INFOGEST Digestion Assay of Raw and Roasted Hazelnuts and Its Impact on Allergens and Their IgE Binding Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Patients’ Sera
2.3. Hazelnut
2.4. Simulated Oral and Gastric In Vitro Digestion Conditions
2.5. SDS-PAGE Analyses
2.5.1. One-Dimensional (1D) SDS-PAGE of Non-Defatted Liquid Gastric Digesta
2.5.2. 1D SDS-PAGE with Defatted Liquid Gastric Digesta
2.5.3. Densitometry with ImageQuant TL Version 8.1
2.5.4. Two-Dimensional (2D) SDS-PAGE of Non-Defatted Liquid Gastric Digesta
2.6. Mass Spectrometry Analysis
2.6.1. Sample Preparation for Nano Liquid Chromatography Coupled to Tandem Mass Spectrometry (nLC-MS/MS)
2.6.2. nLC-ESI-MS/MS with Orbitrap Exploris 240
2.6.3. Identification of Hazelnut Allergens in the Basic Region by PEAKS Studio Xpro
2.7. Purification of Cor a 9 from Raw and Roasted Hazelnuts
2.7.1. CD Spectroscopy of Cor a 9 Purified from Raw and Roasted Hazelnuts
2.7.2. IgE ELISA with Raw and Roasted Cor a 9 with Patients’ Sera Allergic to Hazelnut
2.8. IgE Binding Properties of Hazelnut Gastric Digesta
2.8.1. 1D and 2D Immunoblots of Non-Defatted Hazelnut Gastric Digesta
2.8.2. Inhibitory ELISA of Hazelnut Gastric Digesta with Cor a 9 as an Inhibitor
2.9. 1D and 2D Western Blot of Non-Defatted Gastric Liquid Digesta Probed with Anti-Cor a 8 Antibody
2.10. Statistical Analyses
3. Results & Discussion
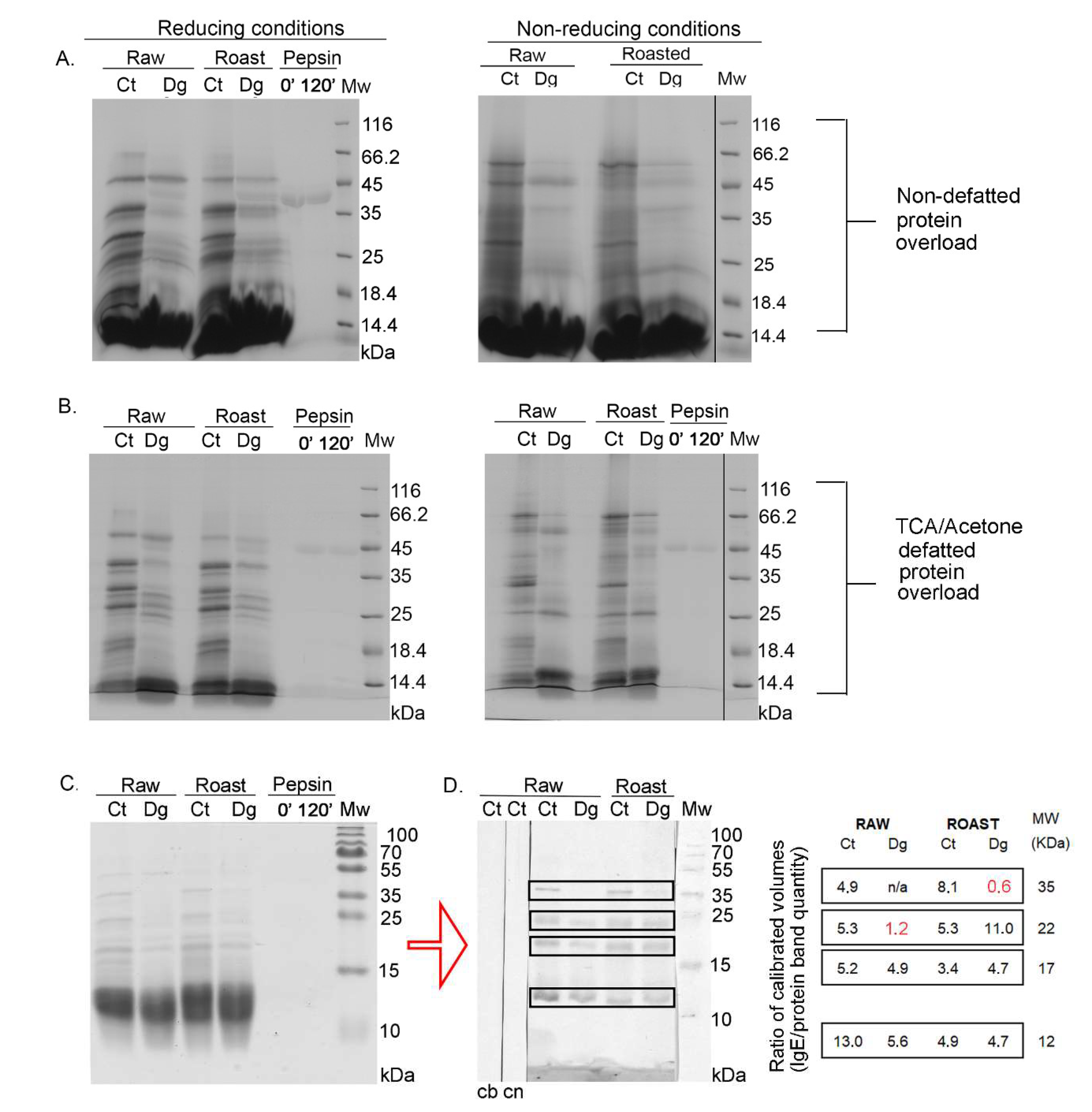
3.1. 1D SDS-PAGE and 1D Immunoblots of Raw and Roasted Gastric Hazelnut Digesta and Their Controls
3.2. 1D Immunoblot of Non-Defatted Gastric Digesta of Raw and Roasted Hazelnuts and Their Controls
3.3. 2D SDS-PAGE and 2D Immunoblots of Non-Defatted Gastric Digesta
3.4. Roasting Induced Slight Structural Changes with No Overall Effect on IgE Binding Capacities of Cor a 9 Purified from Raw and Roasted Hazelnuts
3.5. Assessment of IgE Binding Potency after Simulated Gastric Digestion of Raw and Roasted Hazelnuts
3.6. Specific Antibody Binding to Cor a 8 in Electrophoretically Resolved Raw and Roasted Hazelnut Gastric Digesta
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Uotila, R.; Röntynen, P.; Pelkonen, A.S.; Voutilainen, H.; Kaarina Kukkonen, A.; Mäkelä, M.J. For hazelnut allergy, component testing of Cor a 9 and Cor a 14 is relevant also in birch-endemic areas. Allergy 2020, 75, 2977–2980. [Google Scholar] [CrossRef] [PubMed]
- Geiselhart, S.; Hoffmann-Sommergruber, K.; Bublin, M. Tree nut allergens. Mol. Immunol. 2018, 100, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Grishina, G.; Bardina, L.; Grishin, A.; Sampson, H.A. Identification of an 11S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactions. J. Allergy Clin. Immunol. 2002, 110, 517–523. [Google Scholar] [CrossRef]
- Skypala, I.J.; Asero, R.; Barber, D.; Cecchi, L.; Diaz Perales, A.; Hoffmann-Sommergruber, K.; Pastorello, E.A.; Swoboda, I.; Bartra, J.; Ebo, D.G.; et al. Non-specific lipid-transfer proteins: Allergen structure and function, cross-reactivity, sensitization, and epidemiology. Clin. Transl. Allergy 2021, 11, e12010. [Google Scholar] [CrossRef]
