- Wilderjans, E.; Luyts, A.; Brijs, K.; Delcour, J.A. Ingredient functionality in batter type cake making. Trends Food Sci. Technol. 2013, 30, 6–15. [Google Scholar] [CrossRef]
- Wootton, J.; Howard, N.B.; Martin, J.B.; McOsker, D.E.; Holme, J. The role of emulsifiers in the incorporation of air into layer cake batter systems. Cereal Chem. 1967, 44, 333–343. [Google Scholar]
- Hesso, N.; Garnier, C.; Loisel, C.; Chevallier, S.; Bouchet, B.; Le-Bail, A. Formulation effect study on batter and cake microstructure: Correlation with rheology and texture. Food Struct. 2015, 5, 31–41. [Google Scholar] [CrossRef]
- Sahin, S. Cake Batter Rheology. In Food Engineering Aspects of Baking Sweet Goods; CRC Press: Boca Raton, FL, USA, 2008; pp. 99–119. [Google Scholar]









| Specific Gravity of Cake Batter | Specific Volume of Cake (cm3/g) | |
|---|---|---|
| Shortening-AACC | 0.88 ± 0.02 a | 2.21 ± 0.06 a |
| MAG oleofoam-AACC | 1.17 ± 0.00 b | 1.58 ± 0.02 b |
| Canola oil | 1.19 ± 0.00 c | 1.99 ± 0.02 c |
| Shortening Oleofoam | 1.01 ± 0.02 d | 1.74 ± 0.05 d |
| MAG Oleofoam | 1.04 ± 0.01 d | 1.73 ± 0.03 d |
| MAG+PPC Oleofoam | 1.05 ± 0.03 d | 1.82 ± 0.03 e |
| MAG+FPC Oleofoam | 1.03 ± 0.03 d | 1.79 ± 0.05 ed |
| Sample | Flow Behaviour Index (n) | Consistency Coefficient (K, Pa·sn) | R2 |
|---|---|---|---|
| Shortening-AACC | 0.07 ± 0.01 a | 118.47 ± 2.19 a | 0.9998 |
| MAG oleofoam-AACC | 0.19 ± 0.02 b | 56.29 ± 2.07 b | 0.9984 |
| Canola Oil | 0.55 ± 0.06 c | 70.78 ± 17.07 c | 0.8737 |
| Shortening oleofoam | 0.33 ± 0.03 d | 97.07 ± 10.09 d | 0.9878 |
| MAG Oleofoam | 0.19 ± 0.13 b | 45.19 ± 1.67 e | 0.9620 |
| PPC+MAG Oleofoam | 0.19 ± 0.02 b | 42.60 ± 15.16 b,e | 0.9974 |
| FPC+MAG Oleofoam | 0.25 ± 0.16 b,d | 46.03 ± 2.13 e | 0.9974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanan, A.; Harrison, K.; Cooper, D.M.L.; Nickerson, M.T.; Ghosh, S. Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application. Foods 2022, 11, 2887. https://doi.org/10.3390/foods11182887
Mohanan A, Harrison K, Cooper DML, Nickerson MT, Ghosh S. Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application. Foods. 2022; 11(18):2887. https://doi.org/10.3390/foods11182887
Chicago/Turabian StyleMohanan, Athira, Kim Harrison, David M. L. Cooper, Michael T. Nickerson, and Supratim Ghosh. 2022. "Conversion of Pulse Protein Foam-Templated Oleogels into Oleofoams for Improved Baking Application" Foods 11, no. 18: 2887. https://doi.org/10.3390/foods11182887