- Wigotzki, M.; Steinhart, H.; Paschke, A. Influence of Varieties, Storage and Heat Treatment on IgE binding Proteins in Hazelnuts (Corylus avellana). Food Agric. Immunol. 2000, 12, 217–229. [Google Scholar] [CrossRef]
- Dooper, M.M.B.W.; Plassen, C.; Holden, L.; Moen, L.H.; Namork, E.; Egaas, E. Antibody binding to hazelnut (Corylus avellana) proteins: The effects of extraction procedure and hazelnut source. Food Agric. Immunol. 2008, 19, 229–240. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B. Hazelnut Allergens: Molecular Characterization, Detection, and Clinical Relevance. Crit. Rev. Food Sci. Nutr. 2016, 56, 2579–2605. [Google Scholar] [CrossRef]
- Vieths, S.; Reindl, J.; Müller, U.; Hoffmann, A.; Haustein, D. Digestibility of peanut and hazelnut allergens investigated by a simple in vitro procedure. Eur. Food Res. Technol. 1999, 209, 379–388. [Google Scholar] [CrossRef]
- Pfeifer, S.; Bublin, M.; Dubiela, P.; Hummel, K.; Wortmann, J.; Hofer, G.; Keller, W.; Radauer, C.; Hoffmann-Sommergruber, K. Cor a 14, the allergenic 2S albumin from hazelnut, is highly thermostable and resistant to gastrointestinal digestion. Mol. Nutr. Food Res. 2015, 59, 2077–2086. [Google Scholar] [CrossRef]
- Schimek, E.M.; Zwölfer, B.; Briza, P.; Jahn-Schmid, B.; Vogel, L.; Vieths, S.; Ebner, C.; Bohle, B. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J. Allergy Clin. Immunol. 2005, 116, 1327–1333. [Google Scholar] [CrossRef]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of food allergens to digestion in vitro. Nat. Biotechnol. 1996, 14, 1269–1273. [Google Scholar] [CrossRef]
- Korte, R.; Bräcker, J.; Brockmeyer, J. Gastrointestinal digestion of hazelnut allergens on molecular level: Elucidation of degradation kinetics and resistant immunoactive peptides using mass spectrometry. Mol. Nutr. Food Res. 2017, 61, 1700130. [Google Scholar] [CrossRef]
- Di Stasio, L.; d’Acierno, A.; Picariello, G.; Ferranti, P.; Nitride, C.; Mamone, G. In vitro gastroduodenal and jejunal brush border membrane digestion of raw and roasted tree nuts. Food Res. Int. 2020, 136, 109597. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Prodic, I.; Stanic-Vucinic, D.; Apostolovic, D.; Mihailovic, J.; Radibratovic, M.; Radosavljevic, J.; Burazer, L.; Milcic, M.; Smiljanic, K.; van Hage, M.; et al. Influence of peanut matrix on stability of allergens in gastric-simulated digesta: 2S albumins are main contributors to the IgE reactivity of short digestion-resistant peptides. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 731–740. [Google Scholar] [CrossRef]
- Prodić, I.; Smiljanić, K.; Simović, A.; Radosavljević, J.; Ćirković Veličković, T. Thermal Processing of Peanut Grains Impairs Their Mimicked Gastrointestinal Digestion While Downstream Defatting Treatments Affect Digestomic Profiles. Foods 2019, 8, 463. [Google Scholar] [CrossRef]
- Rao, H.; Tian, Y.; Fu, W.; Xue, W. In vitro digestibility and immunoreactivity of thermally processed peanut. Food Agric. Immunol. 2018, 29, 989–1001. [Google Scholar] [CrossRef]
- Hansen, K.S.; Ballmer-Weber, B.K.; Lüttkopf, D.; Skov, P.S.; Wüthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts--allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy 2003, 58, 132–138. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Apostolovic, D.; Stanic-Vucinic, D.; de Jongh, H.H.; de Jong, G.A.; Mihailovic, J.; Radosavljevic, J.; Radibratovic, M.; Nordlee, J.A.; Baumert, J.L.; Milcic, M.; et al. Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci. Rep. 2016, 6, 29249. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Rigby, N.M.; Marsh, J.; Sancho, A.I.; Wellner, K.; Akkerdaas, J.; van Ree, R.; Knulst, A.; Fernández-Rivas, M.; Brettlova, V.; Schilte, P.P.; et al. The purification and characterisation of allergenic hazelnut seed proteins. Mol. Nutr. Food Res. 2008, 52 (Suppl. S2), S251–S261. [Google Scholar] [CrossRef]
- De Angelis, E.; Bavaro, S.L.; Monaci, L.; Pilolli, R. Effects of the Varietal Diversity and the Thermal Treatment on the Protein Profile of Peanuts and Hazelnuts. J. Food Qual. 2018, 2018, 7635957. [Google Scholar] [CrossRef]
- Guo, F.; Kothary, M.H.; Wang, Y.; Yu, X.; Howard, A.J.; Fu, T.-J.; Zhang, Y.-Z. Purification and crystallization of Cor a 9, a major hazelnut allergen. Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 42–46. [Google Scholar] [CrossRef]
- Ribeiro, M.; Costa, J.; Mafra, I.; Cabo, S.; Silva, A.P.; Gonçalves, B.; Hillion, M.; Hébraud, M.; Igrejas, G. Natural Variation of Hazelnut Allergenicity: Is There Any Potential for Selecting Hypoallergenic Varieties? Nutrients 2020, 12, 2100. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R. Digestion of Raw and Roasted Almonds in Simulated Gastric Environment. Food Biophys. 2009, 4, 365–377. [Google Scholar] [CrossRef]
- Schmitt, D.A.; Nesbit, J.B.; Hurlburt, B.K.; Cheng, H.; Maleki, S.J. Processing can alter the properties of peanut extract preparations. J. Agric. Food Chem. 2010, 58, 1138–1143. [Google Scholar] [CrossRef]
- Smiljanic, K.; Apostolovic, D.; Trifunovic, S.; Ognjenovic, J.; Perusko, M.; Mihajlovic, L.; Burazer, L.; van Hage, M.; Cirkovic Velickovic, T. Subpollen particles are rich carriers of major short ragweed allergens and NADH dehydrogenases: Quantitative proteomic and allergomic study. Clin. Exp. Allergy 2017, 47, 815–828. [Google Scholar] [CrossRef]
- Nitride, C.; Mamone, G.; Picariello, G.; Mills, C.; Nocerino, R.; Berni Canani, R.; Ferranti, P. Proteomic and immunological characterization of a new food allergen from hazelnut (Corylus avellana). J. Proteom. 2013, 86, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Liang, R.; Huang, H.; Ma, X. Maillard Reaction Induced Changes in Allergenicity of Food. Foods 2022, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Chung, S.-Y.; Champagne, E.T.; Raufman, J.-P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.R.; Gaspari, M.; Hefle, S.L.; Koppelman, S.J. Proteolytic processing of the peanut allergen Ara h 3. Mol. Nutr. Food Res. 2005, 49, 744–755. [Google Scholar] [CrossRef]
- Offermann, L.R.; Bublin, M.; Perdue, M.L.; Pfeifer, S.; Dubiela, P.; Borowski, T.; Chruszcz, M.; Hoffmann-Sommergruber, K. Structural and Functional Characterization of the Hazelnut Allergen Cor a 8. J. Agric. Food Chem. 2015, 63, 9150–9158. [Google Scholar] [CrossRef]
- Gatehouse, J.A.; Croy, R.R.D.; Boulter, D.; Shewry, P.R. The synthesis and structure of pea storage proteins. Crit. Rev. Plant Sci. 1984, 1, 287–314. [Google Scholar] [CrossRef]
- Cucu, T.; De Meulenaer, B.; Bridts, C.; Devreese, B.; Ebo, D. Impact of thermal processing and the Maillard reaction on the basophil activation of hazelnut allergic patients. Food Chem. Toxicol. 2012, 50, 1722–1728. [Google Scholar] [CrossRef]
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and functional analysis of various globulin proteins from soy seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502. [Google Scholar] [CrossRef]
- Mills, E.N.; Marigheto, N.A.; Wellner, N.; Fairhurst, S.A.; Jenkins, J.A.; Mann, R.; Belton, P.S. Thermally induced structural changes in glycinin, the 11S globulin of soya bean (Glycine max)—An in situ spectroscopic study. Biochim. Biophys. Acta 2003, 1648, 105–114. [Google Scholar] [CrossRef]
- Dyer, S.; Nesbit, J.B.; Cabanillas, B.; Cheng, H.; Hurlburt, B.K.; Maleki, S.J. Contribution of Chemical Modifications and Conformational Epitopes to IgE Binding by Ara h 3. Foods 2018, 7, 189. [Google Scholar] [CrossRef]
- Müller, U.; Lüttkopf, D.; Hoffmann, A.; Petersen, A.; Becker, W.M.; Schocker, F.; Niggemann, B.; Altmann, F.; Kolarich, D.; Haustein, D.; et al. Allergens in raw and roasted hazelnuts (Corylus avellana) and their cross-reactivity to pollen. Eur. Food Res. Technol. 2000, 212, 2–12. [Google Scholar] [CrossRef]
- Wigotzki, M.; Schubert, S.; Steinhart, H.; Paschke-Kratzin, A. Effects of in vitro digestion on the IgE binding activity of proteins from hazelnuts (Corylus avellana). Internet Symp. Food Allerg. 2000, 2, 1–8. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodić, I.; Smiljanić, K.; Nagl, C.; Ballmer-Weber, B.; Hoffmann-Sommergruber, K.; Veličković, T.Ć. INFOGEST Digestion Assay of Raw and Roasted Hazelnuts and Its Impact on Allergens and Their IgE Binding Activity. Foods 2022, 11, 2914. https://doi.org/10.3390/foods11182914
Prodić I, Smiljanić K, Nagl C, Ballmer-Weber B, Hoffmann-Sommergruber K, Veličković TĆ. INFOGEST Digestion Assay of Raw and Roasted Hazelnuts and Its Impact on Allergens and Their IgE Binding Activity. Foods. 2022; 11(18):2914. https://doi.org/10.3390/foods11182914
Chicago/Turabian StyleProdić, Ivana, Katarina Smiljanić, Christoph Nagl, Barbara Ballmer-Weber, Karin Hoffmann-Sommergruber, and Tanja Ćirković Veličković. 2022. "INFOGEST Digestion Assay of Raw and Roasted Hazelnuts and Its Impact on Allergens and Their IgE Binding Activity" Foods 11, no. 18: 2914. https://doi.org/10.3390/foods11182914
APA StyleProdić, I., Smiljanić, K., Nagl, C., Ballmer-Weber, B., Hoffmann-Sommergruber, K., & Veličković, T. Ć. (2022). INFOGEST Digestion Assay of Raw and Roasted Hazelnuts and Its Impact on Allergens and Their IgE Binding Activity. Foods, 11(18), 2914. https://doi.org/10.3390/foods11182914






